Preparation of Mn3O4from low-grade rhodochrosite ore by chemical bath deposition method
•••
Preparation of Mn3O4from low-grade rhodochrosite ore by chemical bath deposition method
Jing Zhao•Longjun Xu•Taiping Xie•Chao Xie
Mn3O4was prepared with the chemical bath deposition(CBD)method.A MnSO4solution was obtained by the leaching and purifying of low-grade rhodochrosite ore(LGRO),which was used as raw material.The preparation procedures were studied and promoted.The results showed that the Mn3O4with the highest purity and highest specifc surface area could be obtained under the following processes.An MnSO4solution of 1.0 mol/L was added into a beaker under a fow rate of 30 mL/h.The pH of the reaction solution was adjusted to 10 using NH3·H2O at 80°C.Then the solids were washed and dried at 200°C for 2.5 h.The total Mn content(TMC)of Mn3O4was 72.0%. The ionic distributions was formulated as [Mn2+]The average crystallite size of Mn3O4with a tetragonal hausmannite structure was found to be about 35 nm by X-ray diffraction (XRD)analysis.The BET specifc surface area of the Mn3O4measured was 32 m2/g.
Mn3O4·Low-grade rhodochrosite ore· Chemical bath deposition method
1 Introduction
Mn3O4is widely used as electrode materials(Zhao et al. 2012;Yang et al.2012;Dubal et al.2010),soft magnetic materials(Gabriela et al.2012),catalysts(Li et al.2009), corrosion-inhibiting pigments,etc.There are many methods for preparation of Mn3O4powders,such as thermal decomposition(Chang et al.2005),hydrothermal(Zhang et al.2004a;Ahmed et al.2011;Yang et al.2006), solvothermal(Li et al.2009;Zhang et al.2004b),microwave assisted(Apte et al.2006),and ultrasonic irradiation (Gopalakrishnan et al.2005;Bastami et al.2012).However,most of these methods were time consuming and uneconomical,in addition to requiring high cost equipment.As compared to these methods,the CBD method was attractive because it was relatively simple and inexpensive.
Past research on the synthesis of Mn3O4by CBD method had been reported.Peng et al.(2010)produced Mn3O4by the aqueous solution oxidation method.Mn3O4nanoparticles were prepared by a simple chemical route, using cetyltetramethyl ammonium bromide(CTAB)as a template agent(Hassouna et al.2012).Nevertheless,the reaction system in the chemical bath was quite complex and many conditions had a great infuence on the characteristics of the products,such as the concentration,pH and temperature of the reaction solution.Chen et al.(2006) reported that the difference in the dripping speed of the NaOH solution leads to a large difference in the Mn3O4morphologies produced.Therefore,the preparation procedures of Mn3O4by CBD method were worth further studying and promoting.
In recent years,more attention has been paid to developing processes for the economical recovery of manganese from low grade manganese ores and other secondary resources(Mehdilo et al.2013;Zhang and Cheng 2007).Inthis paper,LGRO was used as the raw material to produce Mn3O4.Firstly,the LGRO was leached with sulfuric acid and purifed to obtain the pure MnSO4solution.Then,the Mn3O4was prepared by the CBD method in two stages. During the frst hydrolysis-oxidation stage,the MnSO4solution was hydrolyzed with NH3solution and oxidized by the air to gain the precursor.During the second heatingoxidation stage,the precursor was dried and simultaneously further oxidized by the air.So,the synthetic route of Mn3O4was simplifed effectively as compared to the traditional process.In conclusion,this method was simple and inexpensive.Neither complex apparatuses nor sophisticated techniques were required.This study was of great signifcance to provide a possible high effciency way for the utilization of LGRO.
2 Experimental
2.1 Materials and apparatus
The LGRO samples weres from Xiushan,Chongqing,and the average composition was described in Table 1.
Chemical reagents mainly included H2SO4,NaOH, NH4Cl,NH3solution,Na2C2O4,and EDTA,which were of analytical grade.KMnO4used in this experiment was a guaranteed reagent.
Apparatus included constant temperature bath with mixer(DF-101S),pH meter(pHS-3C),electrothermal blowing dry box(101-1),analysis instrument of specifc surface area and pore diameter(ASAP2010,USA)and X-ray diffractometer(Bruker Advance D8).
2.2 Methods
TheLGRO wasleached with sulfuric acidandfltered(Zhao et al.2013).After oxidizing Fe2+ions to Fe3+ions with MnO2powders,aluminum and iron were removed successivelyfromthefltrateintheformofinsolublesaltsbyadding NaOH solution.Then calcium ions(Ca2+),magnesium ions (Mg2+)and heavy metals were eliminated by the introduction of sulfde and fuoride.The pure MnSO4solution with 191.16 g/Lwasobtained.TheleachingeffciencyofMnwas 96.8%.The removal rates of iron,calcium,and magnesium were 99.8%,99.1%,and 96.8%,respectively.
The fresh aqueous solution of 1.5 mol/L NH3solution, buffer solution at pH=10 and MnSO4solutions at various concentrations were prepared in advance.First,20 mL of the buffer solution was transferred into a beaker immersed in a constant temperature bath.Then the prepared Mn2+solution was added at different fow rates into the beaker under vigorous stirring and at various temperatures with an aging time of 2 h.Meanwhile,the NH3solution was dropwise added to make the Mn2+ions precipitate and to control the pH of the solution.The solid was fltered and carefully washed with distilled water several times to obtain the precursor.This stage was called hydrolysisoxidation.During this stage,infuences of several reaction variables such as concentration and fow rate of the MnSO4solution,temperature and pH were investigated.Finally, the precursor was dried in an electrothermal blowing dry box at various temperatures for different times,and the product was obtained.This stage was called heating-oxidation,and the precursor was dried and further oxidized by the air simultaneously.During this stage,the effects of the heating temperature and time on the total Mn content (TMC)of the product were studied.All experiments were carried out in ambient conditions under atmosphere with air as an oxidizing agent.
The mole ratio of MnSO4to NH3solution was 5:1 in all experiments.After the quantitative NH3solution was used up,a small amount of NaOH solution was introduced to adjust the pH of the reaction solution.This way,the Mn recovery percentage could remain at a high level and the infuence of the Na+could be controlled effectively.
2.3 Product characterization
With the selective dissolution,potassium permanganate titration and EDTA titration method combined(Yu and Huang 2004),the contents of Mn2+,Mn3+,Mn4+and TMC in the product were determined.Next,cation distributions and lattice constants of the Mn3O4were calculated respectively.The X-ray diffraction(XRD)determination of the structures present in the as-prepared samples was carried out on a Bruker Advance D8 X-ray diffractometer with CuKα radiation(λ=0.154 nm)at 40 kV and 30 mA.The scan rate was 4°/min for values of 10°–85°.Brunauer-Emmett-Teller(BET)surface area measurement was performed on Micromeritics ASAP 2010 at 77 K.

Table 1 Chemical composition of LGRO/wt%
3 Results and discussions
When the pure MnSO4and NH3solution were mixed,the solution became yellow to yellow–brown and precipitation occurred.The reaction scheme was described as following,

and the Mn(OH)2was instable and could transform to Mn3O4by the air.

During the hydrolysis-oxidation stage,both reactions existed.MnSO4was hydrolyzed and precipitated as Mn(OH)2.Then most of the Mn(OH)2produced was oxidized.The residuary one could be further oxidized in heating-oxidation stage.
3.1 Effect of the concentration of MnSO4 solution on the TMC
Five 100 mL MnSO4solutions of different concentrations were added to the beaker containing the buffer solution under vigorous stirring at a fow rate of roughly 30 mL/h. The precursor was obtained at 60°C and pH=10 with an aging time of 2 h,and then dried at 150°C for 3 h.The TMC in products are shown in Fig.1.It was around 71%, which had little to do with the concentration of MnSO4solution.However,Mn recovery percentage revealed a decreasing trend with the increase of Mn2+ion concentration.When the concentration of MnSO4solution was higher(1.0 and 1.2 mol/L),Mn2(OH)2SO4was formed and the Mn2+ions were not able to precipitate completely as Mn(OH)2.Meanwhile,it was more diffcult to oxidize Mn2(OH)2SO4than Mn(OH)2by air.Thus,TMC in the products was kept low.Consequently,the concentration of the MnSO4solution should be not too low or too high but appropriate.So,the Mn recovery percentage could reach 97.3%.

Fig.1 Effect of concentration of MnSO4solution on total Mn content
3.2 Effect of the fow rate of MnSO4 solution on the TMC
In order to study the effect of the fow rate,the MnSO4solutions were added into the beaker at different fow rates (ranging from 20 to 60 mL/h).The hydrolysis-oxidation were generated at 60°C and pH=10.As seen from the results in Fig.2,the TMC fell considerably with the increase of fow rates.When the MnSO4solution was quickly dripped into the reaction system(about 50 mL/h), the excessive Mn2+in reaction solution not only lead to an incomplete precipitate,but also resulted in the diffcult oxidation of the formed Mn(OH)2.In consequence,high fow rates had adverse effects on both hydrolysis and oxidation.Considering the time factor,the fow rates were chosen to be 40 mL/h.Thus,the TMC was 71.1%.
3.3 Effect of the temperature on the TMC
To improve the TMC of the product,experiments were carried out at various temperatures.The results in Fig.3 expose that the TMC rose from 70.2%at 50°C to 71.6% at 90°C.As the formation and further oxidation of Mn(OH)2was easier and faster at high temperature,the Mn recovery percentage and TMC of the products were both enhanced.However,the lessening of ammonia due to evaporation at high temperature limited the availability of NH3solution.Even worse,the treatment of ammonia vapours was burdensome.Therefore,the optimum temperature was at 80°C,and with a TMC of 71.6%.

Fig.2 Effect of fow rate of MnSO4solution on TMC
3.4 Effect of the pH in reactive system on the TMC
Under the above optimum conditions,various pHs(from 8 to 12)were examined in the hydrolysis-oxidation stage. The results are shown in Fig.4.It was clear that the curve increased frst then decreased,and the highest TMC (71.57%)was recorded at pH=10.The TMC was extremely low in the beginning because Mn2+was precipitated and oxidized slowly and diffcultly in the weak alkaline reactive system.With the increase of the pH,the rise of TMC was clearly detected.Nevertheless,when the pH was higher than 10 the TMC began to obviously decrease.The reason was that the strong alkaline solution leaded to the over-oxidation of Mn(OH)2.Therefore,the adapted pH of solution was 10.

Fig.3 Effect of temperature on TMC

Fig.4 Effect of pH in reactive system on TMC
3.5 Effects of the heating temperature and time on the TMC
The orthogonal array testing was used to study the infuences of concentration and fow rate of the MnSO4solution,the reaction temperature and the pH on the TMC of the products.The orthogonal array had four factors with three levels.The results indicated that the factor with the biggest effect on the Mn content of the product was temperature,with pH coming in second.The infuence of the concentration of MnSO4solution was minimal.These results were observed from the single-factor testing.The optimum hydrolysis-oxidation parameters of synthesis of Mn3O4were as follows.Concentration of MnSO4solution was 1.0 mol/L,fow rate of MnSO4solution was 30 mL/h, reaction temperature was 80°C,and pH was 10.
According to the single-factor and orthogonal array testing,the TMC could only reach about 71.5%,even under the optimum hydrolysis-oxidation conditions.Since the theoretical value of TMC of Mn3O4was 72.0%,the speculation that the residuary Mn(OH)2was not still oxidized completely was reasonable.Hence,more singlefactor experiments were performed to improve the heatingoxidation conditions.The precursors prepared under the optimum hydrolysis-oxidation conditions were dried at 100–200°C for 3 h.In Fig.5,the largest TMC was observed when the temperature went up to 200°C.Then the precursors were dried at 200°C for a different time, and the results were revealed in Fig.6.It was found that the TMC increased with the increase of temperature and time.Mn(OH)2was instable,and could be further oxidized to Mn3O4during the drying process.That was why the curves in Figs.5 and 6 both increased.The Mn(OH)2couldbe almost entirely transformed to Mn3O4when it was dried at 200°C for 2.5 h.The TMC of the product reached 72.0%.
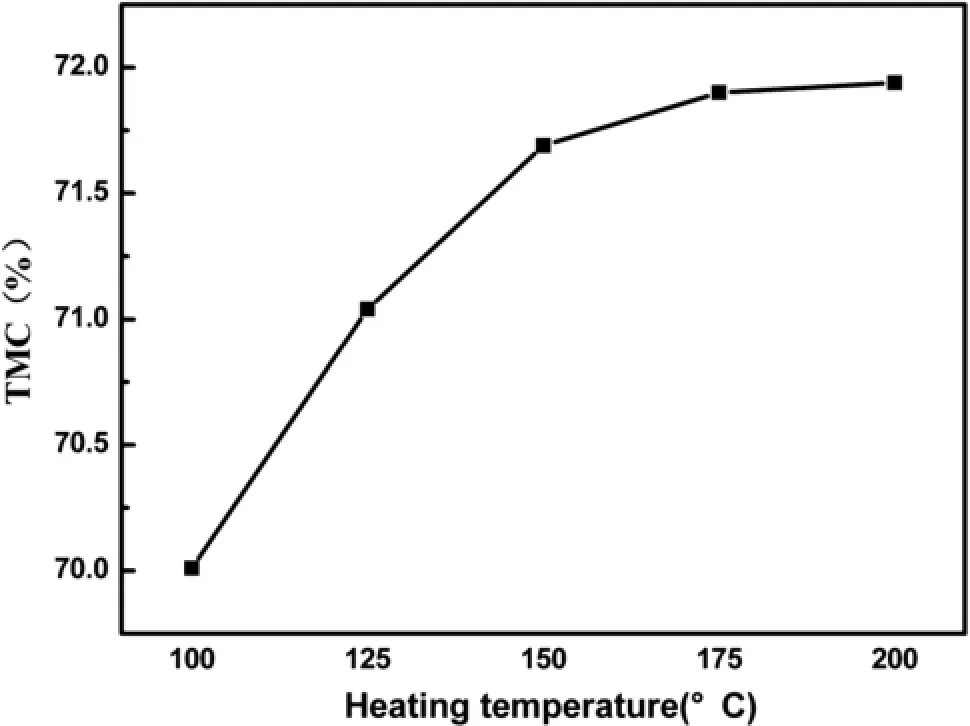
Fig.5 Effect of heating temperature on TMC
3.6 Characterization of the product(Mn3O4)
3.6.1 Ionic distribution
The products were prepared under the optimum hydrolysisoxidation and heating-oxidation conditions.The contents of Mn2+,Mn3+,Mn4+and TMC in the products were determined and listed in Table 2.The highest total content of Mn(exceeding 72.0%)indicated that the as-prepared samples were in a high purity.
As is well known,Mn3O4was a kind of mixed oxide. But there were three different ways to denote it.One view believed that the form of Mn3O4was MnO·Mn2O3including both Mn2+ions and Mn3+ions.The second point argued that Mn3O4was 2MnO·MnO2containing both Mn2+ions and Mn4+ions.The third stated that Mn3O4was consist of 2MnO·MnO2in surface and MnO·Mn2O3inside, and it included Mn2+,Mn3+and Mn4+ions.Gopalakrishnan et al.(2005)reported the ionic structure of Mn3O4synthesized by ultrasonic irradiation was[Mn2+][Mn3+]2-O4.Yu and Huang(2004)confrmed the structural formula of Mn3O4was 2MnO·MnO2.Xiong et al.(2000)proposed the possible distribution of various ions in Mn3O4was

Fig.6 Effect of heating time on TMC

Table 2 Content of different manganese ions and TMC in the products(%,w/w)
The valence state of the manganese in the as-prepared Mn3O4contained Mn2+,Mn3+and Mn4+ions.Based on the spinel structures and the contents of different manganese ions in Mn3O4,the ionic distributions were formulated aswhich was in good agreement with the previous report(Xiong et al.2000).According to the ionic distributions and the previous report(Laarj et al.1996),the lattice constants of the as-prepared product were calculated as aT=0.5741 nm and cT=0.9375 nm,which was consistent with the parameters (a=b=0.5750 nm, c=0.9420 nm) in JCPDS card of Mn3O4(JCPDS No.024-0734).
3.6.2 Crystal structure of Mn3O4
The X-ray diffraction pattern of the product is presented in Fig.7.Allthediffractionpeaksweresuccessfullyrefnedwith the tetragonal hausmannite crystal structure model(JCPDS No.024-0734)of Mn3O4.No peaks of impurities were detected.Thus,the product obtained by CBD method from LGRO was confrmed pure γ-Mn3O4with tetragonal phase. The lattice constants were determined,i.e.a=b=0.5758, c=0.9462 nm.It was in harmony with the calculated above and the value in JCPDS card.Hassouna et al.(2012)reported the preparation of Mn3O4with a crystallite size between 20 and 80 nm using the precipitation method.Anilkumar and Ravi(2005)prepared the nanocrystalline Mn3O4with the averageparticlesizeof~50 nmbygeltocrystallinemethod. The average grain size of the as-prepared Mn3O4was calculated.The result was about~35 nm.
3.6.3 Specifc surface area of Mn3O4
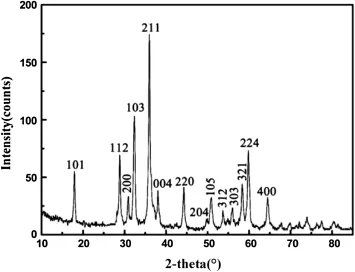
Fig.7 Diffraction analysis of the Mn3O4
Specifc surface area of Mn3O4was determined using the multi-point BET method of adsorption of nitrogen gas(ASAP 2010).The physisorption isotherm was in Fig.8. The adsorption and desorption isotherms showed a hysteresis loop in the relative pressure(P/P0)ranging from 0.70 to 0.98,which was associated with capillary condensation taking place in the mesopores,and the limiting uptake over a range of high P/P0.Therefore,the Mn3O4exhibited adsorption isotherm of Type IV and the product was mesoporous material.The BET surface area and average pore diameter of the as-prepared Mn3O4were 32 m2/g and 15.8 nm,respectively.The average particle size could been calculated with the formula,

where ρ is the theoretical density of the Mn3O4materials (4.86 g/cm3)and S is the specifc surface area of the product.The particle size of the product was 38 nm,which was just slightly bigger than that displayed from the XRD.

Fig.8 Physisorption isotherm of the Mn3O4
4 Conclusions
Mn3O4with high specifc surface area was successfully synthesized by CBD method.The MnSO4solution was obtained by leaching and purifying of LGRO.The CBD method was composed of two stages.During the hydrolysis-oxidation stage,the MnSO4solution was hydrolyzed with NH3solution and oxidized by the air to gain the precursor.During the heating-oxidation stage,the precursor was dried and further oxidized by the air simultaneously.The synthetic route of Mn3O4was simplifed effectively and inexpensively as compared with the traditional process.Neither complex apparatuses nor sophisticated techniques were required.The preparation process of fne Mn3O4provided a potential use for the LGRO.
Through the single-factor and orthogonal array testing, the optimum conditions for synthesis of Mn3O4were obtained.The MnSO4solution of 1.0 mol/L was added into beaker under a fow rate of 30 mL/h.The pH of the reactive system was adjusted to 10 using NH3solution at 80°C.Then the solids were washed and dried at 200°C for 2.5 h.The Mn3O4with high purity and high specifc surface area was obtained and the TMC of Mn3O4was 72.0%.The ionic distribution form was [Mn2+]XRD analysis confrmed the tetragonal hausmannite structure with an average crystallite size of~35 nm.BET specifc surface areas reached to 32 m2/g.
AcknowledgmentsThe study is fnancially supported jointly by the Bureau of Land Resources and Housing Management of Chongqing (Scientifc&Technologic Program in 2011).
Ahmed KAM,Peng H,Wu K,Huang K(2011)Hydrothermal preparation of nanostructured manganese oxides(MnOx)and their electrochemical and photocatalytic properties.Chem Eng J 172:531–539
Anilkumar M,Ravi V(2005)Synthesis of nanocrystalline Mn3O4 at 100°C.Mater Res Bull 40:605–609
Apte SK,Naik SD,Sonawane RS,Kale BB,Pavaskar N,Mandale AB,Das BK(2006)Nanosize Mn3O4(Hausmannite)by microwave irradiation method.Mater Res Bull 41:647–654
Chang YQ,Yu DP,Long Y,Xu J,Luo XH,Ye RC(2005)Largescale fabrication of single-crystalline Mn3O4nanowires via vapor phase growth.J Cryst Growth 279:88–92
Chen ZW,Lai JKL,Shek CH(2006)Shape-controlled synthesis and nanostructure evolution of single-crystal Mn3O4nanocrystals. Scr Mater 55:735–738
Dhaouadi H,Ghodbane O,Hosni F,Touati F(2012)Mn3O4nanoparticles:synthesis,characterization,and dielectric properties.ISRN Spectrosc 10:1–8
Dubal DP,Dhawale DS,Salunkhe RR,Fulari VJ,Lokhande CD (2010)Chemical synthesis and characterization of Mn3O4thin flms for supercapacitor application. J Alloy Compd 497:166–170
Gopalakrishnan IK,Bagkar N,Ganguly R,Kulshreshtha SK(2005) Synthesis of super paramagnetic Mn3O4nanocrystallites by ultrasonic irradiation.J Cryst Growth 280:436–441
Laarj M,Kacim S,Gillot B(1996)Cationic distribution and oxidation mechanism of trivalent manganese ions in submicrometer MnxCoFe2-xO4spinel ferrites.J Solid State Chem 125:67–74
Li X,Zhou L,Gao J,Miao H,Zhang H,Xu J(2009)Synthesis of Mn3O4nanoparticles and their catalytic applications in hydrocarbon oxidation.Powder Technol 190:324–326
Mehdilo A,Irannajad M,Hojjati-rad MR(2013)Characterization and benefciation of Iranian low-grade manganese ore.Physicochem Probl Miner Process 49(2):725–741
Peng T,Xu L,Chen H(2010)Preparation and characterization of high specifc surface area Mn3O4from electrolytic manganese residue.Central Eurpean J Chem 8:1059–1068
Rohani Bastami T,Entezari MH(2012)A novel approach for the synthesis of superparamagnetic Mn3O4nanocrystals by ultrasonic bath.Ultrason Sonochem 19:560–569
Silva GC,Almeida FS,Ferreira AM,Ciminelli VST(2012)Preparation and application of a magnetic composite(Mn3O4/Fe3O4) for removal of As(III)from aqueous Solutions.Mater Res 15(3):403–408
Xiong Q,Huang K,Liu S(2000)Study on determination of Mn2+, Mn3+,Mn4+and their distribution in tetragonal spinel-type Mn3O4.Chin J Anal Lab 19(5):68–70(in Chinese with English abstract)
Yang Z,Zhang Y,Zhang W,Wang X,Qian Y,Wen X,Yang S (2006)Nanorods of manganese oxides:synthesis,characterization and catalytic application.J Solid State Chem 179:679–684
Yang G,Li Y,Ji H,Wang H,Gao P,Wang L,Liu H,Pinto J,Jiang X (2012)Infuence of Mn content on the morphology and improved electrochemical properties of Mn3O4|MnO@carbonnanofber as anode materialfor lithium batteries.J Power Sources 216:353–362
Zhang W,Cheng C(2007)Manganese metallurgy review.Part I: leaching of ores/secondary materials and recovery of electrolytic/chemical manganese dioxide.Hydrometallurgy 89:137–159
Yu KP,Huang BG(2004)Valence state of manganese in manganomanganic oxide and analytical method for its paste sample.Min Metall Eng 24(1):58–60,63(in Chinese with English abstract)
Zhang W,Yang Z,Liu Y,Tang S,Han X,Chen M(2004a)Controlled synthesis of Mn3O4nanocrystallites,MnOOH nanorods by a solvothermal method.J Cryst Growth 263:394–399
Zhang YC,Qiao T,Ya Hu X(2004b)Preparation of Mn3O4nanocrystallites by low-temperature solvothermal treatment of γ-MnOOH nanowires.J Solid State Chem 177:4093–4097
Zhao Y,Nie U,Wang H,Tian J,Ning Z,Li X(2012)Direct synthesis of palladium nanoparticle s on Mn3O4modifed multi-walled carbon nanotubes:a highly active catalyst for methanol electrooxidation in alkaline media.J Power Sources 218:320–330
Zhao J,Xu L,Xie C(2013)Preparation of chemical manganese dioxide from low-grade rhodochrosite ore.Chin J Geochem 32:380–384
Received:23 December 2013/Revised:2 March 2014/Accepted:5 March 2014/Published online:4 February 2015 ©Science Press,Institute of Geochemistry,CAS and Springer-Verlag Berlin Heidelberg 2015
J.Zhao(✉)·L.Xu·T.Xie
State Key Laboratory of Coal Mine Disaster Dynamics and Control,Chongqing University,Chongqing 400044,China
e-mail:20077313@cqu.edu.cn
L.Xu
e-mail:xulj@cqu.edu.cn
C.Xie
School of Environmental Protection and Safety Engineering, University of South China,Hengyang 421001,China
- Acta Geochimica的其它文章
- Coupled alkali feldspar dissolution and secondary mineral precipitation in batch systems:5.Results of K-feldspar hydrolysis experiments
- High pressure equation of state for molten CaCO3from frst principles simulations
- Carbon–oxygen isotopic covariations of calcite from Langdu skarn copper deposit,China:implications for sulfde precipitation
- The O and H isotope characteristics of water from major rivers in China
- Spatial and temporal water quality characteristics of Poyang Lake Migratory Bird Sanctuary in China
- Some aspects of excellent marine source rock formation: implications on enrichment regularity of organic matter in continental margin basins

