Crystal structure and proton-conductivity of a complex based on phosphomolybdic acid and 2-(2-hydroxybenzene)benzimidazole
CHEN Lin, WEI Meilin
(School of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang 453007, Henan, China)
Crystalstructureandproton-conductivityofacomplexbasedonphosphomolybdicacidand2-(2-hydroxybenzene)benzimidazole
CHEN Lin, WEI Meilin*
(SchoolofChemistryandChemicalEngineering,HenanNormalUniversity,Xinxiang453007,Henan,China)
A proton-conductive organic-inorganic complex, [H3L2(PMo12O40)·7H2O·4CH3OH]n(1), was constructed with phosphomolybdic acid and 2-(2-hydroxybenzene)benzimidazole (L) as the starting materials. Single-crystal X-ray diffraction analysis reveals that complex1exhibits a two-dimensional hydrogen-bonding network structure based-on phosphomolybdic acid, L molecules and solvent methanol molecules. Besides, complex1shows proton conductivity of about 10-4S·cm-1at 100 ℃ under 98% relative humidity.
phosphomolybdic acid;benzimidazole; organic-inorganic complex;crystal structure; proton conductivity
Solid-state materials with proton conductivities have interested us from the point of view of transport dynamics and their applications in fuel cells[1-6]. Supramolecular assemblies built by means of hydrogen-bonding interactions have provided numerous solid-state materials with very attractive properties. For a long time, we have focused on organic/inorganic complexes based-on Keggin-type heteropolyacids dispersing in self-ordered hydrogen-bonded networks from the ligands containing 2-substituted benzimidazoles such as 2-(3-pyridyl)benzimidazole molecules[4], which have attracted considerable interest for their versatile coordination modes and potential to form supramolecular aggregates through π-π stacking and hydrogen bonding interactions[4,7]. In the present research, by a self-assembly of phosphomolybdic acid and 2-(2-hydroxybenzene)benzimidazole molecules (L), we have constructed a proton-conductive organic/inorganic hybrid complex, [H3L2(PMo12O40)·7H2O·4CH3OH]n(1). X-ray diffraction analyses at 293 K revealed that complex1presented a two-dimensional(2D) supramolecular framework constructed by L molecules, phosphomolybdic acid and methanol molecules based-on hydrogen-bonding interactions. The results of the impedance measurement show that complex1is a good proton conductor. Interestingly, complex1shows proton conductivities across a wide range of temperatures and relative humidity (RH) and achieve proton conductivity over ~10-4S·cm-1at 100 ℃ under 98% RH. Here we report the synthesis and structural characterization of complex1as well as its proton conductivity evaluation in relation to temperature and RH.
1 Experimental
1.1 Materials and instruments
All organic solvents and materials used for synthesis were of reagent grade and used without further purification.α-H3PMo12O40·6H2O was also prepared according to a literature method[1-4]and characterized by IR spectrum and TG analysis. L was prepared according to a literature method[8]. Elemental analyses (C, H, and N) were carried out on a Perkin-Elmer 240C analyzer. X-ray powder diffraction (XRD) was performed on a Bruker D8 Advance Instrument using Cu-Kαradiation and a fixed power source (40 kV, 40 mA). IR spectrum was recorded on a VECTOR 22 Bruker spectrophotometer with KBr pellets in the 400-4 000 cm-1region at room temperature. Thermogravimetric analysis and differential scanning calorimetry were performed on a Perkin-Elmer thermal analyzer under nitrogen at a heating rate of 10 ℃·min-1. For an electrical conductivity study, the powdered crystalline samples were compressed to 1.0-1.2 mm in thickness and 12.0 mm in diameter under a pressure of 12-14 MPa.Alternatingcurrent (Ac) impedance spectroscopy measurement was performed on a chi660d (Shanghai Chenhua) electrochemical impedance analyzer with copper electrodes[1-6](the purity of Cu is more than 99.8%; the pellet was contacted with two copper plates) over the frequency range from 105Hz to 10 Hz. The conductivity was calculated asσ= (1/R) × (h/S), whereRis the resistance,his the thickness, andSis the area of the tablet.
1.2 Synthesis of the title compound
Complex1was prepared by layering method. A buffer layer of a solution (10 mL) of methanol-water (1∶1,V/V) was carefully layered over 5 mL of an aqueous solution ofα-H3PMo12O40·6H2O (120 mg, 0.06 mmol). Then a methanol (5 mL) of L (25.2 mg, 0.12 mmol) was carefully layered over the buffer layer. Two weeks later, red crystals appeared and were collected and dried in air after quickly being washed with water. Yield: 91 mg, 76% based onα-H3PMo12O40·6H2O. Anal. Calcd (%) for C30H53Mo12N4O53P: calcd(%): C, 14.41; H, 2.14; N, 2.24; Found (%): C, 14.33; H, 2.07; N, 2.16. IR (KBr, cm-1): four characteristic vibrations resulting from heteropolyanions with the Keggin structure: 809ν(Mo-Oc), 881ν(Mo-Ob), 955ν(Mo=Ot), 1 068ν(P-Oa); some vibrations resulting from L molecules: 3 270ν(O-H), 1 625ν(C=N), 1 245ν(C-O), 1 062ν(C-C).
1.3 Structure determination
Intensity data of complex1were collected on a Siemens SMART CCD diffractometer with graphite-monochromated Cu-Kαradiation (λ= 0.071 073 nm) using SMART and SAINT. The structure was solved by direct methods and refined onF2by using full-matrix least-squares method with SHELXTL version 5.1[9]. All non-hydrogen atoms except for solvent molecules were refined anisotropically. Hydrogen atoms of organic molecules were localized in their calculated positions and refined using a riding model. Hydrogen atoms of solvent water molecules were not treated. The crystal parameters, data collection and refinement results for complex1are summarized in Table 1, and the selected hydrogen bond parameters in Table 2 with the lables of atoms shown in Fig.1. CCDC contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from the Cambridge Crystallographic Data Centreviahttp://www.ccdc.cam.ac.uk/data_request/cif.

Table 1 Crystallographic data and refinement parameters for the title complex

Fig.1 Molecular structure unit of complex 1 showing the labeling atoms at 30% probability thermal ellipsoids and hydrogen-bonding interactions(solvent water molecules and hydrogen atoms have been omitted for clarity)
Table2Hydrogenbondlengths(nm)andbondangles(°)
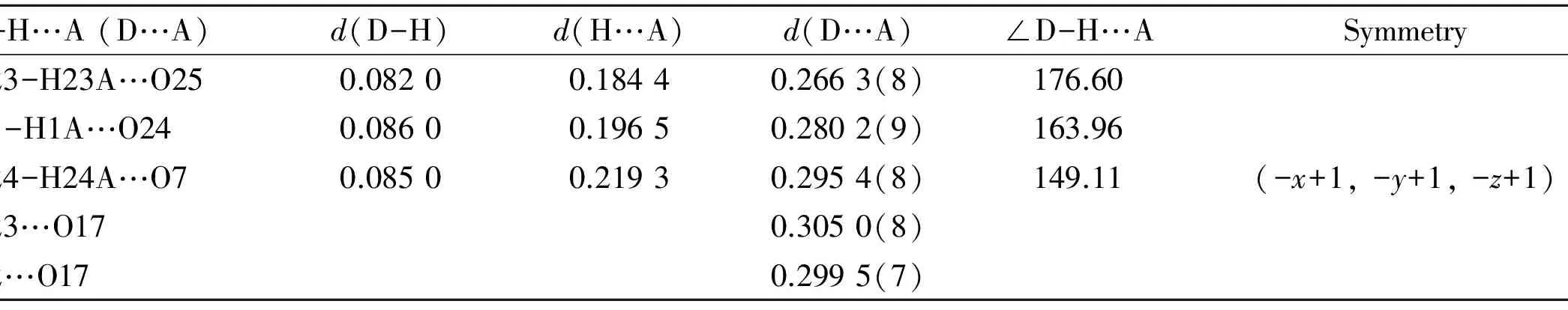
D-H…A(D…A)d(D-H)d(H…A)d(D…A)∠D-H…ASymmetryO23-H23A…O250.08200.18440.2663(8)176.60N1-H1A…O240.08600.19650.2802(9)163.96O24-H24A…O70.08500.21930.2954(8)149.11(-x+1,-y+1,-z+1)O23…O170.3050(8)N2…O170.2995(7)
2 Results and discussion
2.1 Structure description
Complex1, [H3(PMo12O40)L2·7H2O·4CH3OH]n, was synthesized by the reaction of phosphomolybdic acid and L molecules at room temperature. It was characterized by single-crystal X-ray diffraction, infrared spectroscopy, TG and elemental analyses. X-ray diffraction analyses at 293 K revealed that complex1crystallized in the triclinic space groupPī and presented a 2D supramolecular framework constructed by L molecules, phosphomolybdic acid and methanol molecules based-on hydrogen-bonding interactions. The molecular structure of1is shown in Fig. 1. The molecular unit contains two L molecules, one phosphomolybdic acid molecule, four methanol molecules and seven water molecules. In the L molecule, the dihedral angle between the benzimidazole ring and the benzene ring of 2-hydroxybenzene is 6.26°. Bond valence sum (BVS) calculations[10]indicate that the N2 atom of the imidazole ring is the possible binding site of a proton from phosphomolybdic acid. Based on hydrogen-bonding interactions, two L molecules, one phosphomolybdic acid molecule and four methanol molecules form a cluster, [(H3PMo12O40)L2(CH3OH)4]. Moreover, the clusters are connected with each other based-on the hydrogen-bonded interactions between the O7 atoms of [PMo12O40]3-anions and the O24 atoms of methanol molecules to form a 2D layer structure with voids (Fig. 2). Solvent water molecules were just embedded in the voids. In addition, the presence of positively species, H+, from phosphomolybdic acid being embedded in the voids of the 2D anionic framework, could not only attract the polyanions to stabilize the 2D supramolecular framework, but also provide potential proton carriers.
In the [PMo12O40]3-anion, the bond lengths of P-O and Mo-O are 1.480(8)-1.603(9) and 0.163 7(6)-0.248 1(9) nm, respectively. The bond lengths of P-O and Mo-O are respectively comparable to those in the polyoxometalates-based organic-inorganic hybrid materials with Keggin anions as guests. In addition, the O-P-O angles are in the range of 66.7(5)°-112.2(4)°. All these results indicate that the [PMo12O40]3-units have a normal Keggin structure[1-4].
Therefore, in complex1, based on electrostatic and hydrogen-bonding interactions, [PMo12O40]3-anions were stabilized in the supramolecular framework and not easily dissociated from the hybrid network. In addition, the protons from Keggin-type heteropolyacids, the protons belong to L molecules and hydrogen bonding networks indicate that complex1can potentially be a good proton-conducting material.

Fig.2 The 2D hydrogen-bonded network in complex 1 down the b axis
2.2 TG analysis

Fig.3 The curve of the Perkin-Elmer thermal analysis of complex 1 in the atmosphere of N2
Fig. 3 shows the TG result for complex1. Thermal analysis of the powder of the crystalline sample of complex1in an atmosphere of N2reveals that the robustness of the porous network could retain up to 300 ℃ with a weight loss of about 4.91% in the temperature range 20-110 ℃(the weight loss corresponds to the loss of all solvent water molecules). The robustness of the porous network begins to decompose above 300 ℃ due to the loss of methanol molecules and L molecules, indicating that methanol molecules and L molecules in the unit structure are involved in hydrogen-bonding interactions with the supramolecular framework, which is consistent with the result of structural analysis, and could be hold in the supramolecular framework at 300 ℃.
2.3 Proton conductivity
The proton conductivity of complex1was measured at 25 ℃ in the RH range 35%-98% by a complex-plane impedance method using a compacted pellet of the powdered crystalline sample, which has the same structure as the single-crystal. At 25 ℃, complex1showed poor proton conductivities of ~10-9S·cm-1under 35% RH conditions, and its proton conductivities reached ~6.5×10-8S·cm-1with RH up to 98%. The proton conductivities of1were also measured at 100 ℃ in the RH range 35%-98% by a complex-plane impedance method. Fig. 4 shows the lg [σ/(S·cm-1)] versus RH plots of complex1at 25 and 100 ℃ under 35%-98% RH. The conductivities of complex1increase with increasing RH at both temperatures. Again, we measured its ionic conductivities up to 100 ℃ under 98% RH conditions. As the temperature increases, the proton conductivities of complex1increase on a logarithmic scale even with almost saturated humidities. Fig. 5 shows the Arrhenius plots of the proton conductivities of complex1in the temperature range of 25-100 ℃ under 98% RH conditions. The ln(σT) increases almost linearly with temperature range from 25 to 100 ℃, and the corresponding activation energy (Ea) of conductivity was estimated to be 1.25 eV. TheEavalue is high in the temperature range of 25-100 ℃. This is probably due to the fact that protons originating from phosphomolybdic acid and those originating from L molecules need a endothermal process for dissociation as hydrated forms such as H+, H3O+or other proton species[1-4]. Therefore, the fact that complex1exhibits good proton conductivities(5.21×10-5-2.21×10-4S·cm-1) in the temperature range of 85-100 ℃ is indicative of a high carrier concentration based on the dissociating processes of proton from L molecules and phosphomolybdic acid. The powder X-ray diffraction data suggest that the powder sample after the proton-conductive measurement has the same supramolecular framework as that of complex1.

Fig.4 Relative humidity dependence of the proton conductivity of complex 1
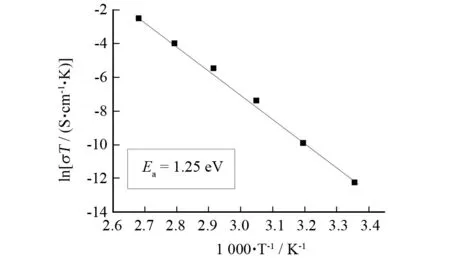
Fig.5 Arrhenius plots of the proton conductivity of complex 1
3 Conclusion
In summary, a proton-conductive organic-inorganic complex based on phosphomolybdic acid and 2-(2-hydroxybenzene)benzimidazole molecules has been constructed. The organic-inorganic hybrid matrix changed the environment around phosphomolybdic acid and influenced the formation of self-ordered hydrogen-bonding network within the resultant structure. Thus, complex1provides a route in increasing the stability and proton conductivity of organic-inorganic hybrid materials based on Keggin-type heteropolyacids and 2-(2-hydroxybenzene)benzimidazole molecules up to 100 ℃.
[1] WEI Meilin, ZHUANG Pengfei, LI Huihua, et al. Crystal structures and conductivities of two organic-inorganic hybrid complexes based on poly-Keggin-anion chains [J]. Eur J Inorg Chem, 2011(9): 1473-1478.
[2] WEI Meilin, ZHUANG Pengfei, MIAO Qiuxiang, et al. Two highly proton-conductive molecular hybrids based on ionized water clusters and poly-Keggin-anion chains [J]. Solid State Chem, 2011, 184: 1472-1477.
[3] WEI Meilin, WANG Xiaoxiang, DUAN Xianying. Crystal structures and proton conductivities of a MOF and two POM-MOF composites based on CuIIions and 2,2′-bipyridyl-3,3′-dicarboxylic acid [J]. Chem Eur J, 2013, 19(5): 1607-1616.
[4] WEI Meilin, WANG Yuxia, WANG Xinjun. Two proton-conductive hybrids based on 2-(3-pyridyl) benzimidazole molecules and Keggin-type heteropolyacids [J]. Solid State Chem, 2014, 209: 29-36.
[5] 孙晶晶,魏梅林. 异烟酸氮氧化物/磷钼酸镍络合物掺杂硅胶复合物的制备及其质子导电性 [J]. 化学研究, 2014, 25(1): 63-66.
[6] 王玉霞,魏梅林. 2,2′-联咪唑磷钨酸盐和氧化石墨复合物的制备及其质子导电性能 [J]. 化学研究, 2014, 25(1): 53-57.
[7] MOON D, LAH M S, DELSESTO R E, et al. The effect of ligand charge on the coordination geometry of and Fe(III) ion: five- and six-coordinate Fe(III) complexes of tris(2-benzimidazolylmethyl)amine [J]. Inorg Chem, 2002, 41: 4708-4714.
[8] CARINAR F, WILLIAMS A F, BERNARDINELLI G. Moleculartricorns: Self-assembly of trinuclear palladium(II) complexes [J]. Inorg Chem, 2001, 40: 1826-1832.
[9] SHELDRICK G M. SHELXL 97, Version 5.1, Program for crystal structure solution and refinement [CP]. University of Göttingen, Germany, 1997.
[10] BROWN I D, ALTERMATT D. Bond-valence parameters obtained from a systematic analysis of the inorganic crystal structure database [J]. Acta Cryst B, 1985, 41: 244-247.
[责任编辑:吴文鹏]
基于磷钼酸和2-(2-羟基苯)苯并咪唑复合物的晶体结构和质子导电性
陈 林, 魏梅林*
(河南师范大学 化学化工学院,河南 新乡 453007)
以磷钼酸和2-(2-羟基苯)苯并咪唑(L)为原料制备了具有质子导电性的有机-无机化合物[H3L2(PMo12O40)·7H2O·4CH3OH]n(1). 单晶X射线衍射分析结果表明化合物1具有基于磷钼酸、2-(2-羟基苯)苯并咪唑及溶剂甲醇分子的二维氢键网络结构;质子导电性能测试结果表明该化合物在100 ℃、相对湿度为98%时的电导率达到10-4S·cm-1.
磷钼酸;苯并咪唑;有机-无机化合物;晶体结构;质子导电性
date:2014-03-11.
National Natural Science Foundation of China(21171050).
Biography:CHEN Lin(1989-), male, postgraduate, majoring in functional coordination compounds.*
, E-mail: weimeilinhd@163.com.
O 611DocumentcodeAArticleID1008-1011(2014)05-0461-05
10.14002/j.hxya.2014.05.006

