Animal Safety Test of Bacillus thuringiensis BT Protein
Weimeng NING, Dingkuo LIU, Fang LIU, Yuan LI, Xiaowei YANG, Zhenguo GUAN, Guijun YANG, Bo ZHANG, Pengcheng HU*
1. Dingzheng Xinxing Biotechnology (Tianjin) Co., Ltd., Tianjin 300383, China; 2. Tianjin Agricultural College, Tianjin 300393, China; 3. Tianjin Key Laboratory of Biological Feed Additives, Tianjin 300383, China; 4. Tianjin Feed Small Molecule Protein Engineering Center, Tianjin 300383, China; 5. Tianjin Agricultural Ecological Environment Monitoring and Agricultural Product Quality Testing Center, Tianjin 300193, China
Abstract [Objectives] To determine the biological safety of BT protein from Bacillus thuringiensis (Bt) fermentation broth to mammals at high doses. [Methods] Healthy mice were randomly divided into 4 groups with 10 mice in each group. The experimental groups were fed with Bt fermentation supernatant at 0.2, 0.6 and 1.0 mL/kg, respectively, once a day for 7 consecutive days. The blank control group was fed normally without intragastric administration. [Results] There was no significant difference in blood routine and blood biochemical analysis between the experimental group and the control group. After intragastric administration, the mice were dissected, and no obvious pathological changes were found; the heart, liver, spleen, lung and kidney were taken to make tissue sections, and no pathological changes were found by microscopic observation. [Conclusions] Mice can tolerate high doses of BT protein from B. thuringiensis fermentation broth.
Key words Bacillus thuringiensis (Bt), BT protein, Biological safety
1 Introduction
Bacillusthuringiensis(Bt) can produce exocrine VIP protein in the vegetative growth stage, and a special parasporal crystal CRY protein will be produced in the spore after entering the budding reproductive stage. These proteins, collectively known as BT protein, have insecticidal effects[1]. The insecticidal mechanism of BT protein has been widely studied. The basic pathway is that there is a specific site in the midgut epithelial cells of the parasite, which can cause membrane perforation after binding with BT protein, eventually leading to the death of the parasite[2-4]. This mechanism mainly acts on lepidopteran insects. Because there is no BT protein receptor on the surface of intestinal cells of mammals, humans and livestock are not sensitive to this protein. Therefore, BT protein is widely used as a harmless insecticide in the field of insecticide[5-6]. However, whether the toxicological effects of BT protein will cause pathological changes in mammals due to its accumulation in the body is still uncertain. In view of these, we intended to increase the concentration ofB.thuringiensis, selected healthy mice as target animals, to understand the effects of BT protein purification liquid on clinical manifestations, physiological and biochemical indicators of animals, so as to provide a theoretical basis for the clinical application of the product.
2 Materials and methods
2.1 Materials and instrumentsForty female healthy mice (experimental animal license: SYXK (Beijing) 2020-0053), weight 18-22 g; BT protein purified solution ofB.thuringiensisfermentation, Dingzheng Xinxing Biotechnology (Tianjin) Co., Ltd.; peptone and yeast powder, Tianjin Beilian Fine Chemicals Development Co., Ltd.
SHY-2A Thermostatic Water Bath Shaker, Shanghai Bilon Instrument Manufacturing Co., Ltd.; H1850R Refrigerated Centrifuge, Hunan Xiangyi Laboratory Instrument Development Co., Ltd.; Countess 3, Thermo Fisher Scientific; FDC NX500iVC Blood Biochemical Analyzer, Fuji Film Medical Systems Co., Ltd.; HS-3315 tissue microtome, Beijing Leibo Ruijie Technology Co., Ltd.; ECLIPSE SI microscope, Nikon Precision Machinery (Shanghai) Co., Ltd.
2.2 Methods
2.2.1Grouping of experimental animals. The experimental mice were randomly divided into 4 groups with 10 mice in each group, and the grouping is shown in Table 1.

Table 1 Grouping and treatment of experimental animals
2.2.2Observation indicators. Generally, the observation period of animal safety experiment is 7-28 d. In this experiment, it is considered that the storage period of fermented products should not be too long, and the longer the storage period, the higher the risk of contamination. In addition, it is considered that BT protein is easily absorbed in the gastrointestinal environment, and 7 d is enough to produce enough accumulation, so it is determined to observe the relevant indicators on the 7thday.
(i) Clinical observation. During the test, the animals were observed daily for drug-related adverse reactions, such as respiratory and behavioral abnormalities, mental depression, and abnormal defecation.
(ii) Body weight. Weighed the mice before and after the experiment and made records.
(iii) Blood sample analysis. First, blood collection and treatment methods. Blood samples were collected from all mice before and after administration, and placed in two tubes; one tube was a centrifuge tube with anticoagulant for blood routine analysis, and the other tube was a centrifuge tube without anticoagulant. Blood samples were kept at room temperature for 2 h, centrifuged at 2 500 r/min for 5 min, and the upper serum was taken and stored at -20 ℃ for blood biochemical analysis.
Second, blood routine analysis. White blood cell count (WBC), white blood cell classification[neutrophil (NE), lymphocyte (LY), monocyte (MO), eosinophil (EO), basophil (BA)], red blood cell count (RBC), hemoglobin (HGB), hematocrit (HCT), platelet count (PLT) and other indicators were analyzed.
Third, analysis of blood biochemical indicators. Serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), blood urea nitrogen (BUN), creatinine (Cr), albumin (ALB), total protein (TP), total cholesterol (CHO), and triglyceride (TG) were analyzed.
(iv) Anatomical examination. To understand the safety of the preparation in clinical application, the target animals were examined by gross anatomy, and the heart, liver, spleen, lung, kidney and other organs were selected for pathological anatomy and pathological tissue observation.
2.3 Statistical analysisThe data were presented in mean±standard deviation, and the differences between groups were analyzed by chi-square test in SPSS 13.0, withP<0.05 andP<0.01 separately indicating significant and extremely significant differences.
3 Results and analysis
3.1 Clinical observation after administrationThe respiration, behavior, spirit, defecation and appetite of the animals in each group were normal after administration, and no adverse reactions occurred.
3.2 Body weight change before and after administrationThe results of animal weight before and after administration are shown in Table 2. The results showed that there was no significant difference in body weight before and after administration (P>0.05).
3.3 Results of blood routine analysisThe results of blood routine indicators of mice in each group are shown in Table 3. It can be seen from Table 3 that the blood routine indicators before and after administration in each dose group were not significantly different from those in the normal group (P>0.05), indicating that the purified supernatant of Bt fermentation in the dose range of 0.2, 0.6 and 1.0 mL/kg had no significant effect on the blood routine indicators.
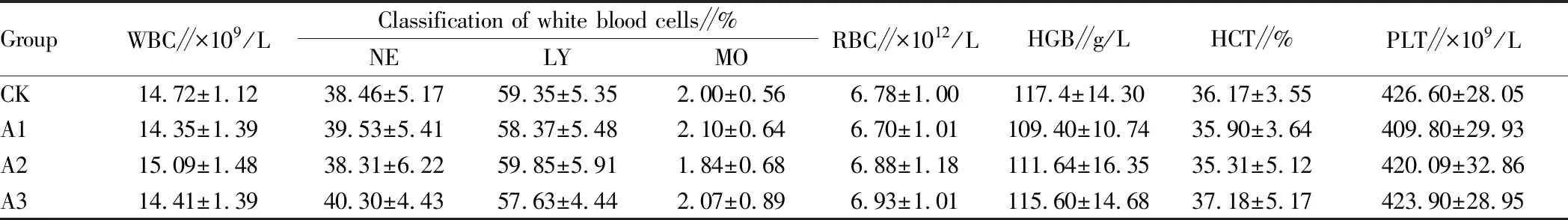
Table 3 Effects of different doses of purified supernatant of Bt fermentation on blood routine indicators of mice
3.4 Results of blood biochemical analysisThe detection results of blood biochemical indicators of mice are shown in Table 4. It can be seen from Table 4 that there was no significant difference (P>0.05) between the blood biochemical indicators of each dose group before and after administration and those of the normal group, indicating that the Bt fermentation and purified supernatant had no significant effect on the blood biochemical indicators in the dose range of 0.2, 0.6 and 1.0 mL/kg.

Table 4 Effects of different doses of purified supernatant of Bt fermentation on blood biochemical indicators of mice
3.5 Autopsy and histological observation resultsThe tissue sections of heart, liver, spleen, lung and kidney in the 1.0 mL/kg dose group and the control group are shown in Fig.1. There were no obvious macroscopic pathological changes in the results of gross autopsy and histological examination.
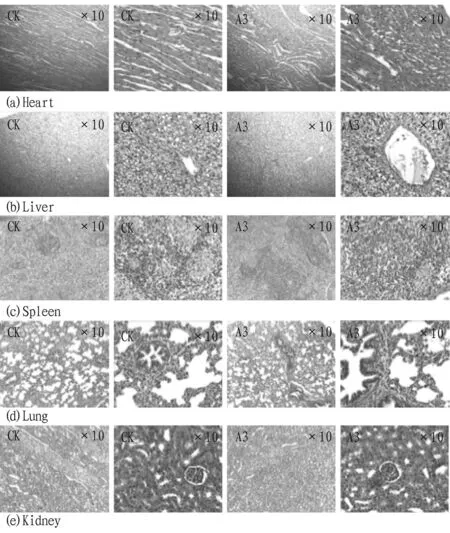
Fig.1 Tissue section
4 Conclusions
In summary, the body characteristics of mice were not abnormal compared with the control group when the purified supernatant of Bt fermentation was administered at the doses of 0.2, 0.6 and 1.0 mL/kg. The results of blood routine and blood biochemical analysis showed that there was no significant difference between the two groups. After the administration, mice were dissected and tissue sections were made, and no pathological changes were observed through 10 times and 40 times microscope. The results showed that the purified supernatant of Bt fermentation had high biological safety.
 Asian Agricultural Research2024年2期
Asian Agricultural Research2024年2期
- Asian Agricultural Research的其它文章
- Food Security Problems and Solutions in China Based on the Strategy of Sustainable Agricultural Development
- Discussion on Dust Removal (Suppression) System of Crushing Station in Open-Pit Coal Mine
- Effects of Fungi Fusarium sp. to Rhizosphere Soil and Physiological Characteristics of Camellia oleifera Abel.
- Promoting High-quality Development of Grain and Oil in Ethnic Areas of the Yangtze River Economic Belt from the Perspective of Agricultural Power
- A Comparative Study on Factors Influencing Food Security in China and India
- Study on Germplasm Resources of Peach Cultivars
