微乳液法制备可共载水溶和脂溶药物的壳聚糖季铵盐乙醇脂质体的载药性能表征
梁晓飞 胡晶莹 陈复华 李宗海 常 津
(1癌基因及相关基因国家重点实验室,上海交通大学医学院附属仁济医院上海市肿瘤研究所,上海200032; 2上海市肿瘤研究所公共实验平台,上海交通大学医学院附属仁济医院,上海200032; 3天津大学材料科学与工程学院纳米生物技术研究所,天津市材料复合与功能化重点实验室,天津430072)
微乳液法制备可共载水溶和脂溶药物的壳聚糖季铵盐乙醇脂质体的载药性能表征
梁晓飞1,*胡晶莹2陈复华2李宗海1,*常 津3,*
(1癌基因及相关基因国家重点实验室,上海交通大学医学院附属仁济医院上海市肿瘤研究所,上海200032;2上海市肿瘤研究所公共实验平台,上海交通大学医学院附属仁济医院,上海200032;3天津大学材料科学与工程学院纳米生物技术研究所,天津市材料复合与功能化重点实验室,天津430072)
采用微乳液法制备了可包载脂溶性和水溶性药物的羧甲基壳聚糖十八烷基季铵盐(OQCMC)乙醇脂质体,研究了OQCMC乙醇高分子脂质体的相图、粒径和电位、对药物的包封及释放能力及共载水溶性和脂溶性荧光染料后的细胞内递送能力.结果表明:OQCMC上长链季铵盐分子的取代度和共乳化剂乙醇的加入量对相图中微乳区域的面积影响不大;微乳液法可制备包载水溶性长春新碱(VCR)、脂溶性消炎痛(IMC)或二者共载的OQCMC载药微球,微球粒径为(52.40±0.55)nm,分布均匀;微乳液体系对VCR的最大载药率为22.7%,对IMC的最大载药率为20.1%,二者共载时,VCR的最大载药率为12.2%,IMC的最大载药率为10.0%;载药微球对药物具有缓控释功能.OQCMC乙醇高聚物脂质体可有效地包载荧光染料异硫氰酸荧光素FITC(水溶性)和尼罗红(脂溶性),并将二者递送到卵巢癌HO8901细胞内.
壳聚糖;表面活性剂;纳米颗粒;药物递送;微乳液;乙醇脂质体
1 Introduction
Chitosan(CS)has attracted a lot of peopleʹs attention due to its physicochemical characteristics and bioactivities.1-5Different chitosan derivatives have been synthesized in order to improve its aqueous solubility,including carboxymethyl chitosan (CMC)and quaternized chitosan.As a new kind of multifunctional amphiphilic polymer,octadecyl quaternized carboxymethyl chitosan(OQCMC)has good solubility both in water and in organic solvents.3,6OQCMC,combined with cholesterol,can form cationic polymeric liposomes,which abroad its application in biomedicine.7,8
To form nanoparticles(NPs)by using polymeric surfactants, there are a lot of methods,such as dialysis for micelles,9-12emulsion,13and microemulsions,14-16etc.Unlike conventional emulsions,microemulsions(ME),which are thermodynamically stable dispersions of oil and water that are stabilized by surfactants and in some cases additionally by co-surfactants,have attracted much interest in recent years because of their great practical importance in terms of their drug delivery potential and interesting physical properties.17,18However,there are few studies about using new polymeric surfactants synthesized from chitosan to form NPs by microemulsion method.In addition,co-delivery of different drugs using the same vector may be a promising application of NPs drug delivery system.19Ethosomes are soft,malleable vesicles composed mainly of phospholipids or other lipids,ethanol and water.Herein,microemulsions were prepared by using the blend of multifunctional surfactant OQCMC(polymeric lipids)and co-surfactant alcohol to form polymeric ethosomes.Hydrophilic drug vincristine (VCR)and lipophilic drug indomethacin(IMC)were selected to study the properties of this OQCMC/alcohol microemulsion system such as drug encapsulation and release ability.
2 Experimental
2.1 Materials
Chitosan was supplied by Yuhuan Aoxing Biochemistry Co., Ltd.in Zhejiang province in China,with a deacetylation degree of above 85%and the molecular weight(Mw)was about 5×104. Vincristine(VCR)(≥99%)was supplied from Shanghai Anticancer Phytochemistry Co.,Ltd(China).Octadecyl quaternized carboxymethyl chitosan(OQCMC)was prepared in our laboratory.8All other chemicals and reagents were of analytical grade and obtained from Sinopharm Chemical Reagent Co., Ltd.
2.2 Preparation of polymeric ethosomes
Methods of submicron emulsion preparation have been described in detail.14Briefly,OQCMC(10 mg)was dissolved in dichloromethane(2 mL)and mixed thoroughly with the water phase(phosphate buffer solutions,0.1 mol·L-1,pH 7.4).The mixture was emulsified with stirring for about 0.5 h.The coarse oil-in-water(O/W)emulsion formed was then homogenized through ultrasonic.Afterward,the co-surfactant of ethanol was added to the emulsion gradually under vigorous agitation until the solution became clear exactly.13The volume of dichloromethane,water phase and ethanol adding in the mixture was recorded for drawing phase diagram.The complex was then stirred for about 12 h,allowing slow evaporation of dichloromethane and formation of NPs.20Polymeric ethosomes encapsulating VCR were obtained by adding VCR to the aqueous phase.Polymeric ethosomes encapsulated IMC were obtained by adding IMC to ethanol or dichloromethane.
2.3 Characterizations
The morphologies of NPs were observed using transmission electron microscopy(TEM).TEM observation was carried out at 200 kV with JEOL-100CXII(Japan)in the NPs solution and placed on a copper grid coated with film.The average particle size and size distribution were determined by quasielastic laser light scattering with a Malvern Zetasizer(Malvern Instruments Limited,United Kingdom)at 25°C.Zeta potentials of NPs were recorded in deionized water solution.
The in vitro release profiles of VCR or IMC from ME NPs were determined as follows:1 mL(1 mg·mL-1)of ME NPs was placed in dialysis bag with 8 mL of phosphate buffer solutions(PBS)(0.1 mol·L-1,pH 7.4)in test tubes and incubated at (37.0±0.5)°C with shaking.21At the designated time intervals, buffer solutions outside the dialysis bag were taken out to determine the amount of drug released from NPs by UV-Vis spectroscopy,and8mLof fresh mediumwas added.
2.4 Fluorescence microscopy
The uptake and intracellular distribution of FITC or Nile Red in HO8901 cells were detected using fluorescence microscopic.HO8901 cells were seeded in a 24-well plate with pre-sterilized cover glass at bottom at a density of 8×104cells· well-1and allowed to attach overnight.The following day,cells were treated with free FITC,free Nile Red,FITC-loaded,Nile Red-loaded,FITC and Nile Red co-loaded ME NPs in medium solution at concentration of 20 μg·mL-1.After 2 h post-treatment,cells were washed three times with PBS and examined by fluorescence microscopy(FV1000,Olimpus,Japan)with appropriate filters for FITC-associated green fluorescence and Nile Red associated red fluorescence.
3 Results and discussion
3.1 OQCMC ME NPs preparation
Ethanol is known as an efficient permeation enhancer reagent.Liposomes,which can aid the transport of hydrophilic and lipophilic compounds,have been considered as an effective NPs system for enhancing percutaneous delivery.22Ethosomes with inclusion of ethanol has already been investigated by many authors.23In our former study,it has been approved that OQCMC with cholesterol can form stable polymeric liposomes.5,6Simliar with conventional ethosomes,vesicles formed from OQCMC with ethanol by ME can be regarded as a kind of polymeric ethosomes.In ME method,dichloromethane has been utilized and was evaporated cleanly in the process of forming ME NPs.
3.2 Influence of DS on the formation of OQCMC ME
Factors,which affect the formation of an O/W ME,include the type of surfactant,co-surfactant,the ratio of surfactant/co-surfactant,and the temperature.24Herein,the degree of substitution(DS)of the octadecyl quaternary ammonium group on the formation of polymeric surfactant ME was investigated.The phase diagrams of oil(dichloromethane)/surfactant(OQCMC)/co-surfactant(ethanol)/water with different DS of OQCMC are shown in Fig.1.The labeled isotropic ME region corresponded to the area of mutual solubility of the oil/ OQCMC/ethanol/water components.The area fractions for the labeled regions were 51.5%,50.8%,53.2%,and 55.5%of the total phase diagram for the different DS degrees of 105.0%, 89.1%,55.8%,and 16.8%,respectively.The results indicated that there are no significant difference on forming ME abilities of difference DS of carboxymethyl chitosan.OQCMC is a kind of polymeric ionic surfactant with positive zeta potential.In compare with other ionic surfactant with low molecular weight,it can be seen that OQCMC has a relatively higher ability to form ME.
3.3 Size and morphology characterization of
OQCMC ME NPs
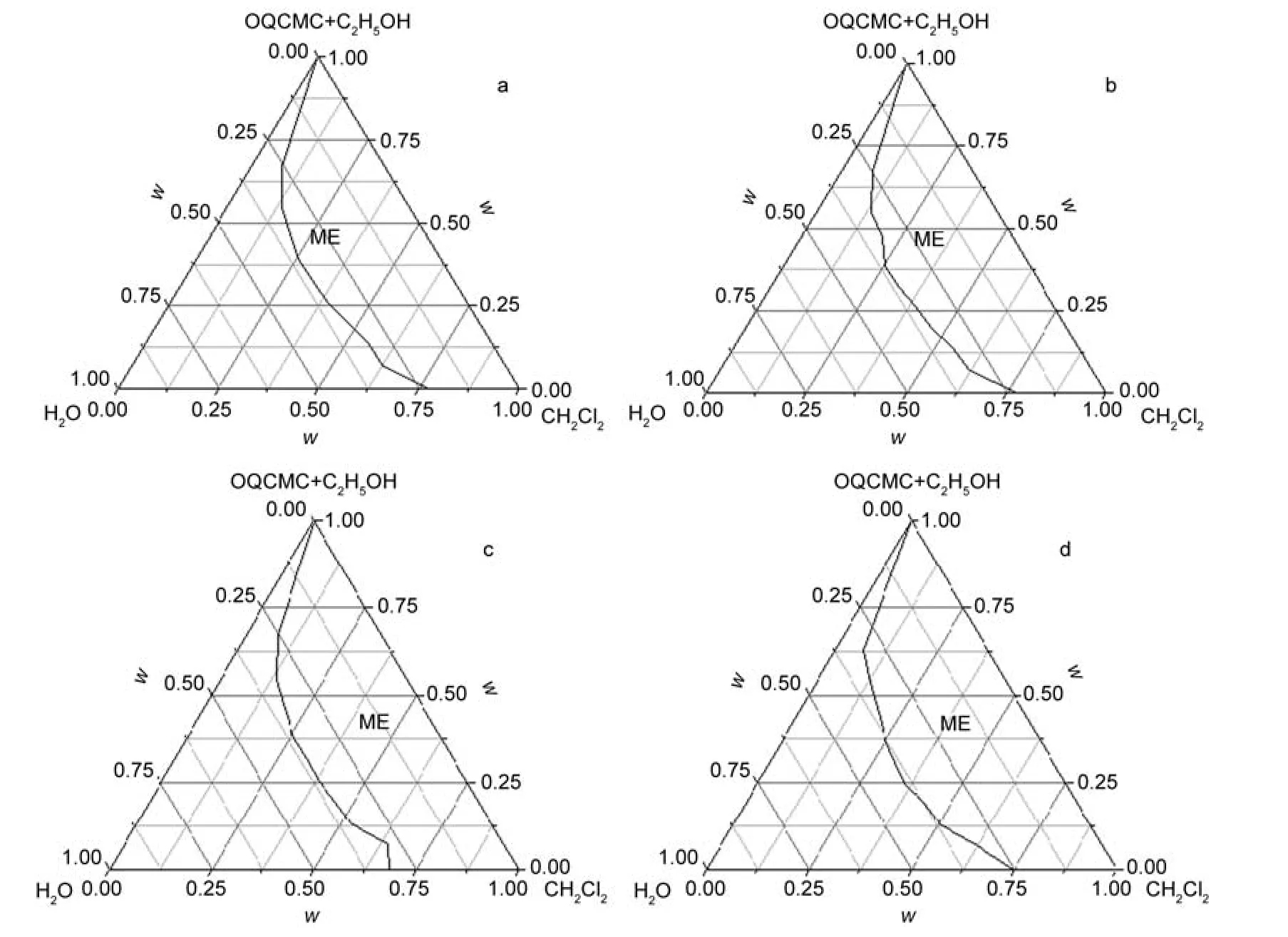
Fig.1 Pseudotemary phase diagrams of microemulsions with dichloromethane/OQCMC/ethanol/water at 25°C with a constant mass ratio of OQCMC:ethanol:water(1:1:1)quaternary substitution(DS)degree(w·w-1):(a)105.0%,(b)89.1%,(c)55.8%,(d)16.8%

Fig.2 TEM image(a)and particle size distribution(b)of VCR and IMC co-encapsulated NPs
A promising application of NPs drug delivery systems is the co-delivery of multiple drugs using the same NPs delivery device in a cell-,tissue-,or disease-specific manner.19Compared with delivering a single drug,co-delivery of multiple drugs has several potential advantages,including:synergistic effects,suppressed drug resistance,and the ability to tune the relative dosage of various drugs to the level of a single NP carrier.Herein, ME method was used to form NPs encapsulated model hydrophobic drug IMC and hydrophilic drug VCR simultaneously. TEM image of ME NPs encapsulated IMC and VCR simultaneously is shown in Fig.2.Drug-loaded ME NPs had irregular shape and different size.The predominant globular vesicles have a small diameter(<30 nm).The mean particle size of OQCMC black NPs in aqueous solution was(52.40±0.55)nm with the polydispersity index 0.303.After encapsulating with VCR or IMC,the diameter of these ME NPs had increased a little with(69.20±0.51)nm and polydispersity index 0.261. Furthermore,zeta potential of OQCMC ME NPs has decreased to (39.36±3.53)mV compared with OQCMC micelles of(49.39± 3.53)mV,which indicated that the quaternary ammonium group of OQCMC had been partially buried in NPs.
3.4 Drug loading efficiency
In order to evaluate drug loading efficiency of OQCMC ME, experiments were designed to obtain the data of the maximal drug loading capacity and drug encapsulating efficiency for IMC and VCR.The chromatograms obtained after elution of free VCR and VCR-loaded ME NPs are shown in Fig.3.It can be seen that the peak of free VCR and VCR-loaded NPs were separated clearly,and there was not latter peak for VCR-loaded ME NPs.The results indicated that the drug encapsulating efficiency for VCR is high(>93%)for ME method.To OQCMC ME,the maximal drug loading efficiencies for VCR and IMC were 22.7%and 20.1%,respectively.The drug loading capacity of the co-delivery OQCMC NPs for VCR and IMC were 12.2%and 10.0%,respectively.
3.5 In vitro release of different drugs in OQCMC ME NPs

Fig.3 Gel exclusion chromatography of free VCR solution and VCR-loaded OQCMC ME NPs with Sephadex G50 column
In vitro drug release profiles of VCR-and IMC-loaded OQCMC ME NPs are shown in Fig.4,which exhibited a two phase release profile with an initial burst of 48.6%and 56.3%in the first 11 h,respectively.This can be considered as a burst effect and was attributed to the unbound excess of VCR or IMC on the surface of NPs.For co-delivery NPs,the co-release behaviors of VCR and IMC are similar with only VCR-or IMC-loaded NPs.All samples of these ME NPs exhibited slow steady drug release action for 60 h.The initial release of drugs from ME NPs was proportional to the square root of time,which indicated that the release of the unbound drugs followed a Fickian diffusion pattern.25
3.6 Fluorescence microscopy
The uptake of the OQCMC ME NPs encapsulated fluorescence probe of FITC(hydrophilic)and Nile Red(hydrophobic) by HO8901 cells with incubation of 2 h was visualized using fluorescence microscopy.The concentration of FITC-loaded,Nile Red loaded,and two fluorescence probe co-loaded OQCMC ME NPs suspension was 20 μg·mL-1(with FITC or Nile Red concentration of 1 μg·mL-1).Drug delivery efficiency of OQCMC ME NPs on fluorescence probe with different property is clearly shown in Fig.5.Fluorescence was not detected in control cells with blank ME NPs adding and free Nile Red adding. The reason is that Nile Red is a kind of lipophilic material and only presents fluorescence in lipophilic environment,such as in lipid or other hydrophobic surfactant.The results in Fig.5(b, e,f)strongly support the conclusion that OQCMC is a kind of polymeric surfactant with liposome character.5Furthermore,a little lack magenta fluorescence overlaid by green fluorescence and red fluorescence which were emitted by FITC and Nile Red were detected in the same cells.It indicated that OQCMC ME NPs can easily delivery and co-delivery two different drugs with hydrophobic or hydrophilic property into tumor cells.
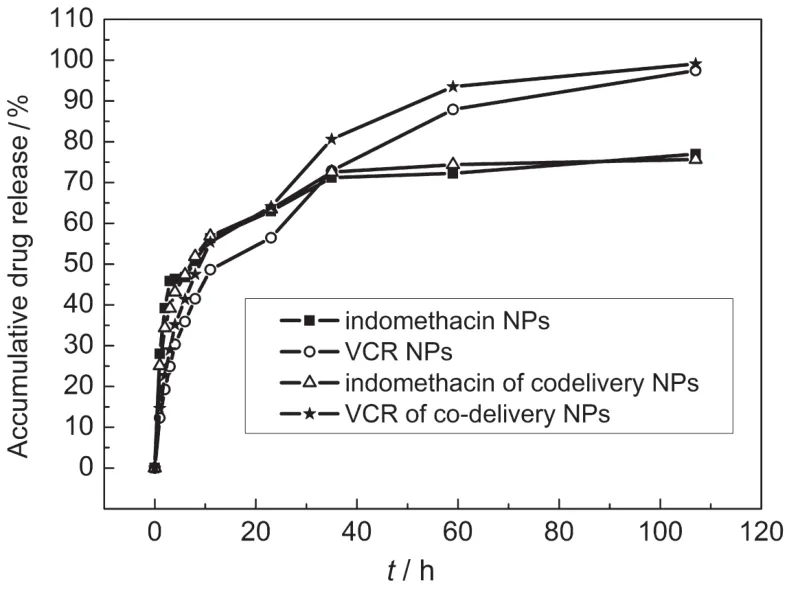
Fig.4 In vitro drug release profiles for IMC and VCR encapsulated in ME NPs

Fig.5 Fluorescence microscopic images of HO8901 cells with different OQCMC ME NPs at a concentration of 20 μg·mL-1after incubation at 37°C for 2 h(a)free FITC,(b)free Nile Red,(c)blank ME NPs,(d)FITC-loaded ME NPs,(e)Nile Red-loaded ME NPs,(f)FITC and Nile Red co-encapsulated ME NPs
4 Conclusions
In summary,polymeric surfactant OQCMC with different DS can form stable polymeric ethosomes by microemulsion method.Different drugs of hydrophobic IMC and hydrophilic VCR can be encapsulated into these ME NPs with high drug encapsulating efficiency(>93%)and drug loading capacity(>20%), simultaneously or respectively.The drug loaded ME NPs had small size(<30 nm)and narrow polydispersity,which exhibited slow steady drug release action for IMC and VCR.Upon incubation of the FITC-loaded,Nile Red loaded,or FITC and Nile Red co-loaded ME NPs with HO8901 cells,bright green and red fluorescences were both observed in the cytoplasm, which indicated that different hydrophobic and hydrophilic drugs carried by the NPs were co-delivered into the tumor cells efficiently.The OQCMC ME NPs may be suitable as a potential one or more different type drugs co-delivery system for cancer therapy.
(1)Tan,W.B.;Zhang,Y.Adv.Mater.2005,17,2375.
(2)Wang,X.D.;Zhang,B.Q.;Liu,X.F.;Lin,J.Y.S.Adv.Mater. 2006,18,3261.
(3) Liang,X.F.;Tian,H.;Luo,H.;Wang,H.J.;Chang,J.Journal of Biomaterials Science 2009,20,115.
(4) Yang,Z.K.;Cheng,S.Y.Journal of Applied Polymer Science 2003,89,924.
(5) Banerjee,T.;Mitra,S.;Singh,A.K.;Sharma,R.K.;Maitra,A. International Journal of Pharmaceutics 2002,243,93.
(6) Liang,X.F.;Wang,H.J.;Tian,H.;Luo,H.;Chang,J.Acta Phys.-Chim.Sin.2008,24,223. [梁晓飞,王汉杰,田 惠,罗 浩,常 津.物理化学学报,2008,24,223.]
(7) Liang,X.F.;Wang,H.J.;Luo,H.;Tian,H.;Zhang,B.B.;Hao, L.J.;Teng,J.I.;Chang,J.Langmuir 2008,24,7147.
(8) Liang,X.F.;Wang,H.J.;Jiang,X.G.;Chang,J.Journal of Nanoparticle Research 2010,12,1723.
(9)Wang,C.;Ge,Q.;Ting,D.;Nguyen,D.;Shen,H.;Chen,J.; Eisen,H.N.;Heller,J.;Langer,R.;Putnam,D.Nature Materials 2004,3,190.
(10)Aliabadi,H.M.;Lavasanifar,A.Expert Opinion on Drug Delivery 2006,3,139.
(11) Sun,K.H.;Sohn,Y.S.;Jeong,B.Biomacromolecules 2006,7, 2871.
(12)Lee,S.C.;Huh,K.M.;Lee,J.;Cho,Y.W.;Galinsky,R.E.; Park,K.Biomacromolecules 2007,8,202
(13) Yang,S.C.;Benita1,S.Drug Development Research 2000,50, 476.
(14) Klier,J.;Tucker,C.J.;Kalantar,T.H.;Green,D.P.Adv.Mater. 2000,12,1751.
(15)Peng,X.H.;Zheng,P.Z.;Ma,Y.M.;Yin,T.X.;An,X.Q.; Shen,W.G.Acta Phys.-Chim.Sin.2011,27,1026.[彭旭红,郑佩珠,马元明,殷天翔,安学勤,沈伟国.物理化学学报, 2011,27,1026.]
(16) Jin,S.P.;Feng,L.;He,W.Y.;Han,Y.Q.;Wei,Y.J.Acta Phys.-Chim.Sin.2010,26,2581. [金淑萍,冯 雷,何文龑,韩玉琦,魏玉娟.物理化学学报,2010,26,2581.]
(17) Bastogne,F.;David,C.Colloids and Surfaces A: Physicochemical and Engineering Aspects 1998,139,311.
(18)Yaghmur,A.;Aserin,A.;Garti,N.Colloids and Surfaces A: Physicochemical and Engineering Aspects 2002,209,71.
(19) Zhang,L.F.;Radovic-Moreno,A.F.;Alexis,F.;Gu,F.X.; Basto,P.A.;Bagalkot,V.;Jon,S.Y.;Langer,R.S.;Farokhzad, O.C.ChemMedChem.2007,2,1268.
(20) Shuai,X.;Merdan,T.;Kissel,T.Bioconjugate Chemistry 2004, 15,441.
(21)Liu,S.Q.;Wiradharma,N.;Gao,S.J.;Tong,Y.W.;Yang,Y.Y. Biomaterials 2007,28,1423.
(22) Lόpez-Pinto,J.M.;Gonzάlez-Rodrίguez,M.L.;Rabasco,A.M. International Journal of Pharmaceutics 2005,298,1.
(23) Godin,B.;Touitou,E.J.Contr.Rel.2004,94,365.
(24) Zhong,F.;Yu,M.;Luo,C.R.;Shoemaker,C.F.;Li,Y.;Xia,S. Q.;Ma,J.G.Food Chemistry 2009,115,539.
(25) Chen,L.Y.;Tian,Z.G.;Du,Y.M.Biomaterials 2004,25,3725.
October 20,2011;Revised:January 16,2012;Published on Web:February 9,2012.
Characterization of Chitosan Polymeric Ethosomes Capable of Encapsulating Hydrophobic and Hydrophilic Drugs Prepared by a Microemulsion Method
LIANG Xiao-Fei1,*HU Jing-Ying2CHEN Fu-Hua2LI Zong-Hai1,*CHANG Jin3,*
(1State Key Laboratory of Oncogenes and Related Genes,Shanghai Cancer Institute,Renji Hospital,School of Medicine,Shanghai Jiao Tong University,Shanghai 200032,P.R.China;2Public laboratory platform,Shanghai Cancer Institute,Renji Hospital, School of Medicine,Shanghai Jiao Tong University,Shanghai 200032,P.R.China;3Institute of Nanobiotechnology, School of Materials Science and Engineering,Tianjin University and Tianjin Key Laboratory of Composites and Functional Materials,Tianjin 300072,P.R.China)
Polymeric ethosomes,formed from amphiphilic octadecyl quaternized carboxymethyl chitosan (OQCMC)with different degrees of quaternary substitution(DS),were prepared by the microemulsion(ME) method.These ethosomes could simultaneously encapsulate both the hydrophobic drug indomethacin (IMC)and the hydrophilic drug vincristine(VCR).The effects of the DS of the OQCMC and primary alcohols as cosurfactants on the phase diagram were elucidated.The prepared nanoparticles(NPs)were small((52.40±0.55)nm)and suitable as drug carriers for different drugs.The maximum drug loading efficiencies of VCR-loaded and IMC-loaded NPs were 22.7%and 20.1%,respectively.The drug loading capacities for co-delivery of VCR and IMC were 12.2%and 10.0%,respectively.OQCMC polymeric ethosomes were stable in aqueous solution and exhibited slow,steady drug release.Hydrophilic fluorescein isothiocyanate(FITC)and hydrophobic Nile Red were encapsulated by the OQCMC ME NPs and simultaneously delivered into HO8901 cells with green and red fluorescence,respectively.This co-delivery system may allow for temporally distinct classes of drugs for cancer therapy.
Chitosan;Surfactant;Nanoparticle;Drug delivery;Microemulsion;Ethosome
10.3866/PKU.WHXB201202091
O648
∗Corresponding authors.LIANG Xiao-Fei,E-mail:xfliang@shsci.org.LI Zong-Hai,Email:zonghaili@gmail.com.CHANG Jin,
Email:jinchang@tju.edu.cn.
The project was supported by the National Natural Science Foundation of China(51003059)and Natural Science Foundation of Shanghai,China (10ZR1428900).
国家自然科学基金(51003059)及上海市自然科学基金(10ZR1428900)资助项目

