β-C(sp3)-H chlorination of amide derivatives via photoinduced copper charge transfer catalysis
Yuhang He ,Chao Tian ,Guanghui An ,Guangming Li
Key Laboratory of Functional Inorganic Material Chemistry (MOE),School of Chemistry and Materials Science,Heilongjiang University,Harbin 150080,China
Keywords: Photoinduced copper catalysis Ligand-to-metal charge transfer 1,4-Hydrogen atom transfer β-Halogenated amide Late-stage functionalization
ABSTRACT An atom economic β-C(sp3)-H chlorination of amide derivatives has been developed.This mild protocol employs CuCl2 instead of palladium catalysts with atom-economic HCl as chlorine sources and enables the late-stage functionalization of medicine derivatives.Mechanism studies suggest a plausible visible light triggered ligand-to-metal charge transfer (LMCT)/1,4-hydrogen atom transfer (HAT) cascade.
Amide moieties are ubiquitous structural motif in various natural products,pharmaceuticals,and fine chemicals [1-3].In particular,β-halogenated amides are key building blocks in many pharmaceuticals,agrochemicals as well as synthetic precursors(Scheme 1a) [4-9].Owing to its atom-and step-economy,Pd catalyzedβ-C-H halogenation was developed as one of the most powerful synthetic methods forβ-haloamides (Scheme 1b)[10-19].Yu and coworkers employed PhI(OAc)2and I2as halogen sources to achieve Pd-catalyzedβ-diiodination of carboxylic acid derivative [20,21].Sahoo [22],Rao [23],Besset [24] and Yu[25] disclosed Pd-catalyzedβ-halogenation of aliphatic amides,usingN-halosuccimide orN-halophthalimide as halogen sources.In these cases,acid additive or special ligand were required.Despite these advances,palladium catalysts were required and large amount of by-products would be produced from these halogen sources.Therefore,a distinct protocol using less-expensive catalysts with atom economic halogen sources would be environmental benign and highly demanding.

Scheme 1.Challenges of β-C(sp3)-H halogenation and the current work.
HCl is a sustainable halogen sources as hydrogen atom would go into its by-product compared withN-halosuccimide orNhalophthalimide.Lu’s group disclosed the only case for bothβchlorination andβ-bromination of pivalic acid,using NaCl or KBr as halogen sources with extra oxidants [26,27].However,utilization of halogens in HCl would be more challenging,as the activation of H-Cl bond is energy costing.
Visible-light-mediated ligand-metal charge transfer (LMCT) processes emerged as a powerful strategy for efficient organic synthesis [28-50].Several photo-induced LMCT systems were developed to generate halogen radicals [28-37].In these works,previous expensive catalysts were replaced by earth-abundant Ni [29],Ce [43],Cu [28,30] or Fe [33,35,37] salts,which upon irradiation would undergo LMCT with readily available halogen sources to generate halogen radicals.In 2021,Wan’s group disclosed chlorine radical generation from the combination of CuCl2and HCl [28].Inspired by Wan’s work and recent visible light enabled copper catalysis[51-55],we envisioned that chlorine radical generated from copper charge transfer catalysis would form amide radicalA,which might trigger 1,4-HAT [56-59] to produceβ-halogenated amides(Scheme 1c).Along our efforts on remote site-selective C-H functionalization [60-63],we herein report photoinduced copper catalyzedβ-C(sp3)-H chlorination of amides derivatives (Scheme 1c).Combination of LMCT and 1,4-HAT processes allowed the utilization of atom economic HCl as halogenation sources with less expensive CuCl2catalysts.Furthermore,this mild halogenation approach enabled the late-stage functionalization of complex medicinal derivatives.
At the outset,the study was initiated by exposingN-phenyl pivalamide (1a) to CuCl2as catalysts andN-chlorosuccinimide(NCS) as halogen sources,providing theβ-chlorinated product2ain 25% yield with aromatic chlorination products (Scheme 2).To avoid halogenation of arenes,we chose electron-deficient aniline derivativeN-(2,5-dichlorophenyl)pivalamide (1b) as model substrate and HCl as halogen sources,affording monochlorinated2band dichlorinated2b’in 70% and<5% yield respesctively (Table 1,entry 1).Diverse metal catalysts,which were reported to undergo LMCT processes [28-30,33,35,37,43],provided inferior yields (entry 2).Replacement of HCl with other halogen sources and readily available halogen salts used in LMCT processes significantly reduced the reaction efficiency (entry 3),probably attributing to good synergistic effect between CuCl2and HCl.In addition,we tested previously established LMCT conditions by other groups (entries 4 and 5) [30,32].In absence of either light or CuCl2,the halogenation processes hardly vanished,indicating that the catalyst and light are critical for this reaction (entry 6).Further investigation of solvent,light sources and temperature confirmed the optimal conditions as: HCl (0.5 mmol,5.0 equiv.,37% in water),CuCl2(40 mol%),in MeCN (0.5 mL) under air,irradiated with 100 W white light LEDs at r.t.for 24 h (entries 7-9).Notably,the reaction afforded slightly higher yield under oxygen and the yield of2bsignificantly decreased under argon (entries 10 and 11).Given the results of control experiments and previous reports [28],oxygen together with HCl oxidizes Cu(I) species to regenerate Cu(II)catalysts.

Table 1Optimization of the reaction conditions.a
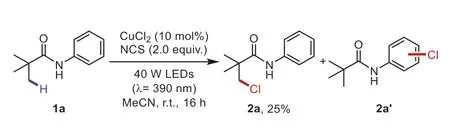
Scheme 2.Preliminary study on the chlorination of amide derivatives.
Having identified the optimized conditions,we set out to explore the scope for various anilides (Scheme 3a).Diverse chlorinated aniline derived amides exhibited good reactivity under optimal conditions (2b-2e).Notably,anilides with trifluoromethyl groups afforded better yields (2f,2h).However,chlorination only occurred on aromatic rings for anilides with electron-donating groups (2i,2j),which could be attributed to electron-rich nature of these arenes.Next,we turned our attention to the scope of 3,5-bis(trifluoromethyl)anilides (2k-2u) deriving from various carboxylic acids (Scheme 3b).Various secondary and tertiary carboxylic amides were successfully chlorinated (2k-2r) under optimal conditions.Interestingly,β-chlorination of 2-methylvaleric acid-derived amides under mildly acidic condition providedβchloroamide2kandδ-chloroamide2k’.β-Halogenation selectively occurred on tertiary carboxylic amides bearing the aromatic rings without substitution on arenes (2p,2q).Given the importance of the bridged 1-adamantanecarboxylic acid in drug discovery,its derivative was selectively chlorinated at the beta position to provide2rin 75% yield.For the three-membered ring-containing anilides,the chlorination did not occur on the cyclopropyl ring (2s,2t),possibly owing to the steric constraint of the reaction.Notably,the unsubstituted cyclopropanecarboxylic acid derivative afforded the ring-opening dichloride product2u.

Scheme 3.Reaction scope.Standard conditions: 1 (0.1 mmol),HCl (5.0 equiv.),CuCl2 (40 mol%),in MeCN (0.5 mL) under air,irradiated with 100 W white LEDs at r.t.for 24 h.a HBr (5.0 equiv.),CuBr2 (40 mol%).b For 2 h.
Encouraging by these results,this protocol was applied for the late-stage structural modification of drugs (Scheme 4a).Ketoprofen,flurbiprofen,and carprofen derivative,nonsteroidal antiinflammatory drugs could be readily converted to their chlorinated derivatives (2v-2x) with recovery of starting materials.As expected,the electron-rich aryl moiety of gemfibrozil underwent an electrophilic chlorination reaction to provide the product2y.A large-scaled reaction by reacting 0.783 g of1r(2 mmol) with HCl afforded2r(0.588 g) in 69% yield.Furthermore,2rcould be successfully transformed into3aand3bthrough nucleophilic substitution (Scheme 4b) [25,64].
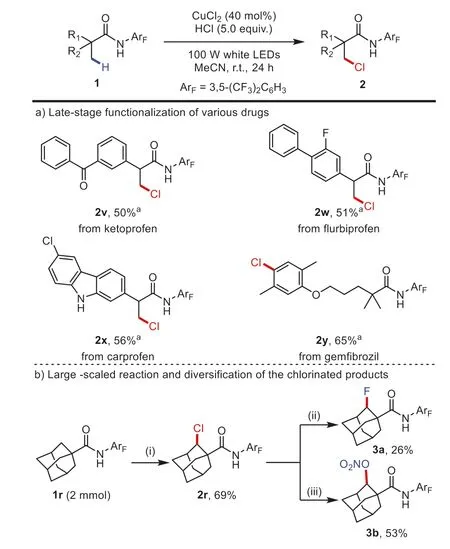
Scheme 4.Standard conditions: 1 (0.1 mmol),HCl (5.0 equiv.),CuCl2 (40 mol%),in MeCN (0.5 mL) under air,irradiated with 100 W white LEDs at r.t.for 24 h.a For 72 h.(i) Standard conditions;(ii) 2r (0.1 mmol,1.0 equiv.),AgF (4.2 equiv.),dry cyclohexane (1.6 mL),120 °C,38 h;(iii) 2r (0.1 mmol,1.0 equiv.),AgNO3 (2.0 equiv.),EtOAc (2.0 mL),120 °C,80 h.
We next sought to interrogate the mechanism of this reaction.Addition of TEMPO inhibited the reaction,and addition of 1,1-diphenylethylene successfully captured chlorine radical,affording the radical adduct4(Schemes 5a and b).To probe the generation of chlorine radical,a series of UV-vis spectra were carried out.UVvis spectroscopy of CuCl2/CH3CN and CuCl2/HCl/CH3CN solution exhibited typical peaks of [(MeCN)2CuCl2] and [(MeCN)CuCl3]-,respectively (Fig.1a) [65],indicating [(MeCN)2CuCl2] would react with HCl to form [(MeCN)CuCl3]-.According to literature [30],[(MeCN)CuCl3]-would readily undergo LMCT to generate chlorine radicals.This was further confirmed by the irradiation of CuCl2/HCl/CH3CN solutions,UV-vis spectroscopy of which showed[(MeCN)CuCl3]-vanished (Fig.1a).To further confirm the change of cupric oxidation state in the catalytic cycle,the X-ray photoelectron spectroscopy (XPS) measurement of the reaction mixture using the CuCl2/HCl system was carried out (Fig.1b).Fig.1b shows the high resolution XPS scans over Cu 2p3/2peak.The peak at 932.5 eV was known as the characteristic of Cu+[66],while the peak at 934.3 eV together with shake-up satellite peaks on the higher binding energy side,942.4,and 944.6 eV,indicated the presence of an unfilled Cu 3d shell and thus confirmed the existence of Cu2+[67,68].The results of UV-vis spectroscopy and XPS suggest that theβ-chlorination reaction may proceed through a Cu(I)-Cu(II) involving LMCT mechanism.To investigate the possibility of 1,4-HAT processes,we subjected substrate5to the standard conditions (Scheme 5c).Notably,β-halogenated product6was not detected,but 27% of product1band 9% of product2bwere obtained.It would be attributed to the lack of N-H bond in5,which is essential to trigger 1,4-HAT forβ-halogenation by converting N-H bond into N-Cl bond with chlorine radical from LMCT.Under the standard condition,1sprovided2swith 6% of ring-opening product7,a typical radical clock product (Scheme 5d) Quantum yield and light on/off experiments suggest that the transformation needed continuous irradiation of visible light and is not a radical chain processes (see Supporting information).

Fig.1.(a) UV-vis characterization of the reaction.(b) The X-ray photoelectron spectroscopy (XPS) data of the reaction mixture.

Scheme 5.Mechanism experiment.
Based on our mechanistic experiments and previous studies [28,30],we proposed the plausible mechanism (Scheme 6).CuCl2is coordinated with the acetonitrile to produce Cu(II) complex [(MeCN)2CuCl2],which is further converted to photoactive Cu(II) species [(MeCN)CuCl3]-by reacting with HCl.Upon irradiation,[(MeCN)CuCl3]-undergoes LMCT to generate chlorine radical,which abstracts N-H hydrogen of1bto affordBand HCl.Bprovides alkyl radicalsCvia1,4-HAT,which reacts with HCl to generate2b.Finally,according to Wang’s report [28],oxygen together with HCl oxidizes Cu(I) complex [(MeCN)2CuCl2]-to regenerate Cu(II) catalysts [(MeCN)CuCl3]-.
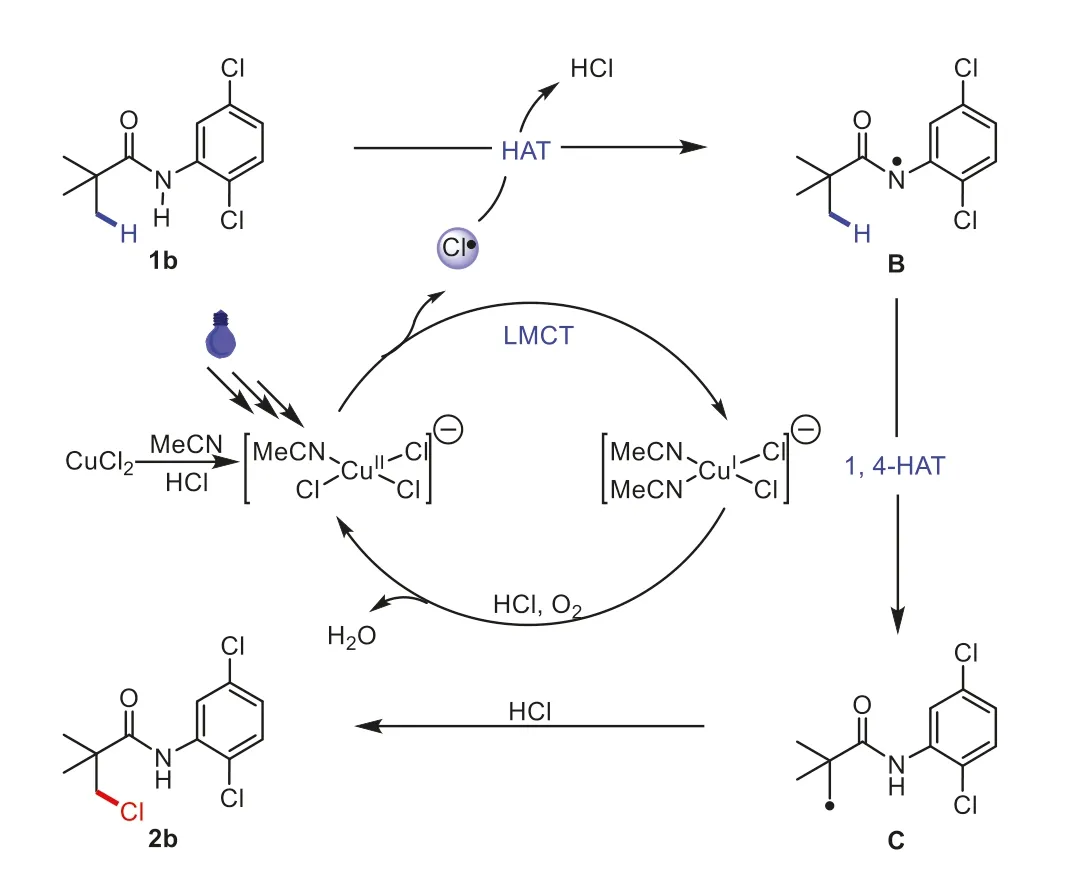
Scheme 6.Proposed mechanism.
In summary,we have achieved additive-freeβ-C(sp3)-H chlorination of amidesviacombination of photoinduced LMCT and 1,4-HAT.CuCl2instead of Pd catalysts has been developed as catalysts with atom-economic HCl as chlorine sources.Furthermore,the reaction enables the late-stage functionalization of medicinal related compounds.In addition,a feasible mechanism is proposed on the basis of several control experiments.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
The authors gratefully acknowledge support from the University Nursing Program for Young Scholars with Creative Talents in Heilongjiang Province (No.UNPYSCT-2017124).
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2023.108546.
 Chinese Chemical Letters2024年2期
Chinese Chemical Letters2024年2期
- Chinese Chemical Letters的其它文章
- The 3rd Xihua Chemistry and Biomedicine Forum
- Professor Hualiang Jiang: A tribute to an esteemed visionary chemist and pharmacist
- Recent advances in visible light-mediated chemical transformations of enaminones
- Development of porphyrin-based fluorescent sensors and sensor arrays for saccharide recognition
- Recent advances of versatile reagents as controllable building blocks in organic synthesis
- Synthetic host-guest pairs as novel bioorthogonal tools for pre-targeting☆
