CsAHL25通过影响CsHB1、LEC1/B3基因表达调控柑橘体细胞胚发生
叶长宁 徐梦梦 刘兰兰 付玉洁 葛晓霞
摘 要:【目的】基于柑橘體细胞胚发生相关基因CsHB1的启动子筛选其上游转录因子,以期为柑橘体细胞胚发生分子机制研究提供可靠的候选基因。【方法】利用CsHB1启动子(-1018~-558 bp)进行酵母单杂筛库实验,筛选出CsHB1上游转录因子CsAHL25;利用亚细胞定位实验,确定CsAHL25在细胞中的位置;通过酵母单杂点对点、双荧光素酶实验验证CsAHL25对CsHB1表达的影响;利用qRT-PCR探究CsAHL25基因在柑橘体细胞胚诱导过程中的表达模式;在柑橘愈伤组织中瞬时表达该基因,并检测体细胞胚发生相关基因的表达变化。【结果】CsAHL25在柑橘体细胞胚诱导过程中呈现先上升后下降的表达模式,该蛋白定位在细胞核中,能与CsHB1启动子结合并下调CsHB1的表达。瞬时表达CsAHL25会导致CsHB1表达量下调,及CsABI3、CsFUS3、CsLEC1、CsL1L等促进体细胞发生的LEC1/B3基因表达量上调。【结论】CsAHL25能直接下调CsHB1的表达,并使LEC1/B3基因表达量上升。CsAHL25可能通过调整CsHB1、LEC1/B3基因的表达促进体细胞胚发生。
关键词:柑橘:体细胞胚发生;HD-ZIP;AT-HOOK
中图分类号:S666 文献标志码:A 文章编号:1009-9980(2024)04-0579-11
CsAHL25 regulates citrus somatic embryogenesis by affecting the expression of CsHB1 and LEC1/B3 genes
YE Changning1, 3, XU Mengmeng1, 3, LIU Lanlan1, 3, FU Yujie1, 3, GE Xiaoxia2, 3*
(1College of Horticulture and Forestry Sciences, Huazhong Agricultural University, Wuhan 430070, Hubei, China; 2Journal Center of Academy of Science and Technology Development, Huazhong Agricultural University, Wuhan 430070, Hubei, China; 3 Center of Applied Boitechnology, Wuhan Institute of Bioengineering, Wuhan 430415, Hubei, China)
Abstract: 【Objective】Somatic embryogenesis (SE) is widely used in the conservation and utilization of plant germplasm resources. However, there is significant variation in the somatic embryogenesis (SE) capacity of calls derived from different citrus varieties. Furthermore, their SE capacity gradually diminishes during culture, posing a significant hindrance to the conservation and utilization of citrus germplasm resources. CsHB1, an HD-ZIP II gene associated with enhancing SE, was isolated from a citrus variety exhibiting robust SE capabilities. In this study, we harnessed the promoter of CsHB1 (pCsHB1) to search for upstream transcription factors to provide reliable candidate genes for the study on plant somatic embryogenesis. 【Methods】 To identify the upstream transcription factors of CsHB1, we cloned pCsHB1 (-1018 to -558 bp) into pAbAi and utilized a yeast one-hybrid (Y1H) assay to obtain the candidate transcription factor CsAHL25 from a yeast library. Using SMART, candidate genes were analyzed for domains and named based on annotations in the Citrus Pangenome Breeding Database. The expression pattern of this gene was measured by qRT-PCR in various somatic embryo developmental stages of citrus, aiming to deduce the function of CsAHL25. The gene was cloned and inserted into pRI121, transferred into GV3101 and Marker mixed annotated Nicotiana benthamiana. After 2 d, the localization in the cells was observed using the laser scanning confocal microscopy. CsAHL25 was cloned and inserted into pGADT7 and transfected into Y1HGold with pCsHB1-AbAi for the Y1H assay. A Y1H assay was performed to determine whether the two were complementary or not based on the growth of the yeast cells in the screening medium. The gene was cloned and inserted into the overexpression vector pCMBAI1300-35S, and pCsHB1 (-2377-0 bp) was cloned and inserted into pGreenII 0800-LUC. The two vectors were then separately transferred into GV3101 and mixed to transiently transform N. benthamiana, with empty vector used as a control. After 2 d, the fluorescence of LUC was observed using an in vivo Plant Fluorescent Imaging System, and the LUC/REN ratio was calculated. This was followed by a comparison with the control to determine the role of this gene in the downstream gene regulation of pCsHB1. To explore the function of this gene, we transiently expressed the gene in the callus of Citrus sinensis ‘Next, and qRT-PCR was used to detect the expression of somatic embryogenesis-related genes. 【Results】 A candidate transcription factor, named CsAHL25, which is involved in the regulation of CsHB1 expression, was identified from the results of Y1H screening. Sequence analysis revealed that CsAHL25 possesses a typeⅠ AT-HOOK domain and a type A PPC domain and belongs to the AHL15-29 subfamily of AT-HOOK. Subcellular localization analysis demonstrated that, similar to other AHL transcription factors, CsAHL25 is a nucleus-localized transcription factor. CsAHL25 exhibited high expression levels at 60 d and 120 d of somatic cell embryo induction. The expression pattern of CsAHL25 suggested that this gene may play a role in SE. The Y1H results showed that yeast cells containing CsAHL25 and pCsHB1 were able to grow well in SD-Leu/ABA200, indicating that CsAHL25 was bound to pCsHB1. The results of plant fluorescent imaging indicated that 1300+pCsHB1-LUC exhibited higher LUC values than CsAHL25-1300+pCsHB1-LUC. The LUC/REN results were consistent with the Plant Fluorescent Imaging outcomes, with the strongest LUC-related activity observed in 1300+pCsHB1-LUC. These results showed that CsAHL25 was bound to the integral pCsHB1 and repressed its transcription. To further investigate the function of CsAHL25 during SE, we performed transient transfection of CsAHL25 in the callus of C. sinensis ‘Anliucheng, and then analyzed gene expression by using qRT-PCR. The results showed that the expression of CsHB1 was significantly downregulated, while the LEC1/B3 genes promoting somatic embryogenesis, such as CsLEC1, CsL1L, CsFUS3 and CsABI3, were significantly upregulated. 【Conclusion】 The results of this study indicated that CsAHL25 was an upstream transcription factor of CsHB1, which can inhibit CsHB1 expression, and transient expression of CsAHL25 can cause upregulation of the expression of LEC1/B3 genes. Based on the expression pattern of CsAHL25, we studied the functions of HD-ZIP, LEC1/B3 and AHL25 in relation to citrus somatic embryogenesis. Finally, we hypothesized that CsAHL25 regulated citrus somatic embryogenesis.
Key words: Citrus; Somatic embryogenesis; HD-ZIP; AT-HOOK
植物体细胞胚发生是植物体细胞形成胚胎的过程,其发育过程与合子胚相似,均会经历球形胚、鱼雷形胚、子叶形胚等发育阶段[1]。随着体细胞胚诱导技术的发展,该技术已经成为植物种质资源保存与创制、重要经济植物大规模生产的重要技术手段之一[2]。柑橘是中国的重要经济作物,近年来,柑橘的种质资源发掘和遗传改良研究为中国柑橘产业发展提供了有力支持[3]。随着诱导出来的柑橘品种的胚性愈伤越来越多,利用体细胞进行柑橘种质资源的保存和育种已成为柑橘种质资源利用的重要手段 [3-4]。然而不同柑橘品种的体细胞胚发生能力有着巨大的差异,部分品种至今无法获得胚性愈伤[5],这对柑橘种质资源保存和育种造成阻碍,因此研究柑橘体细胞胚发生机制有着重要的生物学意义和应用价值。
在体细胞胚发生的过程中,转录因子可以通过影响信号转导调控体细胞胚发生[6]。前期研究表明,LEC[7-9]、FUS[10-12]、ABI[13]、HD-ZIP[14-16]转录因子在拟南芥、龙眼、冷杉、柑橘、油棕、紫花苜蓿等多种植物体细胞胚发生过程中起重要作用。其中HD-ZIP转录因子是一类植物特有的转录因子,具有亮氨酸拉链(ZIP)和与之紧密结合的同源结构域,根据HD-ZIP结构域的同源性、蛋白结构和功能可以将HD-ZIP转录因子分为4类,其中存在CPSCE结构域的HD-ZIP蛋白被归类于Ⅱ型HD-ZIP蛋白[17]。在植物胚胎发育的过程中,Ⅱ型HD-ZIP具有调控生长素转运、维持分生组织、保持植物细胞全能性和控制子叶发育的功能[18- 19]。
AT-HOOK是一类能与DNA序列中富含AT序列区域结合的转录因子[20]。其蛋白包含AT-HOOK结构域与PPC结构域两种保守结构,根据保守结构域的数量和种类可以将AT-HOOK转录因子分为两个亚家族,其中Ⅰ型AT-HOOK蛋白(AHL15-29)包含一个Ⅰ型AHL结构域和一个A型PPC结构域[21-22]。该亚家族成员AHL15、AHL19、AHL20是作用在植物胚胎发生早期的重要转录因子,Ⅰ型AT-HOOK基因的表达受生长素和BBM转录因子的调控,且具有促进植物体细胞胚发生的功能[23]。虽然前人研究已证实Ⅰ型AT-HOOK转录因子具有促进植物胚胎发生的作用,然而Ⅰ型AT-HOOK转录因子调控体细胞发生的分子机制仍未被报道。
在对柑橘体细胞胚发生的研究中,前人分离并鉴定出促进柑橘体细胞胚发生的Ⅱ型HD-ZIP转录因子CsHB1[24-25]。为了探索柑橘体细胞胚发生相关基因CsHB1的上游调控网络,笔者对调控CsHB1基因表达的转录因子进行挖掘,发现一个属于Ⅰ型AT-HOOK亚家族的基因CsAHL25,并对其功能进行初步验证,完善AT-HOOK转录因子的调控网络,为柑橘体细胞胚发生研究提供潜在的候选基因,以期推进柑橘体细胞胚发生的分子机制研究。
1 材料和方法
1.1 试验材料
MT培养基继代保存的纽荷尔脐橙(Citrus sinensis ‘Newhall)、暗柳橙(C. sinensis ‘Anliucheng)胚性愈伤组织。
1.2 CsHB1启动子片段诱饵菌株AbA表达水平检测
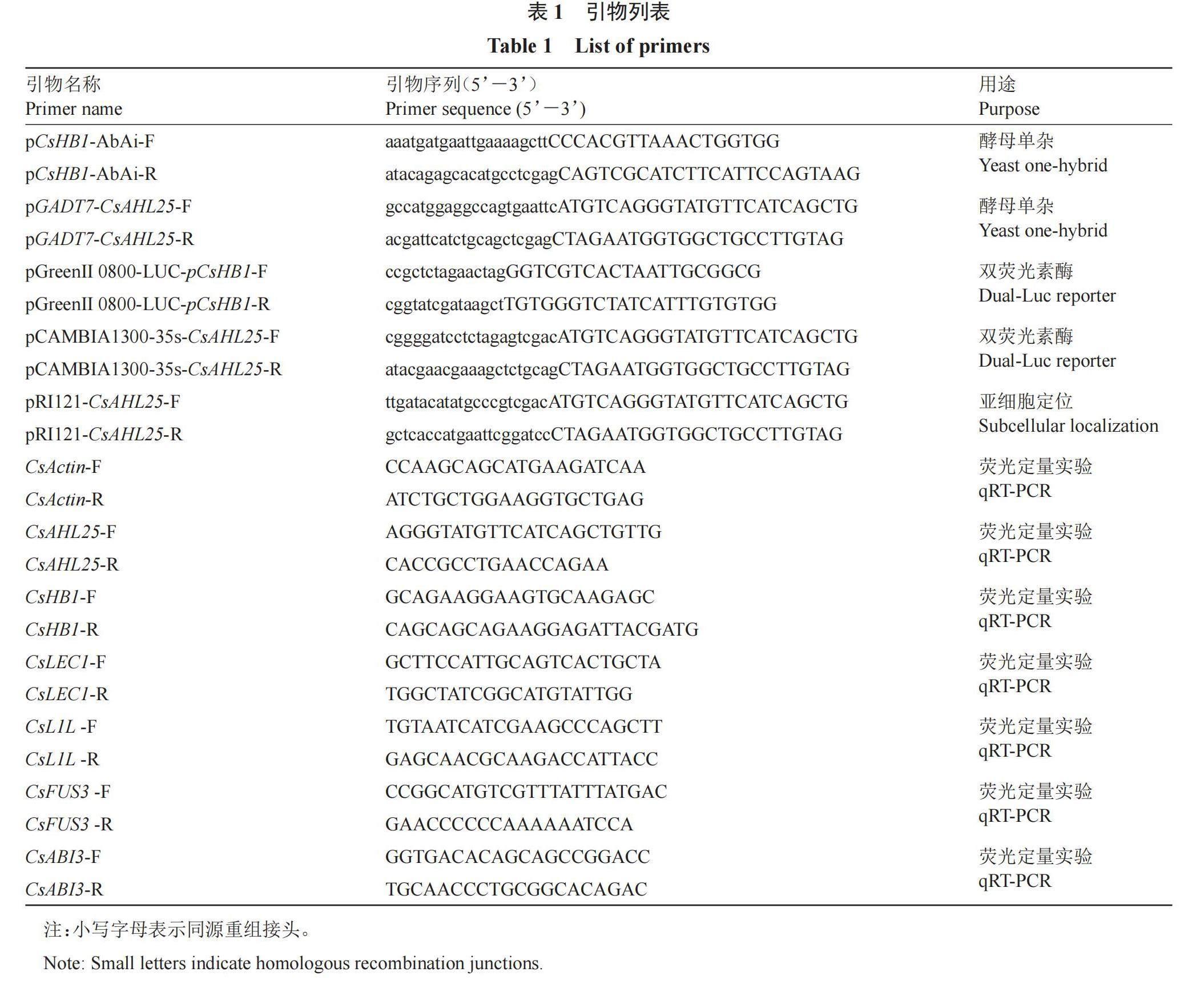
根据距CsHB1基因-1018~-558 bp的启动子片段序列和pAbAi載体序列设计引物(表1),使用Phanta Max聚合酶(诺唯赞,南京)从暗柳橙DNA中扩增目的片段,使用ClonExpress? Ⅱ同源重组试剂盒(诺唯赞,南京)将目的片段克隆到pAbAi载体中,用BstBⅠ限制性内切酶(新景,杭州)酶切后,使用酵母转化试剂盒(酷来搏,北京)将载体转入Y1H Gold酵母细胞中,获得含有pCsHB1-AbAi的酵母细胞。使用0.9%的NaCl 溶液悬浮酵母细胞(OD600=0.002),取100 μL悬浮菌液分别涂在0、50、100 ng·mL-1 AbA的培养基上,30 ℃倒置培养2?3 d,筛选最适浓度作为酵母单杂筛库和酵母单杂点对点实验的AbA浓度。
1.3 酵母单杂筛选CsHB1的上游转录因子
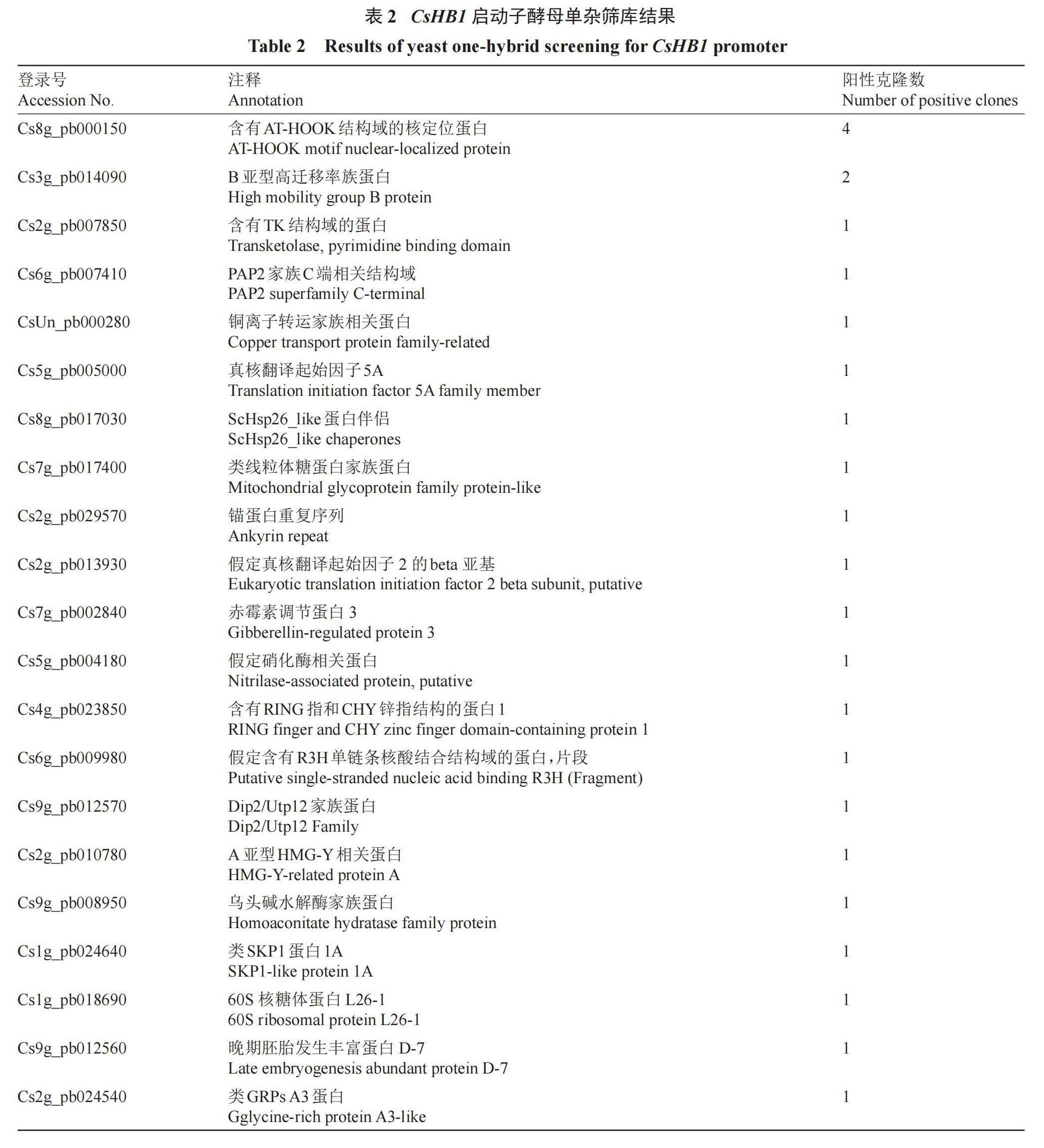
使用酵母转化试剂盒(酷来搏,北京)将各体细胞胚诱导时期的暗柳橙愈伤组织cDNA混合文库质粒转入含有pCsHB1-AbAi的Y1H Gold酵母细胞中,使用SD/-Leu/AbA200培养基筛选阳性克隆,使用Taq酶(翌圣,上海)进行阳性克隆鉴定,将鉴定片段大小500?2000 bp的PCR产物进行测序后,使用CPBD数据库(Citrus Pan-genome to Breeding Database,http://citrus.hzau.edu.cn/)进行比对,分析候选蛋白。
1.4 CsAHL25结构域分析
参考CPBD数据库中甜橙二代基因组注释数据和使用SMART(SMART:Main page)工具分析CsAHL25蛋白的氨基酸序列以确定其结构域。
1.5 CsAHL25系统发育树建立
使用NCBI(https://ncbi.nlm.nih.gov/gene)下载拟南芥(Arabidopsis thaliana)、水稻(Oryza sativa)的Ⅰ型AT-HOOK氨基酸序列。使用MEGA X软件将拟南芥、水稻的AT-HOOK和CsAHL25的氨基酸序列进行多序列比对,利用邻接法构建进化树,Bootstrapping参数值设置为1000次,使用iTOL网站(https://itol.embl.de/)进行数据可视化分析。
1.6 RNA的提取以及cDNA合成
收集甘油培养基(20 mL·L-1)诱导0 d(ALC 0 d)、60 d(ALC 60 d)、120 d(ALC 120 d)的暗柳橙愈伤组织,使用RN38 EASY spin plus植物RNA提取试剂盒(艾德莱,北京)提取组织RNA后,用RT SuperMix反转录试剂盒(诺唯赞,南京)合成cDNA。
1.7 实时荧光定量PCR分析
使用Primer Premier 5软件设计CsAHL25基因的定量引物(表1),以Actin基因作为内参基因。使用LC480实时荧光定量仪器(Roched,美国)和qPCR Master Mix试剂盒(诺唯赞,南京)进行qRT-PCR试验。
1.8 CsAHL25亚细胞定位分析
根据CsAHL25 CDS序列和pRI121载体序列设计引物(表1),从暗柳橙胚性愈伤组织cDNA中扩增序列,将去除终止密码子的CsAHL25 CDS序列克隆到pRI121载体中,获得pRI121-CsAHL25-GFP重组蛋白质粒后转入GV3101农杆菌中。将含有pRI121-CsAHL25-GFP 农杆菌液和核定位marker H2B-RFP菌液混合后注射烟草叶片,2 d后利用TCS SP8激光共聚焦显微镜(Leica,德国)观察荧光信号并拍照。
1.9 酵母单杂验证CsAHL25与CsHB1启动子互作
将CsAHL25 CDS序列克隆到pGADT7载体中(引物见表1),获得猎物载体pGADT7- CsAHL25并转入含有pCsHB1-AbAi的酵母細胞中。以空载猎物载体为阴性对照、pAbAi-P53+pGADT7-P53为阳性对照,将获得的阳性菌株按照10-1梯度稀释并分别接种在SD/-Leu、SD/-Leu/AbA200培养基上,通过观察酵母的生长状态判断CsAHL25转录因子和CsHB1启动子的互作情况。
1.10 LUC活体成像、双荧光素酶验证CsAHL25对CsHB1启动子活性的影响
将CsHB1启动子(-2377~0 bp)克隆到pGreenⅡ 0800-LUC载体中(引物见表1),获得报告子载体pGReenⅡ 0800-LUC-pCsHB1并转入GV3101(pSoup)农杆菌中,将CsAHL25 CDS序列克隆到pCAMBIA1300-35S载体中作效应子(引物见表1),并转入GV3103农杆菌中。以报告子空载pGReen Ⅱ 0800-LUC、效应子空载 pCAMBIA1300-35S作为对照,使用烟草瞬时注射将报告子、效应子菌液按体积比1∶5混合后注射到烟草叶片中,2 d后使用荧光素钾盐试剂盒(翌圣,上海),通过NightSHADE LB 985植物活体成像系统(Berthold、德国)观察其LUC荧光强度并拍照,使用双荧光素酶试剂盒(翌圣,上海),通过Infinite? 200多功能酶标仪(TECAN,瑞士)检测其双荧光素酶活性,计算LUC/REN的比值,得到LUC的相对活性。通过LUC荧光强度和LUC/REN的相对比值判断CsAHL25转录因子对CsHB1启动子活性的影响。
1.11 CsAHL25瞬时超量表达分析
参考张印[26]的方法使用农杆菌介导法将pCAMBIA1300-35s-CsAHL25农杆菌转入纽荷尔脐橙的愈伤组织中,以转pCAMBIA1300-35s载体的愈伤组织为空白对照,在含有AS的MT培养基上培养3 d后,提取愈伤组织的RNA进行荧光定量试验(引物见表1)。
1.12 数据分析
利用GraphPad 8软件、采用t-test进行显著性分析并作图。
2 结果与分析
2.1 CsHB1上游转录因子的筛选
为了筛选调控CsHB1表达的转录因子,利用CsHB1启动子片段(-1018~-558 bp)作为诱饵进行酵母单杂筛库实验。酵母AbA本底表达水平检测表明,在SD/-Ura/AbA100固体培养基上,含有pCsHB1-AbAi的Y1H Gold酵母细胞无法正常生长(图1),最终确定200 ng·mL-1作为酵母单杂筛库的AbA质量浓度值。酵母单杂筛库结果表明,共获得56个酵母克隆,使用PCR鉴定后得到39个插入片段大小在500~2000 bp之间,且条带单一的cDNA片段。测序结果使用CPBD数据库进行Blastx比对分析,去除假阳性克隆后,初步获得21个可能与CsHB1启动子片段结合的蛋白(表2),包括含有AT-HOOK结构域的核定位蛋白、含有RING指和CHY锌指结构的蛋白1、晚期胚胎发生丰富蛋白D-7等。
2.2 CsAHL25基因系统发育与表达模式分析
根据阳性克隆出现次数和相关报道,选择注释为含有AT-HOOK结构域的核定位蛋白Cs8g_pb000150进行研究。Cs8g_pb000150基因CDS长度为918 bp,编码305个氨基酸,进一步分析发现在其61~77 aa处存在一个Ⅰ型AHL结构域、89~214 aa处存在一个A型PPC结构域(图2-A),其结构域具有Ⅰ型AT-HOOK转录因子的特征。该蛋白与拟南芥、水稻Ⅰ型AT-HOOK转录因子进化关系分析表明,该蛋白与AtAHL25、OsAHL25在同一个进化支中(图2-B)。根据CPBD数据库注释与进化分析将该蛋白命名为CsAHL25。
qRT-PCR检测CsAHL25基因在体细胞胚诱导过程中的表达模式,发现在暗柳橙愈伤生胚诱导过程中,CsAHL25的相对表达量随着诱导时间的延长呈先上升后下降的趋势(图2-C),其表达模式暗示CsAHL25基因可能在柑橘的体细胞诱导过程中发挥作用。
2.3 CsAHL25蛋白定位分析
为了检测CsAHL25蛋白在细胞中的定位情况,在烟草叶片中瞬时表达CsAHL25-GFP荧光蛋白和H2B-RFP核marker蛋白,激光共聚焦观察发现与空载对照组相比,CsAHL25-GFP荧光信号集中在细胞核中,并与核marker(H2B-RFP)荧光信号均重叠(图3),结果表明CsAHL25蛋白定位于细胞核中,具有AT-HOOK转录因子的定位特征。
2.4 CsAHL25转录因子与CsHB1启动子互作分析
为了确认CsAHL25转录因子与CsHB1启动子的结合,使用酵母单杂点对点实验对CsAHL25转录因子与pCsHB1启动子片段(-1018~-558 bp)的结合进行验证,含有pGADT7-CsAHL25和pCsHB1-AbAi质粒的酵母细胞和阳性对照能在互作筛选培养基(SD/-Leu/AbA200)上正常生长,阴性对照无法正常生长(图4-A),结果表明在酵母细胞中,CsAHL25具有结合pCsHB1启动子片段的能力。
为了进一步验证CsAHL25转录因子与pCsHB1启动子(-2377~0 bp)的互作,进行植物活体成像实验和双荧光素酶实验,验证其互作关系。植物活体成像结果显示,pCAMBIA1300-35S空载+pCsHB1-LUC组合的LUC荧光值显著高于CsAHL25-1300+pCsHB1-LUC组合与阴性对照(图4-B)。双荧光素酶活性测定结果与植物活体成像结果相同,pCAMBIA1300-35S空载+pCsHB1-LUC组合的LUC相对活性显著高于CsAHL25-1300+pCsHB1-LUC组合(图4-C)。上述实验结果表明,CsAHL25转录因子能与pCsHB1启动子结合并抑制下游基因的表达。
2.5 CsAHL25瞬时表达愈伤系中体细胞胚发生相关基因表达分析
为了进一步探索CsAHL25基因的功能,在纽荷尔脐橙的愈伤组织中瞬时表达CsAHL25。以转pCAMBIA1300-35S空载的愈伤组织为对照,分析已报道的体细胞胚发生相关基因的表达量变化。结果表明,CsAHL25相对表达量均高于或极显著高于对照组(图5-A),且CsHB1表达量均极显著低于对照组(图5-B)。CsLEC1、CsL1L、CsFUS3、CsABI3等参与柑橘体细胞胚发生的LEC1/B3基因相对表达量相较对照组均显著上升,且其表达量变化趋势与CsAHL25基本一致(图5-C~F)。以上结果表明,CsAHL25转录因子能下调CsHB1表达,并影响LEC1/B3调控网络相关基因表达。
3 讨 论
笔者筛选并鉴定到一个直接调控柑橘体细胞发生相关基因CsHB1的Ⅰ型AT-HOOK转录因子CsAHL25。CsAHL25是经过酵母单杂筛库筛选得到的转录因子,是调控CsHB1表达的候选基因之一。经过酵母单杂点对点和双荧光素酶实验验证,发现CsAHL25可以直接与CsHB1启动子结合,进而下调CsHB1的表达。前期研究发现,Ⅰ型AT-HOOK转录因子具有调控植物体细胞胚胎发生的功能,通过过表达AHL15基因,可以诱导拟南芥幼苗直接形成体细胞胚胎,且ahl15 ahl19 amiRAHL20三重突变的拟南芥植株完全无法诱导出体细胞胚[22]。本研究中鉴定出的柑橘Ⅰ型AT-HOOK转录因子CsAHL25,具有和柑橘体细胞胚发生相关的表达模式,该转录因子能够调控体细胞胚发生相关基因的表达,从而影响柑橘体细胞胚发生,与已报道的拟南芥Ⅰ型AT-HOOK转录因子功能相似。Ⅰ型AT-HOOK转录因子能够通过调控GA3OX1基因表达影响GA的合成[27]、通过调控PFI基因表达影响下胚轴的伸长[28]、通过调控SPL基因表达影响植物的寿命[29]。目前Ⅰ型AT-HOOK转录因子促进植物体细胞胚胎发生的分子机制尚未明确,且暂无研究表明Ⅰ型AT-HOOK转录因子调控Ⅱ型HD-ZIP基因表达,本研究中初步证明,Ⅰ型AT-HOOK转录因子调控Ⅱ型HD-ZIP基因的表达,完善了Ⅰ型AT-HOOK转录因子的下游调控网络。
此外,笔者利用瞬时表达实验发现,除了直接下调CsHB1基因的表达外,CsAHL25还影响了LEC1/B3基因的表达。瞬时表达CsAHL25基因会导致CsLEC1、CsL1L、CsFUS3、CsABI3基因的表达量上升,对体细胞胚发生相关基因呈现不同的调控方式。LEC1-FUS3-LEC2-ABI3基因共同构成一个LEC1/B3结构域调控网络,该网络通过调控体细胞胚的形态构成,进而影响植物体细胞胚的发生[30-32]。前人通过分析柑橘体细胞胚发生过程中基因的表达模式,明确LEC1、LEC1 Like、FUS3、ABI3等LEC1/B3调控网络基因在保持柑橘愈伤胚性、促进其胚胎发育中起到了重要的作用[33-34]。进一步研究发现,在柑橘愈伤组织分化的过程中,CsFUS3基因相对表达量逐渐上升,超表达CsFUS3会引起愈伤细胞形态变化、激活体细胞胚发生[35]。过表达CsL1L基因也能够使柑橘的营养组织产生体细胞胚[36]。本研究表明,瞬时表达CsAHL25上调LEC1、L1L、FUS3、ABI3基因的表达量,说明CsAHL25可能通过影响LEC1/B3表达,调控体细胞胚发生的功能。LEC1/B3调控网络基因能够具有细胞发生形态转变、调控体细胞胚形态构建的作用[32,35],而Ⅱ型HD-ZIP基因作用在植物胚胎发生前期,具有维持植物胚胎中干细胞存在的功能[19,37],二者作用在植物胚胎发生过程中的不同方面,其相互关系尚不明确,有待深入研究。此外,研究发现CsFUS3基因可以下调细胞中GA的含量,导致ABA/GA比例上升,从而促进柑橘体细胞胚发育[35],且AHL25基因也具有下调植物中GA含量的功能[27],CsAHL25是否可以通過影响柑橘体内ABA/GA比例来促进柑橘体细胞胚发生还需进一步探讨。
4 结 论
笔者通过酵母单杂点对点实验、双荧光素酶实验等筛选到柑橘体细胞胚发生相关基因CsHB1的上游抑制因子CsAHL25,通过瞬时表达CsAHL25确认其能够下调CsHB1表达量并上调LEC1、LEC1 Like、FUS3、ABI3等LEC1/B3基因相对表达量。笔者认为该基因具有激活柑橘体细胞胚发生的功能,为柑橘体细胞胚发生研究提供了一个潜在的候选基因。
参考文献 References:
[1] MORDHORST A P,TOONEN M A J,DE VRIES S C,MEINKE D. Plant embryogenesis[J]. Critical Reviews in Plant Sciences,1997,16(6):535-576.
[2] SIDDIQUI Z H,ABBAS Z K,ANSARI M W,KHAN M N. The role of miRNA in somatic embryogenesis[J]. Genomics,2019,111(5):1026-1033.
[3] 郭文武,葉俊丽,邓秀新. 新中国果树科学研究70年:柑橘[J]. 果树学报,2019,36(10):1264-1272.
GUO Wenwu,YE Junli,DENG Xiuxin. Fruit scientific research in New China in the past 70 years:Citrus[J]. Journal of Fruit Science,2019,36(10):1264-1272.
[4] 邓秀新. 中国柑橘育种60年回顾与展望[J]. 园艺学报,2022,49(10):2063-2074.
DENG Xiuxin. A review and perspective for citrus breeding in China during the last six decades[J]. Acta Horticulturae Sinica,2022,49(10):2063-2074.
[5] 刘丹. 柑橘优异资源胚性愈伤组织诱导及体细胞杂种创制[D]. 武汉:华中农业大学,2019.
LIU Dan. Induction of nucellar embryogenic callus and generation of somatic hybrid by protoplast fusion in citrus[D]. Wuhan:Huazhong Agricultural University,2019.
[6] ELHITI M,STASOLLA C. Transduction of signals during somatic embryogenesis[J]. Plants,2022,11(2):178.
[7] 蔡英卿,赖钟雄,陈义挺,林玉玲,李惠华,张妙霞. 龙眼胚性愈伤组织LEC1基因cDNA克隆以及在体胚发生过程中的表达分析[J]. 福建农林大学学报(自然科学版),2011,40(5):494-500.
CAI Yingqing,LAI Zhongxiong,CHEN Yiting,LIN Yuling,LI Huihua,ZHANG Miaoxia. Cloning of LEC1 gene from embryogenic callus and its expression analysis during somatic embryogenesis in longan[J]. Journal of Fujian Agriculture and Forestry University (Natural Science Edition),2011,40(5):494-500.
[8] VETRICI M A,YEVTUSHENKO D P,MISRA S. Overexpression of douglas-fir LEAFY COTYLEDON1 (PmLEC1) in Arabidopsis induces embryonic programs and embryo-like structures in the lec1-1 mutant but not in wild type plants[J]. Plants,2021,10(8):1526.
[9] KIM H U,JUNG S J,LEE K R,KIM E H,LEE S M,ROH K H,KIM J B. Ectopic overexpression of castor bean LEAFY COTYLEDON2 (LEC2) in Arabidopsis triggers the expression of genes that encode regulators of seed maturation and oil body proteins in vegetative tissues[J]. FEBS Open Bio,2013,4:25-32.
[10] LEDWO? A,GAJ M D. LEAFY COTYLEDON1,FUSCA3 expression and auxin treatment in relation to somatic embryogenesis induction in Arabidopsis[J]. Plant Growth Regulation,2011,65(1):157-167.
[11] WANG F F,PERRY S E. Identification of direct targets of FUSCA3,a key regulator of Arabidopsis seed development[J]. Plant Physiology,2013,161(3):1251-1264.
[12] LIU Z,GE X X,QIU W M,LONG J M,JIA H H,YANG W,DUTT M,WU X M,GUO W W. Overexpression of the CsFUS3 gene encoding a B3 transcription factor promotes somatic embryogenesis in Citrus[J]. Plant Science,2018,277:121-131.
[13] CHEN B J,FIERS M,DEKKERS B J W,MAAS L,VAN ESSE G W,ANGENENT G C,ZHAO Y,BOUTILIER K. ABA signalling promotes cell totipotency in the shoot apex of germinating embryos[J]. Journal of Experimental Botany,2021,72(18):6418-6436.
[14] KHIANCHAIKHAN K,AROONLUK S,VUTTIPONGCHAIKIJ S,JANTASURIYARAT C. Genome-wide identification of homeodomain leucine zipper (HD-ZIP) transcription factor,expression analysis,and protein interaction of HD-ZIP IV in oil palm somatic embryogenesis[J]. International Journal of Molecular Sciences,2023,24(5):5000.
[15] GE X X,LIU Z,WU X M,CHAI L J,GUO W W. Genome-wide identification,classification and analysis of HD-ZIP gene family in citrus,and its potential roles in somatic embryogenesis regulation[J]. Gene,2015,574(1):61-68.
[16] HU X,ZHANG C R,XIE H,HUANG X,CHEN Y F,HUANG X L. The expression of a new HD-Zip Ⅱ gene,MSHB1,involving the inhibitory effect of thidiazuron on somatic embryogenic competence in alfalfa (Medicago sativa L. cv. Jinnan) callus[J]. Acta Physiologiae Plantarum,2012,34(3):1067-1074.
[17] TRON A E,BERTONCINI C W,CHAN R L,GONZALEZ D H. Redox regulation of plant homeodomain transcription factors[J]. The Journal of Biological Chemistry,2002,277(38):34800-34807.
[18] TURCHI L,CARABELLI M,RUZZA V,POSSENTI M,SASSI M,PE?ALOSA A,SESSA G,SALVI S,FORTE V,MORELLI G,RUBERTI I. Arabidopsis HD-Zip Ⅱ transcription factors control apical embryo development and meristem function[J]. Development,2013,140(10):2118-2129.
[19] ROODBARKELARI F,GROOT E P. Regulatory function of homeodomain-leucine zipper (HD-ZIP) family proteins during embryogenesis[J]. The New Phytologist,2017,213(1):95-104.
[20] PRAVEEN S,PAWAR V,AHLAWAT Y S. Somatic embryogenesis and plant regeneration in Kinnow mandarin[J]. Journal of Plant Biochemistry and Biotechnology,2003,12(2):163-165.
[21] ARAVIND L,LANDSMAN D. AT-hook motifs identified in a wide variety of DNA-binding proteins[J]. Nucleic Acids Research,1998,26(19):4413-4421.
[22] ZHAO J F,FAVERO D S,PENG H,NEFF M M. Arabidopsis thaliana AHL family modulates hypocotyl growth redundantly by interacting with each other via the PPC/DUF296 domain[J]. Proceedings of the National Academy of Sciences of the United States of America,2013,110(48):E4688-E4697.
[23] KARAMI O,RAHIMI A,MAK P,HORSTMAN A,BOUTILIER K,COMPIER M,VAN DER ZAAL B,OFFRINGA R. An Arabidopsis AT-hook motif nuclear protein mediates somatic embryogenesis and coinciding genome duplication[J]. Nature Communications,2021,12:2508.
[24] 謝幸男,赖晓娜,战爽,程来超,许全全,葛晓霞. 柑橘体细胞胚发生基因CsHB1特异肽段多克隆抗体的制备及其蛋白动态检测[J]. 果树学报,2017,34(9):1069-1075.
XIE Xingnan,LAI Xiaona,ZHAN Shuang,CHENG Laichao,XU Quanquan,GE Xiaoxia. Preparation of CsHB1 polyclonal antibody and its protein dynamic changes during somatic embryogenesis in Citrus[J]. Journal of Fruit Science,2017,34(9):1069-1075.
[25] 刘娉婷. 基于柑橘强胚性材料开展CsHB1促体细胞胚发生方式的研究[D]. 武汉:华中农业大学,2022.
LIU Pingting. Study the CsHB1 promotion of somatic embryogenesis uasing citrus strong embryogenic materials[D]. Wuhan:Huazhong Agricultural University,2022.
[26] 张印. 柑橘原花青素积累及ABA代谢的调控机制研究[D]. 武汉:华中农业大学,2021.
ZHANG Yin. Research on the regulation mechanism of proanthocyanidin accumulation and ABA metabolism in citrus[D]. Wuhan:Huazhong Agricultural University,2021.
[27] MATSUSHITA A,FURUMOTO T,ISHIDA S,TAKAHASHI Y. AGF1,an AT-hook protein,is necessary for the negative feedback of AtGA3ox1 encoding GA 3-oxidase[J]. Plant Physiology,2007,143(3):1152-1162.
[28] XIAO C W,CHEN F L,YU X H,LIN C T,FU Y F. Over-expression of an AT-hook gene,AHL22,delays flowering and inhibits the elongation of the hypocotyl in Arabidopsis thaliana[J]. Plant Molecular Biology,2009,71(1):39-50.
[29] RAHIMI A,KARAMI O,BALAZADEH S,OFFRINGA R. miR156-independent repression of the ageing pathway by longevity-promoting AHL proteins in Arabidopsis[J]. The New Phytologist,2022,235(6):2424-2438.
[30] HORSTMAN A,LI M F,HEIDMANN I,WEEMEN M,CHEN B J,MUINO J M,ANGENENT G C,BOUTILIER K. The BABY BOOM transcription factor activates the LEC1-ABI3-FUS3-LEC2 network to induce somatic embryogenesis[J]. Plant Physiology,2017,175(2):848-857.
[31] LOTAN T,OHTO M A,YEE K M,WEST M A L,LO R,KWONG R W,YAMAGISHI K,FISCHER R L,GOLDBERG R B,HARADA J J. Arabidopsis LEAFY COTYLEDON1 is sufficient to induce embryo development in vegetative cells[J]. Cell,1998,93(7):1195-1205.
[32] KWONG R W,BUI A Q,LEE H,KWONG L W,FISCHER R L,GOLDBERG R B,HARADA J J. LEAFY COTYLEDON1-LIKE defines a class of regulators essential for embryo development[J]. The Plant Cell,2003,15(1):5-18.
[33] GE X X,CHAI L J,LIU Z,WU X M,DENG X X,GUO W W. Transcriptional profiling of genes involved in embryogenic,non-embryogenic calluses and somatic embryogenesis of Valencia sweet orange by SSH-based microarray[J]. Planta,2012,236(4):1107-1124.
[34] 繆星辰. 椪柑体细胞胚胎发生关键基因的挖掘与鉴定[D]. 扬州:扬州大学,2023.
MIAO Xingchen. Mining and identification of key genes involved in somatic embryogenesis in ponkan[D]. Yangzhou:Yangzhou University,2023.
[35] 刘政. 柑橘珠心胚起始转录组分析及体细胞胚发生相关基因CsFUS3功能鉴定[D]. 武汉:华中农业大学,2015.
LIU Zheng. Transcriptional analysis of citrus nucellar embryo initiation and functional characterization of CsFUS3 gene preferentially expressed during somatic embryogenesis[D]. Wuhan:Huazhong Agricultural University,2015.
[36] ZHU S P,WANG J,YE J L,ZHU A D,GUO W W,DENG X X. Isolation and characterization of LEAFY COTYLEDON 1-LIKE gene related to embryogenic competence in Citrus sinensis[J]. Plant Cell,Tissue and Organ Culture,2014,119(1):1-13.
[37] TURCHI L,BAIMA S,MORELLI G,RUBERTI I. Interplay of HD-Zip Ⅱ and Ⅲ transcription factors in auxin-regulated plant development[J]. Journal of Experimental Botany,2015,66(16):5043-5053.

