新型含噻唑和吡唑环的醛腙类化合物的合成
吕 警, 王吉德, 解正峰
(新疆大学 化学化工学院,新疆 乌鲁木齐 830046)
噻唑类化合物具有广泛的生理活性,在过去的几十年里,研究者通过各种的方法已合成不同噻唑环衍生物[1~12]。腙类化合物因具有特殊的生物活性和强配位能力, 在农药、医药、材料和分析试剂等方面而备受关注[13], 所以将噻唑环和吡唑环引入到腙类化合物分子中, 很可能由于拼合作用产生更强的生物活性。
本文报道1-苯基-3-甲基-5-取代吡唑-4-甲醛(Ⅰf~Ⅰh)与氨基硫脲缩合制得1-苯基-3-甲基-5-取代吡唑-4-甲醛缩氨基硫脲(Ⅱf~Ⅱh); Ⅱ与α-溴代芳基乙酮(1a~1e)反应合成了15个新型含噻唑和吡唑环的醛腙——N-(1-苯基-3-甲基-5-氯吡唑-4-基)-N′-(4-芳基-噻唑-2-基)醛腙(2a~2e),N-(1-苯基-3-甲基-5-苯氧基吡唑-4-基)-N′-(4-芳基噻唑-2-基)醛腙(3a~3e)和N-(1-苯基-3-甲基-5-对甲苯氧基吡唑-4-基)-N′-(4-芳基噻唑-2-基)醛腙(4a~4e, Scheme 1),其结构经1H NMR, IR和元素分析表征。2c作X-射线单晶衍射测试。
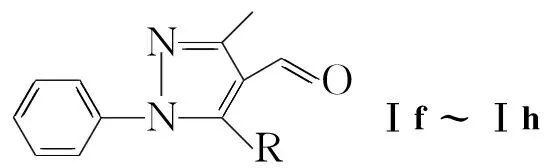
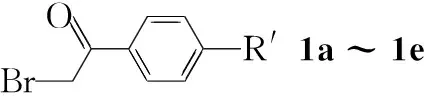
Scheme1
1 实验部分
1.1 仪器与试剂
FP52型显微熔点仪(温度计未校正);VARIAN INOVA-400 MHz型核磁共振谱仪(CDCl3为溶剂,TMS为内标); BRUKER EQUINOX 55型傅里叶变换红外光谱仪(KBr压片);Thermo Flash EA-1112型元素分析仪;R-AXIS SPIDER型X-射线单晶衍射仪。
Ⅱf~Ⅱh[14,16]和1a~1e[15]按文献方法制备;其余所用试剂均为分析纯。
1.2 2a~5e的合成(以2a为例)
在三颈烧瓶中加入Ⅱf1 mmol的无水乙醇(25 mL)溶液和1a1 mmol,搅拌下回流反应1 h。冷却至室温,滴加氨水至不再析出沉淀,过滤,滤饼用混合溶剂[V(DMF) ∶V(EtOH)=3 ∶2]重结晶得2a。
用类似方法合成2b~2e,3a~3e和4a~4e。2~4的实验结果见表1,表征数据见表2。
2 结果与讨论
2.1 表征
由表2可见,2~4的IR谱图具有共同特征:3 178 cm-1~3 071 cm-1出现υN-H特征吸收峰,1 640 cm-1~1 547 cm-1出现υC=N吸收峰,1 300 cm-1~1 210 cm-1出现υN-N-C吸收峰,695 cm-1~660 cm-1的强峰指派为υC-S-C特征吸收峰,另外芳氢吸收峰出现在3 100 cm-1~3 000 cm-1,芳环骨架吸收峰出现1 600 cm-1~1 460 cm-1。
2.2 2c的结体结构
将2c用乙醇溶解,于室温自然挥发,数天后得棕黄色柱状单晶。将2c单晶(0.57 mm×0.52 mm×0.45 mm)置衍射仪上,用MoKα射线(λ=0.071 073 nm)以ω~2θ扫描方式在3.02°≤θ≤27.49°收集9 055个强反射数据,其中独立衍射点71 089个(Rint=0.066 0)。全部强度数据均经Lp因子校正,并做经验吸收校正. 晶体结构用直接法解出,全部非氢原子的坐标及各向异性参数经最小二乘修正,用SHELXL-97程序对F2进行精修获得非氢原子坐标及各向异性参数,氢原子由差值Fourier合成。和理论计算得到,他们的坐标和各向同性温度因子参与结构计算,但不参与修正。最后得到R1=0.054 5,ωR2=0.148 9,Rsigma=0.027,Rint=0.066 0,GOF=1.049,最后残余电子密度峰最大最小值为0.647 e·nm-3~0.941 e·nm-3。2c的非氢原子坐标和等效温度因子见表3,晶体学参数见表4,部分键长和键角见表5;分子结构及其在晶胞中的二维堆积分别见图1和图2。由图1和图2可看出,在2c晶体中, 噻唑环氮与另一分子上的NH相互作用分别形成分子间的N-H┈N氢键,N(5)-H(9A)为2.941 Å, N(10)-H(4A)为2.983 Å。由于2c具有较强分子间氢键,使其分子堆积紧密。
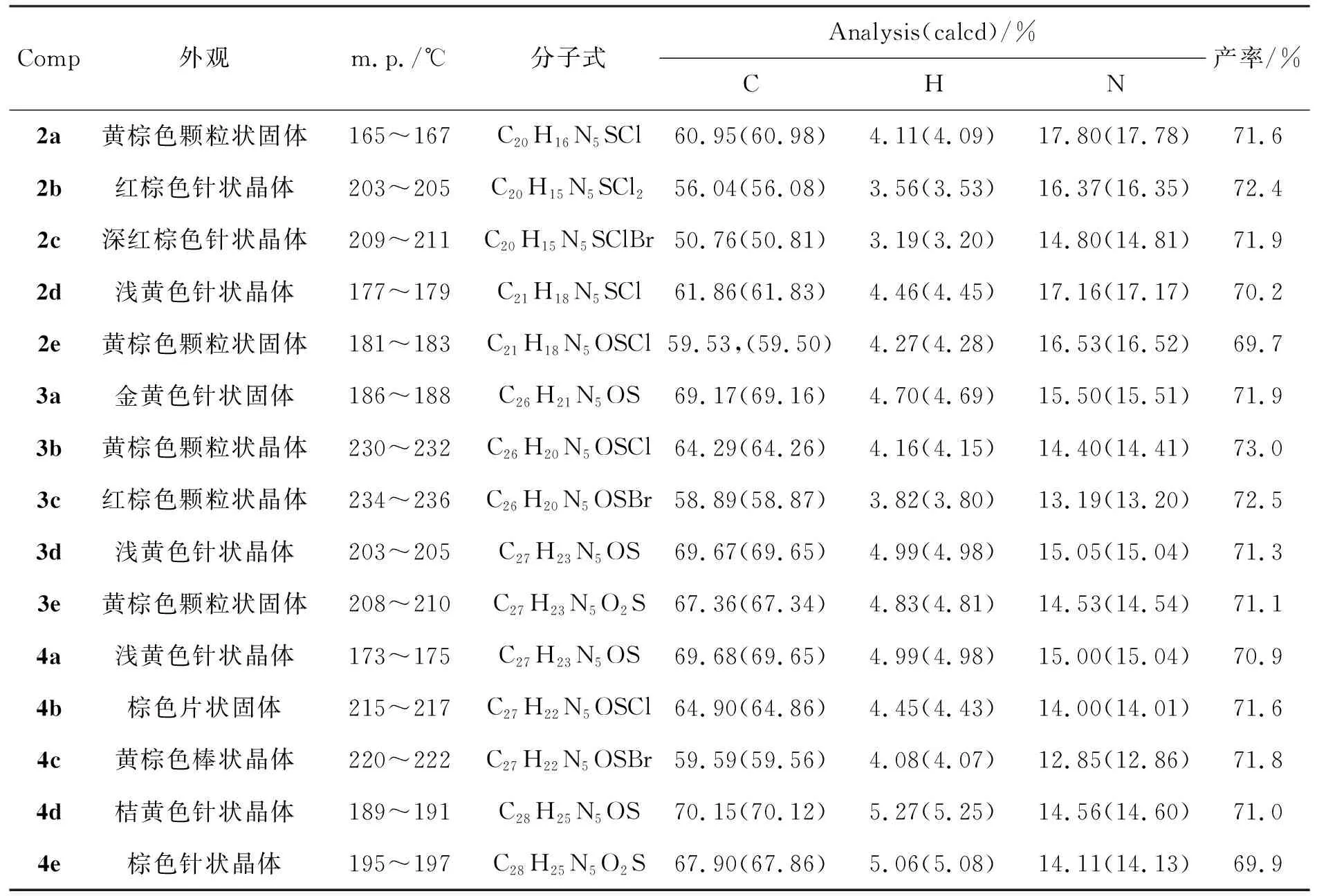
表 1 合成2~4的实验结果Table 1 Experamental results of synthesizing 2~4
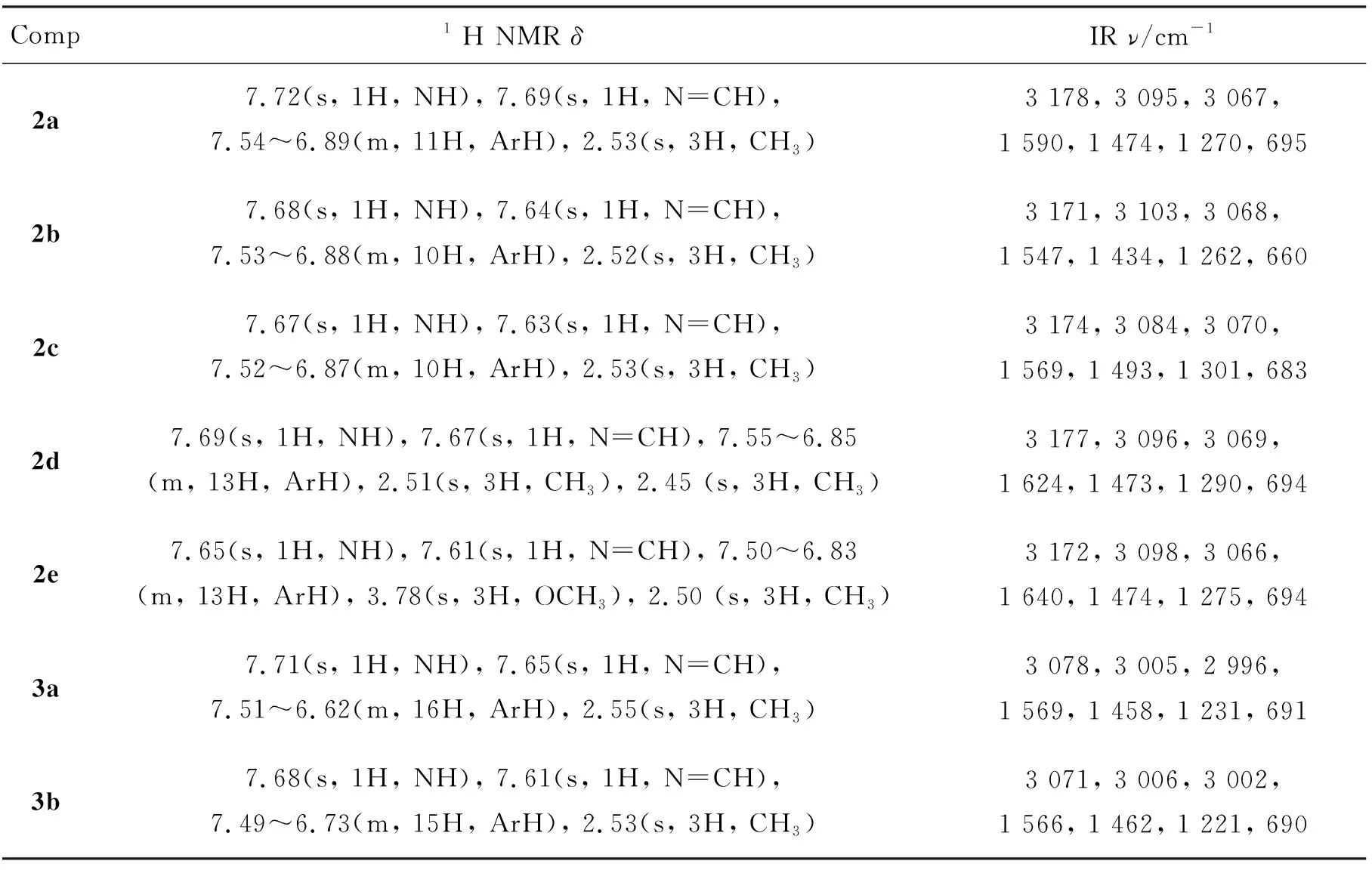
表 2 2~4的表征数据Table 2 Characteristic data of 2~4
续表2
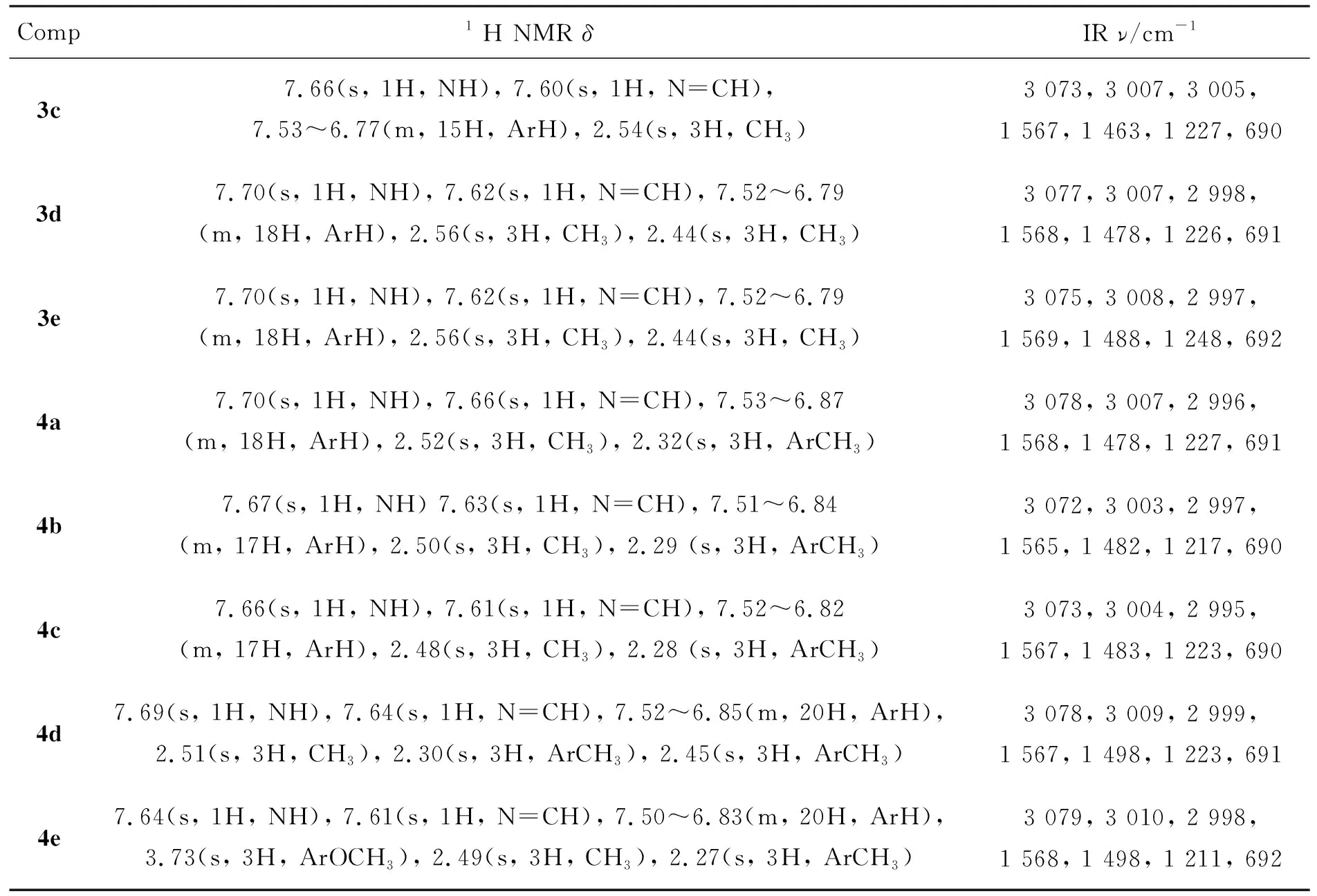
Comp1 H NMR δIR ν/cm-13c7.66(s, 1H, NH), 7.60(s, 1H, N=CH),7.53^6.77(m, 15H, ArH), 2.54(s, 3H, CH3)3 073, 3 007, 3 005, 1 567, 1 463, 1 227, 6903d7.70(s, 1H, NH), 7.62(s, 1H, N=CH), 7.52^6.79(m, 18H, ArH), 2.56(s, 3H, CH3), 2.44(s, 3H, CH3)3 077, 3 007, 2 998, 1 568, 1 478, 1 226, 6913e7.70(s, 1H, NH), 7.62(s, 1H, N=CH), 7.52^6.79(m, 18H, ArH), 2.56(s, 3H, CH3), 2.44(s, 3H, CH3)3 075, 3 008, 2 997, 1 569, 1 488, 1 248, 6924a7.70(s, 1H, NH), 7.66(s, 1H, N=CH), 7.53^6.87(m, 18H, ArH), 2.52(s, 3H, CH3), 2.32(s, 3H, ArCH3)3 078, 3 007, 2 996, 1 568, 1 478, 1 227, 6914b7.67(s, 1H, NH) 7.63(s, 1H, N=CH), 7.51^6.84(m, 17H, ArH), 2.50(s, 3H, CH3), 2.29 (s, 3H, ArCH3)3 072, 3 003, 2 997, 1 565, 1 482, 1 217, 6904c7.66(s, 1H, NH), 7.61(s, 1H, N=CH), 7.52^6.82(m, 17H, ArH), 2.48(s, 3H, CH3), 2.28 (s, 3H, ArCH3)3 073, 3 004, 2 995, 1 567, 1 483, 1 223, 6904d7.69(s, 1H, NH), 7.64(s, 1H, N=CH), 7.52^6.85(m, 20H, ArH), 2.51(s, 3H, CH3), 2.30(s, 3H, ArCH3), 2.45(s, 3H, ArCH3)3 078, 3 009, 2 999, 1 567, 1 498, 1 223, 6914e7.64(s, 1H, NH), 7.61(s, 1H, N=CH), 7.50^6.83(m, 20H, ArH), 3.73(s, 3H, ArOCH3), 2.49(s, 3H, CH3), 2.27(s, 3H, ArCH3)3 079, 3 010, 2 998, 1 568, 1 498, 1 211, 692
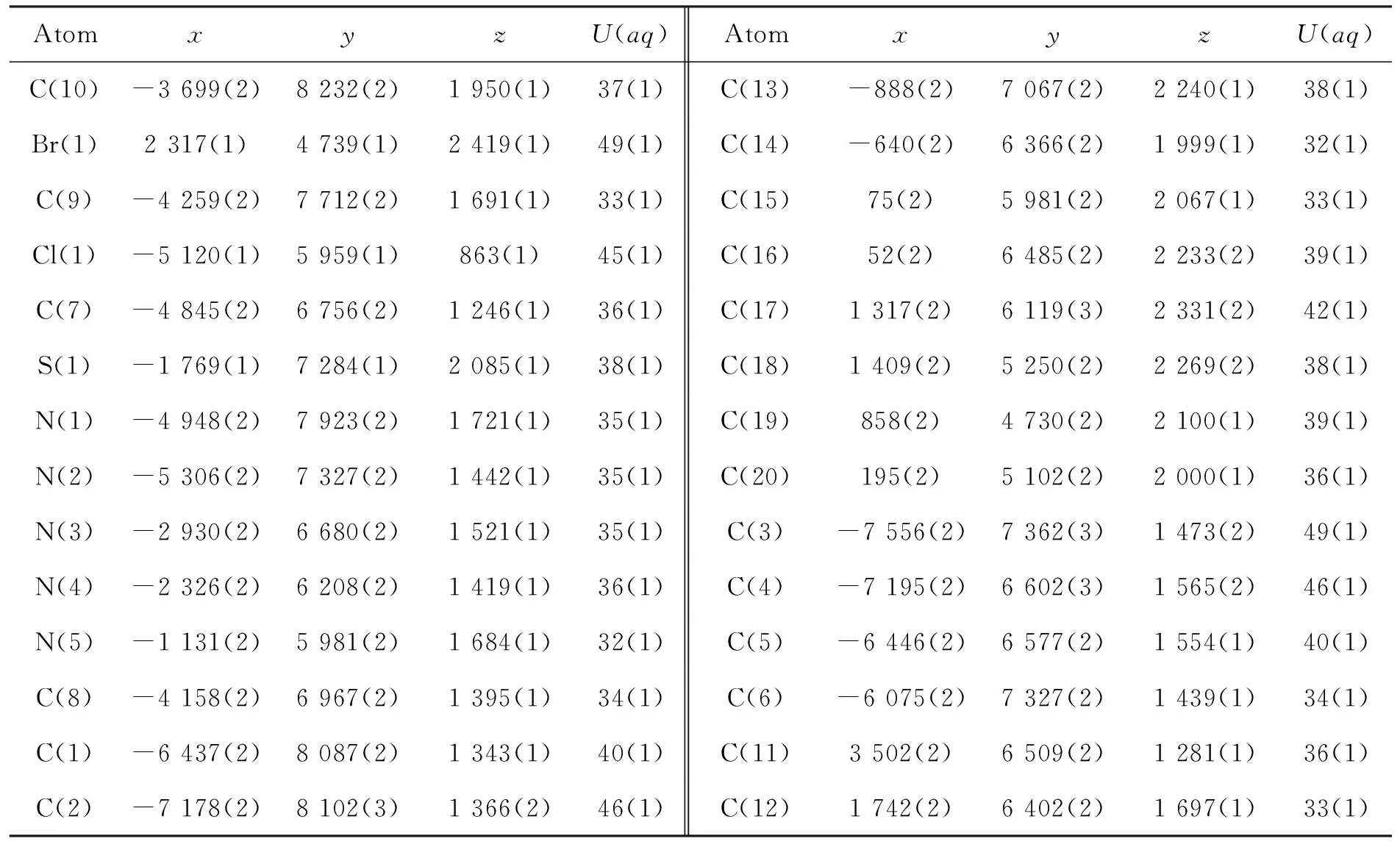
表 3 2c的原子坐标(×104)和各向同性热参数(×103)Table 3 Atomic coordinates(×104) and equivalent isotropic displacement parameters(×103) of 2c

表 4 2c的晶体学参数Table 4 Crystal data and refinement details of 2c

表 5 2c的部分键长和键角Table 5 Selection bond lengths and angles of 2c
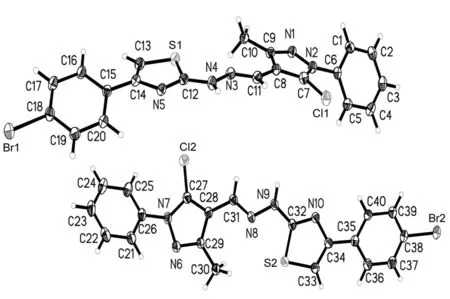
图 1 2c的分子结构图Figure 1 Molecular structures of 2c

图 2 2c的晶胞堆积图Figure 2 Packing drawing of 2c
[1] Holla B S, Malini K V, Rao B S,etal. Synthesis of some new 2,4-disubstituted thiazoles as possible antibacterial and anti-inflammatory agents[J].Eur J Med Chem,2003,38:313-318.
[2] Karegoudar P, Karthikeyan M S, Prasad D J,etal. Synthesis of some novel 2,4-disubstituted thiazoles as possible antimicrobial agents[J].Eur J Med Chem,2008,43:261-267.
[3] Cukurovali A, Yilmaz I, Gur S,etal. Synthesis,antibacterial and antifungal activity of some new thiazolylhydrazone derivatives containing 3-substituted cyclobutane ring[J].Eur J Med Chem,2006,41:201-207.
[4] Siddiqui H L, Iqbal A, Ahmad S,etal. Synthesis and spectroscopic studies of new schiff bases[J].Molecules,2006,11:206-211.
[5] Potewar T M, Ingale S A, Srinivasan K V, Efficient synthesis of 2,4-disubstituted thiazoles using ionic liquid under ambient conditions:A practical approach towards the synthesis of Fanetizole[J].Tetrahedron,2007,63:11066-11069.
[6] Karade H, Sathe M, Kaushik M P. An efficient method for the synthesis of 2-aminothiazoles using silica chloride as a heterogeneous catalyst[J].Catal Commun,2007,8:741-746.
[7] Narender M, Reddy M S, Sridhar R,etal. Aqueous phase synthesis of thiazoles and aminothiazoles in the presence ofb-cyclodextrin[J].Tetrahedron Lett,2005,46:5953-5955.
[8] Das B, Reddy V S, Ramu R. A rapid and high-yielding synthesis of thiazoles and aminothiazoles using ammonium-12-molybdophosphate[J].J Mol Catal A:Chem,2006,252:235-237.
[9] Kazzouli S E, Berteina-Raboin S, Mouaddib A. Solid support synthesis of 2,4-disubstituted thiazoles and aminothiazoles[J].Tetrahedron Lett,2002,43:3193-3196.
[10] Kabalka G W, Mereddy A R. Microwave promoted synthesis of functionalized 2-aminothiazoles[J].Tetrahedron Lett,2006,47:5171-5172.
[11] El-Subbagh H I, Al-Obaid A M. 2,4-Disubstituted thiazolesⅡ.A novel class of antitumor agents,synthesis and biological evaluation[J].Eur J Med Chem,1996,31:1017-1021.
[12] Easmon J, Heinisch G, Hofmann J,etal. Thiazolyl and benzothiazolyl hydrazones derived froma-(N)-acetylpyridines and diazines:Synthesis,antiproliferative activity and CoMFA studies[J].Eur J Med Chem,1997,32:397-408.
[13] Sayed L E, Iskander M F. Coordination compounds of hydrazine derivatives with transition metals——Ⅲ:The reaction of aroyl hydrazones with Ni(Ⅱ) and Cu(Ⅱ) salts[J].J Inorg Nucl Chem,1971,33(2):435-443.
[14] 李在国. 有机中间体制备[M].北京:化学工业出版社,2000.
[15] 史延年,卢彦昌,方建新,等。ω-芳氧乙酸氧基-ω-(1H-1,2,4-三唑-1-基)苯乙酮衍生物的研究[J].高等学校化学学报,1995,16(11):1710-1713.
[15] 黄泰山. 呋喃醛缩氨基硫腙的合成和结构测定[J]厦门大学学报(自然科学版),1994,33(6):827-831.

