A Computational Study on Coumarin Aza-crown Ether Photochemical Sensors for Ca2+ Based on ICT*
-, -
(Key Laboratory of Environmentally Friendly Chemistry and Applications of Ministry of Education,College of Chemistry, Xiangtan University, Xiangtan 411105 China)
A Computational Study on Coumarin Aza-crown Ether Photochemical Sensors for Ca2+Based on ICT*
WANGSu-chun,WANGXue-ye*
(Key Laboratory of Environmentally Friendly Chemistry and Applications of Ministry of Education,College of Chemistry, Xiangtan University, Xiangtan 411105 China)
香豆素;密度泛函理论;分子内电荷转移;含时密度泛函理论
As we all known, calcium ion is abundant in human cells, and involves in a series of physiological processes, such as cell division and differentiation[1, 2], metabolism[3]. The dysregulation of calcium ions in human body is likely to cause some diseases[4, 5]. What’s more, many people are interested in food with high content of calcium. Therefore, detecting and monitoring of calcium ion are very important not only in chemistry, biology and medicine, but also in food science.
The common techniques for detecting calcium ion include EDTA titration method[6,7], radiotracer method[8], selective electrode method[9]and photochemical sensing method[10,11]. Therein, photochemical sensor method develops rapidly owing to its characters of high selectivity, high sensitivity and easy operating. And there are two main photophysical mechanisms, photo-induced electron transfer (PET) and intramolecular charge transfer (ICT). The former is often based on signal variation in the fluorescence emission spectra, while ICT probes can be detected by both absorption and emission properties. For this reason, ICT probes have drawn serious interest from researchers.
In many ICT systems, coumarins have been used as luminophores[12~14]because they are easily available and posses high luminous efficiency. In addition, there is a significant improve in the selectivity and sensitivity for ions, when coupling with crown ethers[15~18]. After coordinating, metal ions would interact with the electron-donor or electron-acceptor of the probe. As a result, the efficiency of intramolecular charge transfer would be affected, thus changing the absorption and emission properties. Besides, the stereochemistry structure of the probe may also change its photochemical properties. Planar intramolecular charge transfer (PICT)[19,20]model has been widely used to explain the electron transfer. In this model, the planarization between electron donor and acceptor is considered to be the main reason for charge transfer. The planarization can enhance the electronic coupling, that makes the charge transfer more easy. Taking account of these researches, we can get a clear understanding of the selectivity and sensitivity for metal cations.
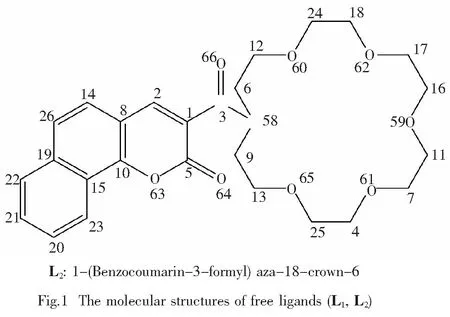
In this paper, two novel azo-crown ethers containing coumarin group[21,22](Fig.1) and their complexes with metal cations (Na+, K+, Mg2+, Ca2+) have been studied by computational method. The UV-vis absorption calculating results of the two systems show high selectivity for Ca2+. Furthermore, by comparing the two systems, a conclusion can be made that the modification of coumarin can make great effect on the selectivity for the same cation. This may provide a theoretical foundation for designing and synthesizing new ICT sensors.

1 Calculation method
In this paper, all calculations were performed in gas phase using the Gaussian03 software package[23]. The B3LYP (Becke-Lee-Yang-Parr) method[24, 25]was used, because it had been proved to be successful in reproducing various molecular properties[25, 26]. The geometry optimization and natural bond orbital (NBO) calculations were completed at DFT B3LYP/6-31+G (d, p) level without any restriction. Moreover, according to the structures of the ligands, the UV-vis absorption spectra were calculated by time-dependent density functional theory (TD-DFT) method, which could provide an excellent accuracy and computational efficiency of properties for large and medium-size molecules[27~29].

2 Results and discussion
2.1Optimized geometric structures
The fully optimizedgeometric structures of free ligands (L1, L2) and their complexes with Na+, K+, Mg2+, Ca2+are shown in Fig.2. Comparing the two systems, a conclusion can be made that the crown ether parts in L/Na+and L/Mg2+show obvious folding and twisting, while in L/K+and L/Ca2+, they are asymmetric. This is due to the smaller radius of Na+and Mg2+, so the ring needs more severe folding and twisting to interact with the cations.
What’s more, in order to observe the dihedral angles between the carbonyl, luminophore and N in crown ethers, the surface of coumarin group is used as the reference plane, and the data are listed in Tab.1. Both the structures and relevant parameters show the planarization between N in the ring and coumarin group from L to L/Mn+, especially in L1/Ca2+and L2/Ca2+. This indicates that the complexing enhances the conjugation, which may reflect the electrical characteristics of the molecules.
Tab.1 The dihedral angles (in degree) between the N, linking group and luminophore of the geometry structures optimized at DFT B3LYP/6-31G (d, p) level
2.2Thermodynamic energies of the complexes
To demonstrate the stability of the complexes in gas phase, the binding energies (ΔE), enthalpies (ΔH) and Gibbs free energies (ΔG) were also calculated and listed in Tab.2. All these datas are negative values, indicating that all complexes are chemically feasible in gas phase. Besides, ΔAL1/M+(A=E,H,G) is more negative than ΔAL2/M+. This may be caused by the electron donor, diethylamine group in L1.
Moreover, bothalkali metal cations and alkaline earth metal cations display the same rule, that the smaller the radius of cation is, the more negative the values are. This phenomena agrees with the fact that the crown ether parts in L/Na+and L/Mg2+show bigger geometric distortions than in L/K+and L/Ca2+.

Tab.2 The binding energies ΔE, binding enthalpies ΔH and Gibbs free energies ΔG of the complexes calculated in gas phase at 298 K (kcal·mol-1)
2.3Natural bond orbital analysis
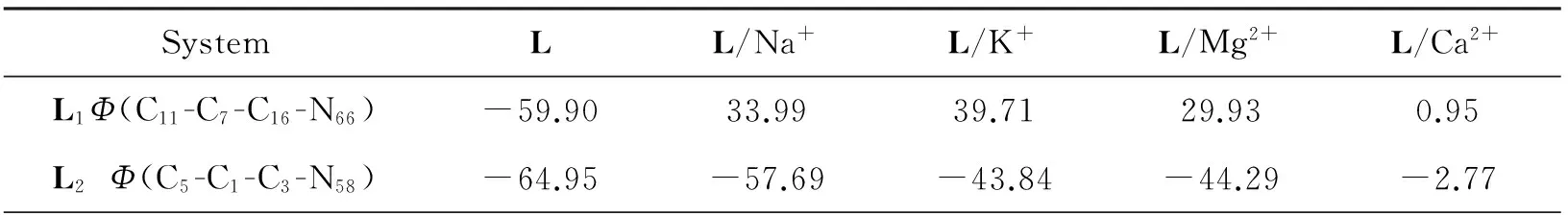
The interaction energies(E2) of host-guest molecules are mainly caused by the lone pair electrons of O or N atoms in crown ether parts and LP*orbitals of the metal cations (Na+, K+, Mg2+, Ca2+). And the bigger value indicates stronger interaction. Hence, in order to make a further exploration for the interactions between the metal cations and ligands, the NBO analysis results are summarized in Tab. 3. Although the interactions between metal cations and N66or N58are not as strong as O, all nitrogen atoms in the crown ether ring participate the coordination with metal cations either in L1/Mn+or L2/Mn+. Besides, compared with other cations, Ca2+has more stronger interaction with N66or N58. This will surely affect the electron transfer from nitrogen atom in the crown ether ring to the coumarin group, thus changing the electrical properties of the molecule.
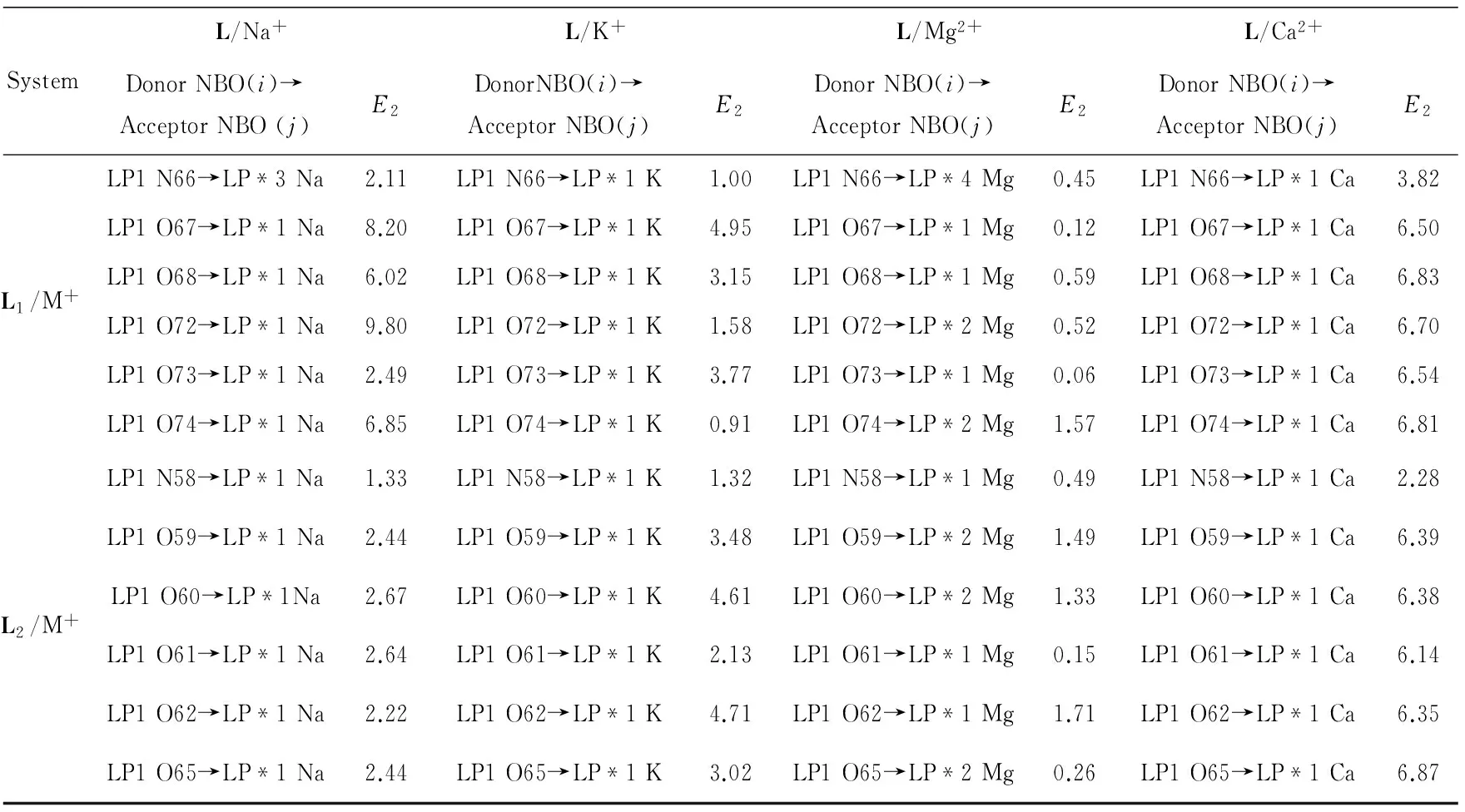
Tab.3 Selected stabilization interaction energy E2 (kcal/mol) of L/Mn+(Mn+=Na+, K+, Mg2+, Ca2+) calculated at at B3LYP/6-31+G (d, p) level (kcal·mol-1)
2.4UV-vis absorption spectra
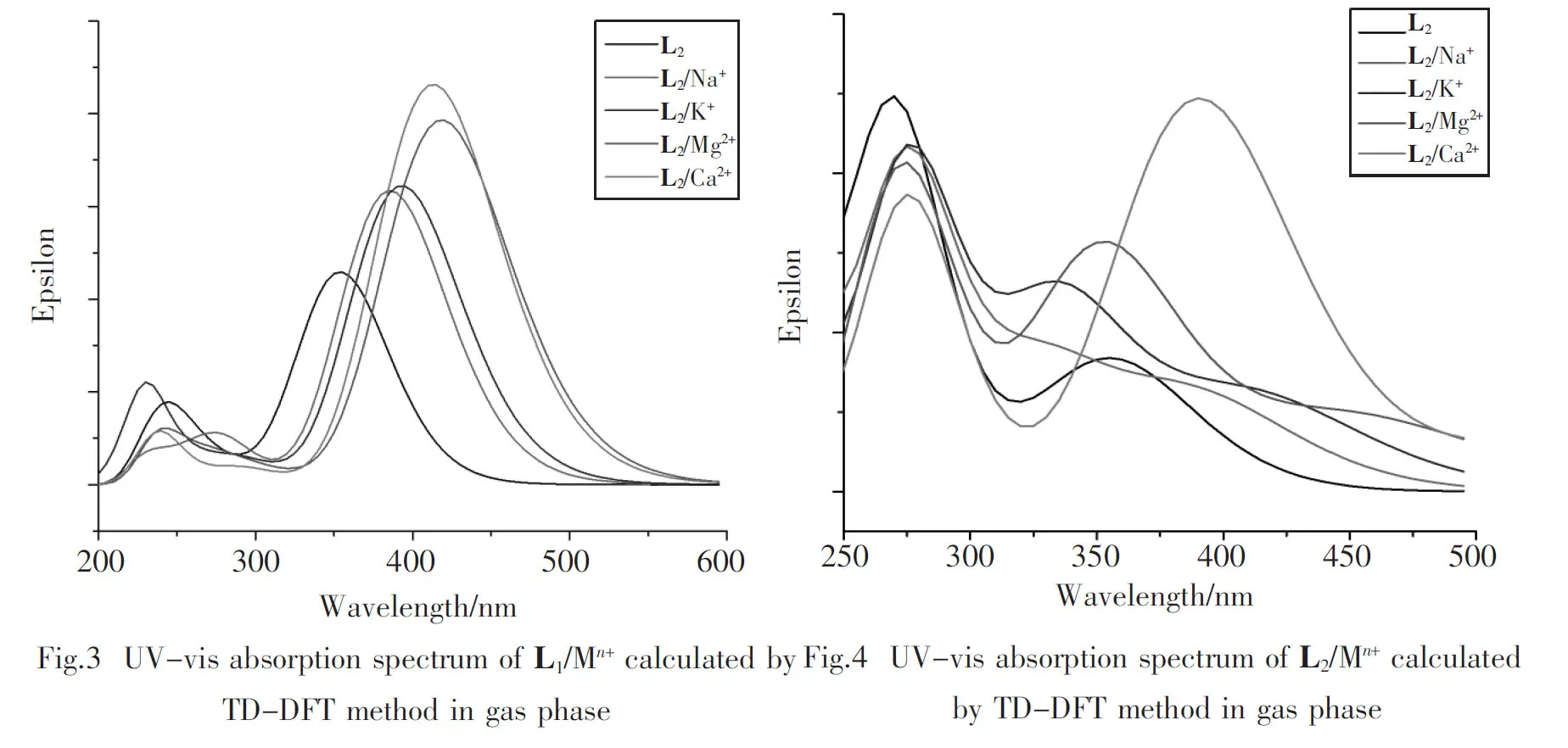
To demonstrate the selectivity for Ca2+, the UV-absorption spectra of the two systems are shown in Fig.3 and Fig.4, respectively, and Tab.4 also lists partial excitation data. From Fig.3, we find that the peaks of L1/Mn+are similar to L1, and the maximum absorption peaks get broaden and red-shifted, which can be ascribed to charge transfer. The interaction between N66and cations weakens its electron-donating property, and decreases the electron transfer to the coumarin group. But, on the other hand, the positive charge possesses electron-withdrawing property, which will enhance the electron transfer from the diethylamine group to the coumarin. Additonally, the increasing of conjugation caused by the planarization makes the process more easier. Therefore, the peaks of L1/Mn+change as Fig.3, and L1/Ca2+has the biggest red-shift and absorption strength.
Tab.4 The selected excitation data of L (L1, L2) and L/Ca2+ calculated by TD-DFT B3LYP/6-31+G (d, p) method.

CoefficientE/eVλ/nmfL1H→L0.690363.49354.870.5547L1/Ca2+H→L0.596062.99413.371.0605L2H→L0.684813.45359.070.1842L2/Ca2+H-1→L0.621783.17391.020.6038
However,L2just has one electron donor, and the phenyl coupled to the coumarin group dramatically enhances the conjugation of the molecule. As a result, after complexing, the absorption property of coumarin almost keep the same, but the electron-donating property of N58decreases. So the second peaks of L2/Na+, L2/K+and L2/Mg2+are blue-shifted slightly. But only the peak of L2/Ca2+remove to the long wavelength, which is mainly caused by the highly planarization between N58and coumarin group. The exception indicates that electron density and the structure of molecule affect ICT process together, and the highly planarization in L2/Ca2+plays a major role here. These analyses demonstrate the high selectivity for Ca2+of the two coumarin aza-crown ethers.
3 Conclusions
The NBO analysis of the two systems indicates that the coordination decreases the electron transfer from nitrogen atom in the ring to thecoumarin g roup. However, it also facilitates the planarization between the donor and the acceptor, which enhances the electronic coupling. For L1/Mn+, the electron withdrawing ability of the cation induces electron transfer from another electron donor, diethylamine. And the overall result is that the absorption induced by charge transfer moves to long wavelength. On the contrary, it would be blue-shifted in L2/Mn+. But due to the quite high planarization in L2/Ca2+, red-shift occurs. In summary, both in L1/Mn+and L2/Mn+, UV-vis absorption spectra show high selectivity for Ca2+. The results also demonstrate that not only electron density, electronic properties of the linking group ,but also the geometric structures have a great effect on the intramolecular charge transfer.
References
[1]KATO T. Role of mitochondrial DNA in calcium signaling abnormality in bipolar disorder[J].Cell Calcium, 2008, 44: 92-102.
[2]MCKINSEY T A,ZHANG C L, OLSON E N. MEF2: a calcium-dependent regulator of cell division, differentiation and Death[J]. Trends Biocheml Sci, 2002, 27: 40-47.
[3]DENTON R M. Regulation of mitochondrial dehydrogenases by calcium ions[J]. Biochimica et Biophysica Acta, 2009, 1787: 1 309-1 316.
[4]MOOREN F C, KINNE R K H. Cellular calcium in health and disease[R]. Biochimica et Biophysica Acta, 1998,1 406: 127-151.
[5]PETERS U, CHATTERJEE N, MCGLYNN K A, et al. Calcium intake and colorectal adenoma in a US colorectal cancer early detection program[J]. Am J Clin Nutr, 2004, 80:1 358-1 365.
[6]BARROWS H L, SIMPSON E C. An EDTA method for the direct routine determination of calcium and magnesium in soils and plant tissue[J]. Soil Sci Soc AM J, 1962, 26: 443-445.
[7]FAN F N, MA Y C, WANG Z K, et al. Improvement of volumetric method for continuous determination of calcium and magnesium using EDTA[J]. Rock and Mineral Analysis, 2002, 21: 307-310.
[8]STÜRUP S. Uncertainty calculation on the determination of calcium absorption in nutritional experiments with enriched stable isotopes and detection by double focusing sector field ICP-MS with a shielded torch[J]. J Anal At Spectrom, 2002, 17: 1-7.
[9]BERGER C E M, HORROCKS B R, DATTA H K. Application of ion-selective microelectrodes to the detection of calcium release during bone resorption[J]. Electrochim Acta, 1999, 44: 2 677-2 683.
[10]SHALOM S, STRINKOVSKI A, PELEG G, et al. An optical submicrometer calcium sensor with conductance sensing capability[J]. Anal Biochem,1997, 244: 256-259.
[11]JOHN E, CHENG C, BORNSCHLEGEL A, et al. Optical sensing device for online calcium detection in extracorporealblood purification[J]. Sensor Actuat B, 2015, 209: 1 023-1 029.
[12]SONG Y X, CHEN Z, LI H Q. Advances in coumarin-derived fluorescent chemosensors for metal lons[J]. Curr Org Chem, 2012, 16: 2 690-2 707.
[13]HELAL A, CHOI C H, KIM H S, et al. Chromogenic and fluorogenic sensing of Cu2+based on coumarin[J]. Tetrahedron, 2011, 67: 2 794-2 802.
[14]SU Z, CHEN K Y, GUO Y, et al. A coumarin-eased fluorescent chemosensor for Zn2+in aqueous ethanol media[J]. J Fluoresc, 2010, 20: 851-856.
[15]LIM N C, YAO L L, FREAKE H C, et al. Synthesis of a fluorescent chemosensor suitable for the imaging of zinc(II) in live cells[J]. Bioorg Med Chem Lett, 2003, 13: 2 251-2 254.
[16]KASAPBASI E, YURTSEVER M. Dependence of the optical absorption and Na+binding energies of coumarin-crown ethers on the size and attachment position of ether ring: density functional investigation[J].J Mol Model, 2013, 19: 173-178.
[18]ERKÇ.Cation recognition with fluorophore crown ethers[J]. Ind Eng Chem Res, 2000, 39: 3 582-3 588.
[19]YOSHIHARA T, DRUZHININ S I, ZACHARIASSE K A. Fast intramolecular charge transfer with a planar rigidized electron donor/acceptor molecule[J]. J AM Chem Soc, 2004, 126: 8 535-8 539.
[20]ZACHARIASSE K A, DRUZHININ S I, BOSCH W, et al. Intramolecular Charge Transfer with the Planarized 4-aminobenzonitrile 1-tert-Butyl-6-cyano-1,2,3,4-tetrahydroquinoline (NTC6)[J]. J AM Chem Soc, 2004, 126: 1 705-1 715.
[21]YAN L Q, XIE M S, PENG M S, et al. A coumarin fluorescent probe for Ca2+containing aza-crown ether unit[J]. Aust J Chem, 2013, 66: 1 584-1 586.
[22]YAN L Q, PENG M S. Synthesis of a fluorescent probe for ca2+containing aza crown unit[J]. Chemical Reagents, 2014, 6: 114-116.
[23]FRISCH M J, TRUCKS G W, SCHLEGEL H B, et al. Gaussian 03, Revision C. 02[M], Wallingford CT: Gaussian Inc, 2003.
[24]LEE C, YANG W T, PARR R G. Development of the Colic-Salvetti correlation-energy formula into a functional of the electron density[J]. Physical Review B, 1988, 37: 785-789.
[25]BECKE A D. Density-functional thermochemistry. III. The role of exact exchange[J]. J Chern Phys, 1993, 98: 5 648-5 652.
[26]HUDSON G A, CHENG L, YU J M, et al. Computational studies on response and binding selectivity of fluorescence sensors[J].J Phys Chem B, 2010, 114: 870-876.
[27]CASIDA M E, JAMORSKI C, CASIDA K C, et al. Molecular excitation energies to high-lying bound states from time-dependent densityfunctional response theory: Characterization and correction of the time-dependent local density approximation ionization threshold[J]. J Chem Phys, 1998, 108: 4 439-4 449.
[28]GUILLAUMONT D, NAKAMURA S. Calculation of the absorption wavelength of dyes using time-dependent density-functional theory (TD-DFT)[J]. Dyes Pigments, 2000, 46: 85-92.
[29]KAUR P, SINGH K. Supramolecular analyte recognition: experiment and theory interplay[J]. RSC Adv, 2014, 4: 11 980-11 999.
责任编辑:朱美香
Two new intramolecular charge transfer (ICT) probes, aza-18-crown-6 with a coumarin group (L), and their complexes with metal cations, L/Mn+(Mn+=Na+, K+, Mg2+, Ca2+) were studied using density functional theory (DFT) in gas phase. All optimized structures have been proved to be stable by real frequencies obtained at B3LYP/6-31+G(d, p) level. Their structures and relevant parameters show the planarization between N in the crown ether ring and coumarin group from L to L/Mn+, which enhances the conjugation. The binding energies, enthalpies and Gibbs free energies of the complexes L/Mn+have been calculated. Besides, natural bond orbitals (NBO) analysis indicates interaction between N and Mn+. Then time-dependent density functional theory (TD-DFT) calculations achieved the absorption spectra and partial excitation data, illustrating high selectivity for Ca2+. In all, comparing the two ICT probe systems, a conclusion will be made that the modification of coumarin and the structure of the molecule can make great effect on ICT process.
coumarin; density functional theory (DFT); intramolecular charge transfer (ICT); time-dependent density functional theory (TD-DFT)
基于ICT原理氮杂冠醚光化学传感器的理论研究
王素春,王学业*
(湘潭大学 化学学院,环境友好化学与应用省部共建教育部重点实验室,湖南 湘潭 411105)
[摘要]采用DFT方法,对两个基于ICT原理的氮杂18-冠-6光化学传感器及其与Na+、K+、Mg2+、Ca2+的配合物进行了理论研究.并在B3LYP/6-31+G(d,p)理论水平上对所有分子进行了几何构型优化,优化构型及部分参数表明配合物中冠醚环上氮原子与香豆素基团平面化程度显著增加,尤其是两配体与Ca2+的配合物.另外,据NBO分析结果可知,冠醚环上的氮原子不同程度地参与了与金属离子的配位.以上两种现象均会对分子内电荷转移产生影响.为了说明两现象相互作用使配体对金属离子产生的选择性,本文还利用TD-DFT方法,对配体及配合物的紫外吸收进行了计算和分析,结果显示两个配体均对Ca2+有较高选择性.
2015-11-28
湖南省教育厅重点项目(12A132)
王学业(1963-),男,湖南 津市人,博士,教授.E-mail:wxueye@xtu.edu.cn
O426.3
A
1000-5900(2016)01-0054-07

