番茄红素β-环化酶基因(LcyB)启动子调控LcyB RNAi双元载体构建
莫爱琼, 文 了, 黎海燕, 马 丽, 万小荣
(仲恺农业工程学院生命科学学院,广州 510225)
番茄红素β-环化酶基因(LcyB)启动子调控LcyB RNAi双元载体构建
莫爱琼, 文了, 黎海燕, 马丽, 万小荣*
(仲恺农业工程学院生命科学学院,广州 510225)
根据番茄基因组DNA序列信息设计引物进行PCR扩增了Micro-Tom中番茄红素β-环化酶(Lycopeneβ-cyclase, LcyB)基因起始密码子上游1 534 bp启动子区域序列(LcyBp),生物信息学分析表明,该启动子序列中存在TATA-盒、CAAT-盒、昼夜节律响应元件Circadian、光响应元件Box I、真菌激发子响应元件Box-W1、低温响应元件LTR、响应赤霉素的作用元件P-box、乙烯响应元件ERE、响应生长素的作用元件TGA-element等顺式作用元件. 依据番茄LcyB基因序列,设计2对含有不同酶切位点的特异引物进行PCR扩增LcyB基因3’端特异的276 bp DNA片段,利用RNAi载体pKANNIBAL构建了“LcyB启动子-LcyB基因正义片段(Sense)-PDK内含子-LcyB基因反义片段(Antisense)-OCS终止子”的RNAi表达框,并将这一RNAi表达框插入植物双元表达载体pART27的NotI位点,构建成本研究的LcyB启动子驱动的LcyB基因RNAi植物双元表达载体pART-LcyBp-RNAi-LcyB. 为利用RNAi技术特异性敲除LcyB基因进而提高番茄果实中番茄红素含量奠定实验基础.
番茄; 番茄红素β-环化酶(Lycopeneβ-cyclase, LcyB); 启动子; RNAi双元载体
番茄红素(Lycopene)具有淬灭单线态氧、清除自由基、诱导细胞间连接通讯、调控细胞增殖等多种功能,尤其是对某些癌细胞增殖的抑制作用比α-胡萝卜素和β-胡萝卜素更强,因而成为现在最受关注的类胡萝卜素色素之一,是目前国际功能食品研究和化妆品与食品添加剂研究的焦点,有希望成为最重要的一个化学防癌物质,对人类健康有重要意义[1-2].
在高等植物中番茄红素是由八氢番茄红素脱氢转变而来的,番茄红素的代谢途径主要是其环化反应,特别是番茄红素β-环化酶(Lycopeneβ-cyclase, LcyB)催化其环化形成β-胡萝卜素,是其主要代谢途径,PECKER等[3]克隆鉴定了番茄中编码番茄红素β-环化酶的LcyB基因,发现其表达在果实后熟阶段降低,从而利于果实中番茄红素的积累. 目前成功的相关转基因植物报道的工作是在类胡萝卜素合成品种中过表达正向催化番茄红素前体合成的关键酶基因,希望提高转基因植株中番茄红素的含量,但由于向番茄红素合成支路的流向增大,往往导致其它以异戊二烯类化合物为前体的合成途径底物缺乏,而对转基因植株的生长发育造成不利影响[4-5]. 例如组成型表达八氢番茄红素合成酶基因的转基因番茄中,因为与赤霉素的生物合成途径竞争牻牛儿牻牛儿焦磷酸(GGPP)前体导致植株矮化等现象,因而无法应用于农业生产[4]. 2000年以色列科学家利用番茄Beta突变体的研究[6]表明,该突变体果实“后熟”期间番茄红素水平明显低于野生型,进一步研究发现这种突变表型是由于第6染色体上编码番茄红素β-环化酶的LcyB基因高表达所致,即番茄红素环化反应增强,大量转变生成β-胡萝卜素了. 迄今尚无番茄中LcyB基因启动子研究的有关报道.
一些小的双链RNA可以高效、特异地阻断体内特定基因表达,使特异mRNA降解,诱使细胞表现出特定基因缺失的表型,这一过程称为双链RNA干扰(Double-stranded RNA interference, 简称RNAi)[7-8]. RNAi作为一种反向遗传学的研究方法,为后基因组时代基因功能的分析提供了一种可靠、快速的应用技术平台.
本实验从新型模式植物微型番茄(Micro-Tom)中克隆LcyB基因5’上游启动子序列,并构建其驱动的特异静默LcyB基因的RNAi植物双元表达载体,为在此基础上利用RNAi技术特异性敲除番茄果实中的LcyB基因,通过阻断番茄果实中番茄红素的环化反应来终止以番茄红素为底物继续进行的代谢途径,进而获得番茄红素高富集的优质番茄奠定实验基础.
1 材料与方法
1.1植物材料
微型番茄(Lycopersiconesculentum,称作Micro-Tom)是一种新型模式植物,其生命周期短,从播种到果实成熟只需约70 d,且生长密度高,可达约1 357株/m2;农杆菌介导的Micro-Tom子叶转化频率高,约达80%;Micro-Tom中只有2个主要基因(DwarfGene和MiniatureGene)与普通番茄不同[9-10]. 上述特征大大方便了番茄的突变和转基因,且使基因敲除的应用更为便利. Micro-Tom种子播种在泥炭土中,生长条件为:光周期,16 h光/8 h暗;温度,25±1 ℃. 萌发生长约20 d后取番茄叶片备用.1.2Micro-TomLcyB基因5’上游启动子序列克隆
采用SDS法提取Micro-Tom叶片基因组DNA[11]. 根据DNA数据库中报道的番茄基因组DNA序列信息(GenBank Accession No. KP233172)设计一对引物(LcyBp-F: 5’-CGRYCGTTCAGTCGTCTTAGGC-3’和LcyBp-R: 5’-CTCGAGACCATTATAGAGAATG-3’),以Micro-Tom基因组DNA为模板,进行PCR扩增LcyB基因5’上游启动子序列,将PCR产物克隆到pMD 19-T (Simple) 载体(TaKaRa)上,通过PCR和酶切检测获得阳性克隆(含质粒pMD-LcyBp)后,挑阳性克隆送上海生工生物技术有限公司测序,获得Micro-TomLcyB基因5’上游启动子序列(命名为LcyBp).
1.3LcyB基因启动子序列的生物信息学分析
将上述克隆的Micro-TomLcyB基因启动子序列在植物顺式作用元件数据库中的信号扫描程序进行生物信息学分析,搜寻该启动子序列中可能响应外界环境刺激和发育信号的顺式作用元件.
1.4LcyB基因启动子驱动的特异静默LcyB基因的RNAi双元表达载体构建
构建LcyB基因RNAi植物表达载体时,本研究选用质粒pKANNIBAL作为基本克隆载体. 以引入的McrI和XhoI 2个限制性内切酶酶切质粒pMD-LcyBp,获取LcyBp片段替代质粒pKANNIBAL上的CaMV 35S 启动子,构建成含Micro-TomLcyB基因启动子的中间RNAi质粒pK-LcyBp.
根据DNA数据库中报道的番茄LcyB基因序列信息(GenBank Accession No. AEKE02020044)设计引物RNAi-S1(5’-CTCGAGGATCTTGATCCTAAATACTGGC-3’)和RNAi-S2(5’-GGTACCTGACAGTATGTAGCTCTTATCTCAC-3’)、以及RNAi-AS1(5’-AAGCTTGATCTTGATCCTAAATACTGGC-3’)和RNAi-AS2(5’-ATCGATTGACAGTATGTAGCTCTTATCTCAC-3’)扩增LcyB基因3’端276 bp片段,在上述4条引物5’端分别引入XhoI、KpnI和HindIII、ClaI酶切位点. 将2个PCR产物分别克隆到载体pMD 19-T (Simple) (TaKaRa)上,通过PCR、酶切检测及测序验证获得阳性克隆(分别含质粒pMD-RNAiS及质粒pMD-RNAiAS).
以XhoI和KpnI 2个限制性内切酶双酶切质粒pK-LcyBp及质粒pMD-RNAiS,分别回收质粒pK-LcyBp的大片段和质粒pMD-RNAiS酶切后的LcyB基因片段,连接构建成中间RNAi质粒pK-LcyBp-RNAiS;以HindIII和ClaI 2个限制性内切酶双酶切pK-LcyBp-RNAiS及pMD-RNAiAS这2个质粒,分别回收质粒pK-LcyBp-RNAiS的大片段和质粒pMD-RNAiAS酶切后的LcyB基因片段,连接构建成中间RNAi质粒pK-LcyBp-RNAi-LcyB.
再利用NotI从质粒pK-LcyBp-RNAi-LcyB切下LcyBp::LcyBRNAi表达框插入植物双元表达载体pART27的NotI位点,最后构建成本研究的RNAi植物双元表达载体pART-LcyBp-RNAi-LcyB.
2 结果与分析
2.1Micro-TomLcyB基因启动子序列克隆与生物信息学分析
根据DNA数据库中报道的番茄基因组DNA序列信息设计一对引物,以Micro-Tom基因组DNA为模板进行PCR扩增,结果扩增出一条约1 500 bp的DNA片段(图1),将此片段回收后克隆到载体pMD 19-T (Simple)上,通过PCR和酶切检测、筛选,获取含质粒pMD-LcyBp的阳性克隆. 挑阳性克隆送上海生工生物技术有限公司测序,测序结果表明PCR产物为1 551 bp的DNA序列. 对此序列进行BLASTn分析(http://blast.ncbi.nlm.nih.gov/Blast.cgi),结果表明其与GenBank DNA数据库中报道的番茄基因组DNA序列信息完全吻合,说明所克隆的DNA序列为Miro-TomLcyB基因起始密码子ATG上游启动子区域序列(图2).

M:DL5 000 DNA marker (TaKaRa);1:LcyB基因启动子
Figure 1PCR amplification of promoter ofLcyBgene from Micro-Tom
将克隆的LcyB基因启动子区域序列在国际植物顺式作用元件数据库PlantCARE[12]中进行生物信息学分析,搜寻该启动子序列中可能的响应发育信号和外界环境刺激的顺式作用元件(图2). 在该启动子序列-137~-132(LcyB基因起始密码子ATG上游)处有典型的TATA-box,核心序列为ATATAA[13];-107~-104处有CAAT-box,核心序列为CAAT[14];在-298~-289、-275~-266及-116~-107处有典型的响应昼夜节律的顺式作用元件Circadian,核心序列分别为CAAAAATATC、CAAACACATC及CAAAAGCATC[15];-326~-320处有光响应元件Box I,保守序列为TTTCAAA[16];在-783~-778及-315~-310处有真菌激发子(Elicitor)响应元件Box-W1,核心序列为TTGACC[17];在-712~-707及-383~-378处有低温响应元件LTR,核心序列为CCGAAA[18];另外,在该启动子序列中存在一些响应几种植物激素的顺式作用元件,如-1 352~-1 346及-920~-914处响应赤霉素的作用元件P-box,核心序列为CCTTTTG[19];-327~-320处的乙烯响应元件ERE,核心序列为ATTTCAAA[20];-995~-990响应生长素的作用元件TGA-element,核心序列为AACGAC[13](表1). 序列分析结果表明,所克隆的DNA序列为Micro-TomLcyB基因起始密码子上游包含各种响应植株发育信号和外界环境刺激的顺式作用元件的启动子区域序列.

图2番茄LcyB基因启动子序列包括的主要顺式作用元件
Figure 2Predictedcis-acting elements in the promoter of tomatoLcyBgene in the database of plantcis-acting regulatory element
注:下划线序列为预测的主要顺式作用元件

表1 番茄LcyB基因启动子区域序列中的主要顺式作用元件
2.2LcyB启动子驱动的LcyB基因RNAi双元表达载体构建
以限制性内切酶McrI和XhoI双酶切质粒pMD-LcyBp,回收LcyBp启动子片段克隆到质粒pKANNIBAL的McrI和XhoI位点,替换其中的CaMV 35S 启动子,构建成含番茄LcyB基因启动子的中间RNAi质粒pK-LcyBp. 对构建的载体pK-LcyBp进行PCR和双酶切检测,结果以LcyBp-F和LcyBp-R为引物可特异地扩增出1 551 bp的LcyBp片段,以McrI和XhoI 2个限制性内切酶双酶切质粒pK-LcyBp可切下相应大小的DNA片段(图3),说明载体pK-LcyBp构建正确.

M: DL10 000 DNA marker (TaKaRa);1:McrI和XhoI双酶切检测质粒pK-LcyBp;2:质粒pK-LcyBp(酶切负对照);3:PCR检测质粒pK-LcyBp
图3pK-LcyBp质粒PCR及酶切检测
Figure 3Confirmation of the constructed pK-LcyBp plasmid by PCR and double enzyme digestion
以Micro-Tom基因组DNA为模板,分别以RNAi-S1和RNAi-S2以及RNAi-AS1和RNAi-AS2为引物,进行PCR扩增LcyB基因3’端276 bp的DNA片段.
按图4的流程构建LcyB启动子驱动的LcyB基因RNAi植物双元表达载体. 用限制性内切酶XhoI和KpnI双酶切质粒pK-LcyBp,将LcyB基因片段用同样的酶从质粒pMD-RNAiS上切下,然后将2个片断用连接酶连接,构建成质粒pK-LcyBp-RNAiS. 用限制性内切酶HindIII和ClaI双酶切pK-LcyBp-RNAiS,并以同样的酶从质粒pMD-RNAiAS上切下LcyB基因片段,再回收2片段并连接,构建成中间RNAi质粒pK-LcyBp-RNAi-LcyB. 再利用NotI从质粒pK-LcyBp-RNAi-LcyB切下LcyBp::LcyBRNAi表达框插入载体pART27的NotI位点,最后构建成Micro-TomLcyB启动子驱动的LcyB基因RNAi双元表达载体pART-LcyBp-RNAi-LcyB.
图4pART-LcyBp-RNAi-LcyB RNAi双元表达载体构建流程图Figure 4Outline of RNAi binary vector pART-LcyBp-RNAi-LcyB construction
注:高清图参见:http://journal.scnu.edu.cn/si/?3905
对构建的载体pART-LcyBp-RNAi-LcyB进行PCR、酶切及测序检测,结果以LcyBp-F和LcyBp-R为引物可特异地扩增出1 551 bp的LcyBp片段;分别以XhoI/KpnI和HindIII/ClaI双酶切质粒pART-LcyBp-RNAi-LcyB,均可切下276 bp的LcyB基因片段;以NotI单酶切质粒pART-LcyBp-RNAi-LcyB,得到与预期大小一致的2个片段(图5). 进一步对质粒pART-LcyBp-RNAi-LcyB所有经连接的接合处(Junction Area)进行测序,结果表明,构建质粒的接合处序列都与预期一致,构建过程中未发生碱基插入、缺失等造成的读码框变化. 说明已成功构建Micro-TomLcyB启动子驱动的LcyB基因RNAi植物双元表达载体(图6).
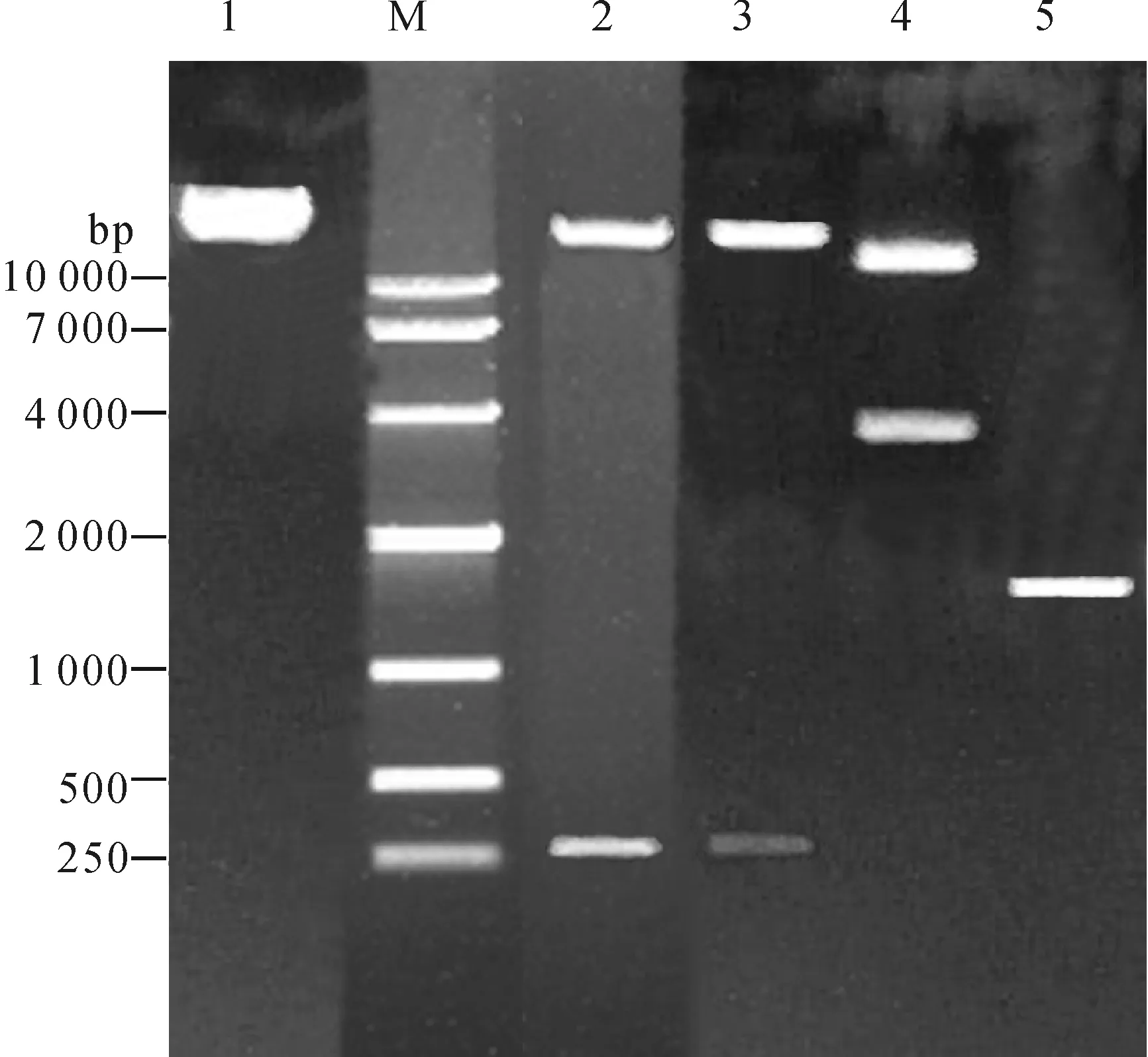
M: DL10 000 DNA marker (TaKaRa);1:质粒pART-LcyBp-RNAi-LcyB (酶切负对照);2:XhoI/KpnI双酶切检测;3:HindIII /ClaI双酶切检测;4:NotI酶切检测;5:PCR检测
图5双元表达载体pART-LcyBp-RNAi-LcyB-RNAi PCR及酶切检测
Figure 5Identification of RNAi binary construct pART-LcyBp-RNAi-LcyB by PCR and enzyme digestion

图6Micro-TomLcyB基因启动子调控的LcyBRNAi植物双元表达载体示意图
Figure 6Schematic structure of RNAi binary vector targeting to SilencingLcyBgene driven by nativeLcyBpromoter
3 讨论
近年来伴随番茄红素重要生理功能的发现,利用基因工程技术改造番茄红素合成途径,提高农作物番茄红素含量的研究成为类胡萝卜素研究领域的新热点. 植物中转入番茄红素合成关键酶同源序列很强的基因非常容易发生基因静默(Gene silencing),从而会降低番茄红素的含量.
RNAi具有高度的特异性,只引起与dsRNA同源的mRNA的降解,在由21~23个核苷酸构成的siRNA(small interfering RNA)中只要改变1个核苷酸,就可以使该siRNA序列不对靶向mRNA起作用[21]. 已有大量研究[7-8, 21-24]证实RNAi可高效特异地抑制特定基因的表达,获得功能性丧失,从而成为研究基因功能的良好工具. 本实验从Micro-Tom中克隆了LcyB基因起始密码子上游1 534 bp的启动子区域序列,利用RNAi中间载体pKANNIBAL构建了“番茄LcyB启动子-LcyB基因正义片段(Sense)-PDK内含子-LcyB基因反义片段(Antisense)-OCS终止子”的结构,并将这一结构以NotI从质粒pK-LcyBp-RNAi-LcyB上切下,插入植物双元表达载体pART27的NotI位点,最后构建成本文的RNAi植物双元表达载体pART-LcyBp-RNAi-LcyB. 故可使将来转基因植物中经转录就形成了具有“LcyB基因正义片段-PDK内含子-LcyB基因反义片段”结构的mRNA,LcyB基因正、反义片段通过链内退火,形成dsRNA,激发RNAi机制,形成siRNA,能够与内源LcyB基因转录的mRNA发生特异性作用,使LcyB基因在转录后水平沉默(PTGS).
许多报道的转基因实验中所用的启动子多为组成型启动子,如CaMV 35S,在它的调控下,外源基因在转基因植物中所有的发育阶段和所有的部位都能表达,对于需要组织特异性表达的基因来说,在该启动子调控下表达造成营养浪费而常导致植株生长不良,如上述Fray和Grierson将番茄八氢番茄红素合成酶基因在组成型启动子调控下转入番茄,结果幼果异常生长,植物矮化. 在基因工程研究中对于组织或器官特异性启动子的需求是很大的,也越来越受到研究人员的重视. 因此本研究是采用番茄LcyB基因本身的启动子调控LcyB基因RNAi片段的表达,将可更加特异地阻抑LcyB基因在番茄中的时空表达.
[1]谭新平, 王银娜, 刘昕. 番茄红素与癌 [J]. 天然产物研究与开发, 2001, 13(4): 71-75.
TAN X P, WANG Y N, LIU X. Lycopene and cancer [J]. Natural Product Research and Development, 2001, 13(4): 71-75.
[2]FRASER P D, ROMER S, SHIPTON C A, et al. Evaluation of transgenic tomato plants expressing an additional phytoene synthase in a fruit-specific manner [J]. Proceedings of the National Academy of Sciences of the Uni-ted States of America, 2002, 99(2):1092-1097.
[3]PECKER I, GUBBAY R, CUNNINGHAM F X, et al. Cloning and characterization of cDNA for lycopene β-cyclase from tomato reveals a decrease in its expression du-ring tomato ripening [J]. Plant Molecular Biology, 1996, 30: 806-819.
[4]FRAY R G, GRIERSON D. Identification and genetic analysis of normal and mutant phytoene synthase genes of tomato by sequencing, complementation and co-suppre-ssion [J]. Plant Molecular Biology, 1993, 22: 589-602.
[5]刘仲齐, 薛俊, 金凤媚. 番茄果实中类胡萝卜素的合成及其调控 [J]. 天津农业科学, 2005, 11(1): 6-11.
LIU Z Q, XUE J, JIN F M. Regulation and formation of carotenoid in tomato fruit [J]. Tianjin Agricultural Sciences, 2005, 11(1): 6-11.
[6]RONEN G, CARMEL-GOREN L, ZAMIR D, et al. An alternative pathway to β-carotene formation in plant chromoplasts discovered by map-based cloning of Beta and old-gold color mutations in tomato [J]. Proceedings of the National Academy of Sciences of the United States of America, 2000, 97(20): 11102-11107.
[7]HELLIWELL C A, WATERHOUSE P M. Constructs and methods for hairpin RNA-mediated gene silencing in plants [J]. Methods in Enzymology, 2005, 392: 24-35.
[8]EARLEY K W, HAAG J R, PONTES O, et al. Gateway-compatible vectors for plant functional genomics and proteomics [J]. The Plant Journal, 2006, 45: 616-629.[9]MEISSNER R, JACOBSON Y, MELAMED S, et al. A new model system for tomato genetics [J]. The Plant Journal, 1997, 12(6): 1465-1472.
[10]刘小花, 张岚岚, 朱长青, 等. Micro-Tom番茄矮化微型机制及其在植物功能基因组学研究中的应用 [J]. 遗传, 2008, 30(10): 1257-1264.
LIU X H, ZHANG L L, ZHU C Q, et al. Mechanisms for miniature dwarf characteristics of Micro-Tom tomato and its application in plant functional genomics studies [J]. Hereditas, 2008, 30(10): 1257-1264.
[11]WAN X R, LI L. Regulation of ABA level and water-stress tolerance of Arabidopsis by ectopic expression of a peanut 9-cis-epoxycarotenoid dioxygenase gene [J]. Biochemical and Biophysical Research Communications, 2006, 347(4): 1030-1038.
[12]LESCOT M, DEHAIS P , MOREAU Y, et al. PlantCARE: a database of plantcis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences [J]. Nucleic Acids Research, 2002, 30(1): 325-327.
[13]PASTUGLIA M, ROBY D, DUMAS C, et al. Rapid induction by wounding and bacterial infection of anSgene family receptor-like kinase gene inBrassicaoleracea[J]. The Plant Cell, 1997, 9(1): 49-60.
[14]STRAUB P F, SHEN Q, HO D T H. Structure and promo-ter analysis of an ABA- and stress-regulated barley gene,HVA1 [J]. Plant Molecular Biology, 1994, 26(2): 617-630.
[15]PICHERSKY E, BERNATZKY R, TANKSLEY S D, et al. Molecular characterization and genetic mapping of two clusters of genes encoding chlorophyll a/b-binding proteins inLycopersiconesculentum(tomato) [J]. Gene, 1985, 40(2): 247-258.
[16]KUHLEMEIER C, FLUHR R, GREEN P J. et al. Sequences in the pearbcS-3Agene have homology to constitutive mammalian enhancers but function as negative regulatory elements [J]. Genes and Development, 1987, 1(3): 247-255.
[17]RUSHTON P J, TORRES J T, PARNISKE M, et al. Interaction of elicitor-induced DNA-binding proteins with elicitor response elements in the promoters of parsleyPR1 genes [J]. The EMBO Journal, 1996, 15(20): 5690-5700.
[18] WHITE A J, DUNN M A, BROWN K, et al. Comparative analysis of genomic sequence and expression of a li-pid transfer protein gene family in winter barley [J]. Journal of Experimental Botany, 1994, 45: 1885-1892.
[19]TAKAIWA F, OONO K, WING D, et al. Sequence of three members and expression of a new major subfamily of glutelin genes from rice [J]. Plant Molecular Biology, 1991, 17(4): 875-885.
[20]ITZHAKI H, WOODSON W R. Characterization of an ethylene-responsive glutathione S-transferase gene cluster in carnation [J]. Plant Molecular Biology, 1993, 22(1): 43-58.
[21]WESLEY S V, HELLIWELL C A, SMITH N A, et al. Construct design for efficient effective and high-throughput gene silencing in plants [J]. The Plant Journal, 2001, 27(6): 581-590.
[22]CHUANG C F, MEYEROWITZ E M. Specific and heritable genetic interference by double-stranded RNA inArabidopsisthaliana[J]. Proceedings of the National Academy of Sciences of the United States of America, 2000, 97: 4985-4990.
[23]WANG M B, WATERHOUSE P M. Application of gene silencing in plants [J]. Current Opinion in Plant Biology, 2001, 5: 146-150.
[24]李红艳. 核糖体蛋白S6对果蝇发育的影响 [J]. 华南师范大学学报(自然科学版), 2014, 46(3): 107-111.
LI H Y. Effect of ribosomal protein S6 onDrosophiladevelopment [J]. Journal of South China Normal University (Natural Science Edition),2014, 46(3): 107-111.
【中文责编:成文英文责编:李海航】
Construction of RNAi Binary Vector to Silence Lycopene β-cyclase Gene LcyB Driven by Native LcyB Promoter
MO Aiqiong, WEN Liao, LI Haiyan, MA Li, WAN Xiaorong*
(College of Life Sciences, Zhongkai University of Agriculture and Engineering, Guangzhou 510225, China)
Suppressing the expression of gene encoding lycopeneβ-cyclase (LcyB), the key enzyme regulating the metabolism of lycopene toβ-carotene, is an effective way to increase the content of lycopene. In the present study, a 1 534 bp promoter of 5’ upstream ofLcyBgene was cloned from Micro-Tom tomato (Lycopersiconesculentum) and bioinformatically analyzed in the database of plantcis-acting regulatory element (PlantCARE). The result showed that several importantcis-acting elements were identified in the promoter, including the TATA-box, CAAT-box, Circadian (response to circadian rhythm), Box I (response to light), Box-W1 (response to fungal elicitor), LTR (response to low-temperature), P-box (response to gibberllin), ERE (response to ethylene) and TGA-element (response to auxin). TheLcyBpromoter was inserted into the plasmid pKannibal betweenMcrI andXhoI restriction sites to replace the CaMV 35S promoter, resulting in pK-LcyBp vector. Based on tomato genomic DNA sequence reported in the GenBank (Accession No. AEKE02020044), two pairs of primers containing various restriction sites were designed to amplify the 3’ terminal 276 bp fragment ofLcyBgene from Micro-Tom tomato. The two 276 bpLcyBfragments were separately cloned into the plasmid pK-LcyBp betweenXhoI/KpnI andHindIII/ClaI sites, in sense or antisense directions, generating pK-LcyBp-RNAi-LcyB construct harboringLcyBRNA interfering expression frame driven byLcyBpromoter. The RNA interfering expression frame was then cloned into theNotI site of the binary expression vector pART27 to achieve the RNAi binary vector targeting to silencingLcyBgene driven by its native promoter. This investigation lays the foundation for future increasing lycopene content through specific knock-out ofLcyBgene by RNAi in tomato fruit.
tomato; Lycopeneβ-cyclase (LcyB); promoter; RNAi binary vector
2015-10-10 《华南师范大学学报(自然科学版)》网址:http://journal.scnu.edu.cn/n
广东省科技计划项目(2013B020303003);广东省教育厅科技创新项目和创新强校专项工程项目(2013KJCX0104);广东省自然科学基金项目(2016A030313370);2015年度国家级大学生创新创业训练计划项目(201511347003)
万小荣,教授,Email: biowxr@126.com.
Q945.1
A
1000-5463(2016)04-0050-07

