溶胶-凝胶法制备多孔晶体材料C12A7-Cl-
孙剑秋 宫 璐 沈 静 林 舟 李全新
(中国科学技术大学化学物理系,生物质洁净能源实验室,合肥 230026)
As one of the important chemical intermediates,the chlorine anion(Cl-)has attracted much attention in many fields including atmospheric chemistry,biochemistry,material modifications, electrochemistry,and semiconductor industry[1-8].For example, the presence of two isotopes(35Cl-and37Cl-)favors the interpretation of anionic mass spectra,which has been of considerable value in chemical ionization mass spectrometry[1].Because the major reactions between Cl-and organic substances are generally to form the anionic attachment products[MCl]-[1,4-6],Cl-has been considered to identify saccharides[5],and to monitor barbiturates[6].On the other hand,it has been reported that Cl-can enhance the plasma etching of polycrystalline silicon(poly-Si)in semiconductor chemistry[8].More recently,halogen anions have been proposed as an alternative to positive ions for ion fusion drivers in inertial confinement fusion[9],because electron accumulation would be prevented in negative-ion beams.
The material of Ca12Al14O32Cl2(or named C12A7-Cl-),one of thederivateofC12A7,wasfirstpreparedfromco-sinteringC12A7 with CaCl2by Jeevaratnam et al.and reported a lattice constant of 1.2004 nm[10].Its chemical character was further investigated by Sango et al.[11].Similar to C12A7[12],the material of C12A7-Cl-, belonging to I43d space group,possesses a body-centered cubic structure and also has typical positively charged framework of [Ca24Al28O64]4+with a unit-cell content of Ca24Al28O64Cl4[10].Besides conventional method,C12A7 has also been successfully synthesized by sol-gel method[13-16],which was utilized to prepare C12A7-Cl-sample in this article.
Recently,we have developed an approach to generate high pure and sustainable chlorine anion flux,which is emitted from the surface of the synthesized C12A7-Cl-material[17].But both of the high sintering temperature(up to 1350℃)and the using of chlorine gas,which is harmful to the environment and human body,were disadvantages of application.In this work,we confirmed experimentally that the sol-gel method could be useful in preparing C12A7-Cl-material and avoid the two disadvantages mentioned above.
1 Experimental
1.1 Sample preparation
The material of Ca12Al14O32Cl2(C12A7-Cl-)was prepared by the sol-gel method.The starting solution was prepared from hydrated calcium nitrate(AR),hydrated aluminum nitrate(AR), calcium chloride(AR),urea(AR),ethylene glycol(AR),and distilled water.Molar ratio of Ca(NO3)2·4H2O∶Al(NO3)3·9H2O∶CaCl2∶CO(NH2)2∶HOCH2CH2OH is 11∶14∶1∶76∶380.Firstly,appropriate amounts of Ca(NO3)2·4H2O,Al(NO3)3·9H2O,and CaCl2were dissolved in distilled water.The solution was stirred for 2-3 h.Then,a given amount of urea was added into the above solution,stirring for 2-3 h too.At last,ethylene glycol was added to the solution,stirring in the same condition to form the sol. The resulting sol was aged for 24 h to form gel.Thermal treatment was done in air at 350℃for 2 h.The resulting powder sample was further temperature-programmed with a heating rate of 10℃·min-1,sintered at 1000℃for 6 h,and finally cooled to room temperature under flowing argon atmosphere(flowing rate: 100 mL·min-1).
1.2 Characterization
X-ray diffraction(XRD)measurement was carried out to investigate the structure of C12A7-Cl-.Powder X-ray diffraction patterns were recorded on a MAC Science MXP18AHF diffractometer with a Cu-Kα1source(λ=0.154056 nm)in the 2θ range of 15°-75°.The crystal morphology was investigated using the fieldemissionscanningelectronmicroscopy(FESEM;FEI,Sirion-200,America).
Thermogravimetric analysis(TGA)(Shimadzu DTG-60H thermal analyzer)was carried out with a heating rate of 10℃·min-1in nitrogen atmosphere for the precursor powder which was obtained by thermally treating the dried gel in air at 350℃for 2 h.
The absolute Cl-concentration in the C12A7-Cl-sample(after dissolving in the deionized water)was analyzed by ion chromatography(IC;DX-120,Dionex Co.),and the IC results were calibrated with a standard NaCl solution of 4×10-4mol·L-1. Electron paramagnetic resonance(EPR)measurement was performed to investigate the anionic species(e.g.,O-and O-2)in the C12A7-Cl-bulk.The experiment was conducted at ca 9.1 GHz (X-band)using a JES-FA200 spectrometer at 120 K.Spin concentrations were determined from the second integral of the spectrum using CuSO4·5H2O as a standard with an error of about 20%.
For time-of-flight(TOF)observation,the powder of precursor was pressed to a slice with a diameter of 15 mm and a thickness of 2 mm under a pressure of(1.5-2)×1010Pa,and then were temperature-programmed to 1000℃with a heating rate of 10℃·min-1,sintered at 1000℃for 6 h and cooled to room temperature under flowing argon atmosphere.The TOF experimental apparatus,described previously[18],consists essentially of a sampling chamber and an ion detection chamber equipped with a TOF mass spectrometer.The sample(diameter:1.5 cm,thickness: 1.5 mm)was supported by a quartz tube(length:60 cm,diameter:3 cm),which had a circinal flat(diameter:1.5 cm,depth:1.8 mm)with a hole of 2.5 mm in the center of the flat.The quartz tube was installed in the sample chamber,ensuring that the sample was located in the center of the sample chamber.The anions and electrons emitted from the frontal surface were extracted by an extraction electrode,and then analyzed by the TOF mass spectrometer.
2 Results and discussion
2.1 XRD/SEM analysis
The XRD analysis was carried out to investigate the structure of the as-prepared C12A7-Cl-.Fig.1 shows the XRD pattern obtained from the C12A7-Cl-sample sintered at 1000℃.The results of comparing the measured positions and intensities of the XRD patterns with the standard data in the PDF 05-4568 card (International Centre for Diffraction Data(ICDD),2002)are as follows:all the diffraction peaks have appeared,the X-ray diffraction structure for the samples completely accords with that of Ca12Al14O32Cl2.This means that the material structure prepared by the sol-gel method has a cubic structure with a unit-cell content of Ca24Al28O64Cl4and belongs to I43d space group.The unit cell constant for the sample sintering at 1000℃,derived from the stronger diffraction peaks,was about(1.2002±0.0006) nm.
The mean particle size(d)was estimated by analyzing the broadening of the diffraction peaks.The mean particle size was calculated by the Scherrer equation:

where K is a constant(0.89),λ is the wavelength of X-ray source (Cu Kα1,λ=0.154056 nm),β is the full-width at half maximum (FWHM),and θ is the diffraction angle of the X-ray diffraction. The mean particle diameter of the C12A7-Cl-sample calculated by Eq.(1)was 48.95 nm.

Fig.1 XRD pattern of C12A7-Cl-sample prepared by sol-gel method(sintered at 1000℃for 6 h)
The microstructure of the as-prepared C12A7-Cl-sample was observed by the FESEM.Fig.2 shows typical FESEM image of the sample which was formed in further sintering temperature at 1000℃for 6 h.From the SEM image,the C12A7-Cl-sample had high density of voids presented with a random distribution, indicating that the prepared C12A7-Cl-is a porous material.
2.2 TGA analysis
The TGA analysis was carried out to study the phase transformation during the formation of C12A7-Cl-.Fig.3 shows the TGA diagram of the precursor powder of the material.For the dried gel has been thermally treated in air at 350℃for 2 h,the mass loss in the temperature region lower than 360℃is slight. The mass loss between 50 and 150℃was assigned to the loss of the residual water[19].The mass loss in the range from 360 to 690℃was obviously identified from the TGA curve.This mass loss would mainly response for the combination of the decomposition of the residual aluminum nitrate into Al2O3[20-21]and calcium nitrate into CaO with the existence of Al2O3[22-23].The mass loss over 800℃may be attributed to the gasification of small amount solid residuals such as carbon[24].
2.3 EPR/IC analysis

Fig.2 Typical FESEM image of the C12A7-Cl-material prepared by sol-gel method(sintering at 1000℃for 6 h)

Fig.3 TGA of the material precursor powder
The EPR measurement was performed to investigate the active oxygen species such as O-and O-2in the C12A7-Cl-material.Fig.4 shows the EPR spectrum from the C12A7-Cl-sample. The experimental spectrum was simulated by a simple superposition with the Lorentzian line shape of O-(gxx=gyy=2.043 and gzz=1.997)and O-2(gxx=2.001,gyy=2.009,and gzz=2.071)[12]in common set of g-values and different intensity ratios.By simulating the EPR spectrum and using CuSO4·5H2O as a spin concentration standard,we estimated the O-and O-2concentrations in the C12A7-Cl-sample to be about 2.39×1017cm-3and 1.91×1017cm-3, respectively.
Note that this EPR method can not measure the Cl-species in the C12A7-Cl-material because there are no unpaired electrons for the Cl-.The absolute Cl-concentration in the C12A7-Cl-was analyzed by using IC method and calibrated with a standard NaCl solution of 4×10-4mol·L-1,the C12A7-Cl-aqueous solution was obtained by dissolving 28.8 mg C12A7-Cl-sample into 100 mL deionized water.Absolute Cl-concentration for the sample was estimated by the integral of the calibrated IC peaks using NaCl solution as a standard.The Cl-concentration in the C12A7-Clsample was 1.88×1021cm-3.Due to the total negative charge concentration(2.33×1021cm-3)[18],the above IC result further confirmed that the Cl-anions were dominating anionic species stored in the C12A7-Cl-material.
2.4 TOF observations
The anions emitted from the C12A7-Cl-surface were identified by anionic TOF mass spectrometer.Fig.5 shows a typical anionic TOF-MS from the C12A7-Cl-sample at a emission temperatureof700℃andextractionelectricfield of 800 V·cm-1.Thedominant peaks have the mass numbers of 35 and 37,which correspond to chlorine isotopes of35Cl-and37Cl-,respectively,and the other two peaks at m/z=0,16 were simultaneously observed, attributed to emission of the electrons and O-anions,respectively. It was found that the anionic species emitted from the C12A7-Clmaterial surface were dominating with the Cl-anions together with an amount of O-anions and electrons in the investigated range.The emission of O-anions and electrons were mainly caused by the decomposition of remaining O2-in the bulk of the material:
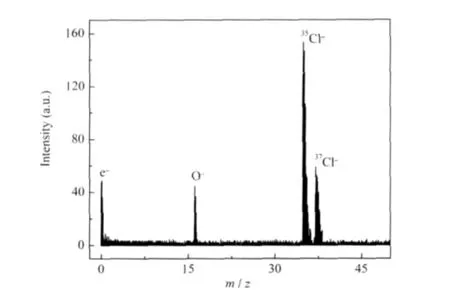
Fig.5 Typical TOF mass spectrum for anions emitted from C12A7-Cl-sample(sintered at 1000℃for 6 h)surfaces at 700℃and 800 V·cm-1

where c,s,g stand for the anions in the cage,surface of the material,gas phase,respectively.The TOF result shows that the C12A7-Cl-material has the ability to emit Cl-anion.The anionic distribution emitted from the C12A7-Cl-would indirectly demonstrate that Cl-anions should be the major anions stored in the materials of C12A7-Cl-.
Comparing with the common direct synthesis method of C12A7-Cl-which we have reported before[17],present sol-gel method has successfully decreased the sintering temperature for about 350℃(from 1350 to 1000℃).Moreover,the chlorine gas is not needed in sol-gel method,which lowers much danger.So the sol-gel method could be considered as an alternative method for the energy-saving and safety.
3 Conclusions
In this work,we prepared porous crystal functional material of C12A7-Cl-by sol-gel method,The XRD result shows that the C12A7 structure has been successfully synthesized via the nano particles of CaO and Al2O3formed from the gel.The transformation temperature from CaO/Al2O3to 12CaO·7Al2O3(C12A7)by present sol-gel method decreased about 350℃,comparing with common direct synthesis of C12A7 via CaO and Al2O3powder. According to IC result,Cl-anions are the dominant anion species stored in the C12A7-Cl-material.TOF observation proves that Cl-anions can be emitted under suitable temperature and electric field conditions.Present sol-gel method has shown as an alternative synthesis method for decreasing sintering temperatureandavoidingchlorinegas.Potentially,presentsol-gel method is useful to develop a Cl-anion source,which may be applied to the fields such as etching,film preparation,sterilization,material modification,and anion mass spectrometer,etc.
1 Zhang,W.;Beglinger,C.;Stone,J.A.J.Phys.Chem.,1995,99: 11673
2 Richardson,S.L.;Francisco,J.S.;Mebel,A.M.;Morokuma,K. Chem.Phys.Lett.,1997,270:395
3 Haas,B.M.;Crellin,K.C.;Kuwata,K.T.;Okumura,M.J.Phys. Chem.,1994,98:6740
4 Cole,R.B.;Zhu,J.H.Rapid Commun.Mass Spectrom.,1999,13: 607
5 Kato,Y.;Numajiri,Y.J.Chromatogr.,1991,562:81
6 Horning,E.C.;Horning,M.G.;Carroll,D.I.;Dzidic,I.;Stillwell, R.N.Anal.Chem.,1973,45:936
7 Ito,K.;Ohshima,M.;Kurokama,H.;Sugiyama,K.;Miura,H. Catal.Commun.,2002,3:527
8 Ahn,T.H.;Nakamura,K.;Sugai,H.Plasma Sources Sci.Technol., 1996,5:139
9 Grisham,L.R.;Kwan,J.W.;Hahto,S.K.;Hahto,S.T.;Leung,K. N.;Westenskow,G.Rev.Sci.Instrum.,2006,77:03A501
10 Jeevaratnam,J.;Glasser,F.P.;Glasser,L.S.D.J.Am.Ceram.Soc., 1964,47:105
11 Sango,H.;Miyakawa,T.;Minamisawa,H.;Machinaga,O.;Kasai, J.Gypsum&Lime,1991,233:13
12 Hayashi,K.;Hirano,M.;Matsuishi,S.;Hosono,H.J.Am.Chem. Soc.,2002,124:738
13 Tas,A.J.Am.Ceram.Soc.,1998,81:2853
14 Wang,D.;Liu,Y.X.;Liu,Y.C.;Xu,C.S.;Shao,C.L.;Li,X.H. J.Nanosci.Nanotechnol.,2008,8:1458
15 Wang,D.;Liu,Y.X.;Xu,C.S.;Liu,Y.C.;Wang,G.R.;Li,X.H. J.Rare Earths,2008,26:433 [王 丹,刘玉学,徐长山,刘益春,王国瑞,李兴华.稀土学报,2008,26:433]
16 Zahedi,M.;Ray,A.K.;Barratt,D.S.J.Phys.D-Appl.Phys., 2008,41:035404
17 Sun,J.Q.;Song,C.F.;Ning,S.;Lin,S.B.;Li,Q.X.Acta Phys.-Chim.Sin.,2009,25:1713 [孙剑秋,宋崇富,宁 珅,林少斌,李全新.物理化学学报,2009,25:1713]
18 Li,Q.X.;Hosono,H.;Hirano,M.;Hayashi,K.;Nishioka,M.; Kashiwagi,H.;Torimoto,Y.;Sadakata,M.Surf.Sci.,2003,527: 100
19 Lopez,T.;Ramos,E.;Bosch,P.;Asomoza,M.;Gomez,R.Mater. Lett.,1997,30:279
20 Pacewska,B.;Keshr,M.Thermochim.Acta,2002,385:73
21 Pacewska,B.;Keshr,M.J.Therm.Anal.Calorim.,2004,75:113
22 Ettarh,C.;Galwey,A.K.Thermochim.Acta,1995,261:125
23 Migdal-Mikuli,A.;Hetmanczyk,J.;Hetmanczyk,L.J.Therm. Anal.Calorim.,2007,89:499
24 Tancredi,N.;Cordero,T.;Rodriguez-Mirasol,J.;Rodriguez,J.J. Fuel,1996,75:1505

