Response Surface Analysis of Pb(Ⅱ) Rhodotorula Mucilaginosa Biosorption
SIVAKUMAR K M,JIANG Bin-hui, ZHAO Yan, LEI Lei, WANG Li
(1.University of Wollongong, Wollongong 2500, Australia; 2.Northeastern University, Shenyang 110819, China; 3.Wuhan University of Science and Technology, Wuhan 430081, China;4.Shenyang University of Chemical Technology, Shenyang 110142, China)
Response Surface Analysis of Pb(Ⅱ) Rhodotorula Mucilaginosa Biosorption
SIVAKUMAR K M1,JIANG Bin-hui2, ZHAO Yan2, LEI Lei3, WANG Li4
(1.University of Wollongong, Wollongong 2500, Australia; 2.Northeastern University, Shenyang 110819, China; 3.Wuhan University of Science and Technology, Wuhan 430081, China;4.Shenyang University of Chemical Technology, Shenyang 110142, China)
The optimization of Pb2+biosorption process was performed among three main parameters through Box-Behnken design under response surface analysis.SEM,EDX,and FTIR were used to analyze mechanism and characteristics of Pb2+biosorption on biomass surface.The morphology and elements ofRhodotorulamucilaginosabiomass were observed by SEM and EDX,which indicated that lead ions were existing after adosrption and biosorption on biomass occurred indeed.From response surface plots,the optimum value of lead ions uptake of 1.45 mg/g biomass was achieved with RSM under Design-Expert software on the condition that initial Pb2+concentration were 30 mg/L,initial pH was 5.45 and adsorption time was 25 min,respectively.The analysis of variance(ANOVA) of quadratic model demonstrated that the model was highly significant.The coefficient of varianceC.V.=2.4 % obtained revealed the experiments were carried out more precisely and reliably.
Rhodotorulamucilaginosa; biosorption; Pb(Ⅱ) removal; response surface methodology; wastewater treatment
Conventional methods for removing heavy metals from wastewater include precipitation,coagulation,ion-exchange,reverse osmosis,evaporation,membrane processes[1]and adsorption[2].Most of these technologies have some drawbacks,such as high reagent requirements and operational cost,the disposal of residual toxic sludges,and un-suitable for large scale application.Biosorption possessing more efficient,high selectivity for specific metal,no secondary pollution[3],less investment and effective cost compared with other mentioned conventional methods,is able to eliminate heavy metal ions especially when their concentrations are lower from aqueous solution[4].
Microorganisms including fungi,algae,bacteria[5],which can afford to the uptake of heavy metallic contaminations has been reported intensively[6].Andrea Yipmantin[7]chose a red algaChondracanthuschamissoito carry out the biosorption of metal ions Pb(Ⅱ) and Cd(Ⅱ).Recently,yeast species belonging toRhodotorulasp. as biosorbents for heavy metals removal have attracted more attention to the researchers,due to being easily cultivated in inexpensive growth medium,high growth rate and being amenable to genetic and morphological manipulations[8].For example,lead uptake and potentiometric titration studies with live and dried cells ofRhodotorulaglutinishas been reported[9].Biosorption of cadmium[10]by aRhodotorulasp.[11]and characterization of Pb2+biosorption byRhodotorulaglutinis[12]andRhodotorulaaurantiaca[13]have also been discussed.Different strains ofRhodotorulamucilaginosawere used to remove silver from aqueous solution[14]and the performance ofRhodotorularubrato remove cadmium and lead was tested[15].These studies mentioned above have largely concentrated on analyzing the effects of experimental parameters such as pH and temperature,the equilibrium isotherms and kinetics ofRhodotorulasp. on biosorption of heavy metals and characterizing the biosorption properties ofRhodotorulasp.cells by SEM or FTIR.A great potential ofRhodotorulasp.for treatment of contaminated wastewater containing toxic metal ions.
Response surface methodology(RSM) is an optimizing mathematical statistic method with synthetically experiment-designed and mathematical modeling,making use of the multivariate quadratic regression equation to fit the functional relationship between factors and response values[16].The accurate and reliable method has been applied by researchers in more and more experimental studies due to its saving on experimental conditions and expenses with fewer experiments and less time in contrast to other traditional optimizing and statistic methods[17].The experiment-designed approaches including central composite design(CCD),Box-Behnken design(BBD),Doehlert Matrix(DM) and so on,are often used in RSM,which has some applications in optimization of growth parameters for the production of carotenoids[18],of removal of nickel by cone biomass ofPinussylvestris[19],and of microbial media[20],or some other chemical experimental processes[21],but it has not yet been reported to optimize operational parameters of biosorption on heavy metal ions such as Pb2+by response surface methodology.
In this work,Rhodotorulamucilaginosausually used for producing Carotenoids was screened out of a certain molybdenum ore tailing soil and the designed approach of Box-Behnken design(BBD) was chosen to optimize experimental conditions of biosorption on lead ions forRhodotorulamucilaginosawith response surface methodology,aiming at large-scale practical application for biosorption on metal ions pollutants.Then,the biosorption mechanism and properties ofRhodotorulamucilaginosawere analyzed by infrared spectroscopy(FTIR),scanning electron microscopy(SEM) and energy dispersive X-ray spectrometer(EDS).
1 Experimental
1.1 Microorganism,Cell and Storage Pb2+Solutions Preparation
Rhodotorulamucilaginosa(WT6-5) was screened from a tailings soil where located in Huludao(Liaoning Province of China) and tested by morphological characteristics,biochemical properties,carbon assimilation.The 16SrDNA sequencing and paired-end(PE) sequencing on DNA of WT6-5 strains by using Illumina technology were carried out in the Institute of Microbiology Chinese Academy of Sciences and Shanghai Majorbio Bio-pharm Biotechnology Co.,Ltd,respectively,which identified the WT6-5 strains asRhodotorulamucilaginosathat could endure a wide range of pH(2~8).The cells ofRhodotorulasp. were maintained on yeast malt agar plates at 4 ℃ and grown in 250 mL triangular flasks containing YPD growth medium consisted of 20 g/L of glucose,10 g/L of yeast extract and 20 g/L of peptone.Then,cultures were incubated at 28 ℃ on an orbital shaker at 160 r/min.After 48 h incubation at the exponential growth state,the cells derived from centrifugation at 10 000 r/min for 10 min was washed twice with deionized distilled water.TheRhodotorulamucilaginosalive cells obtained were reserved for Pb2+biosorption studies.
Stock Pb2+solutions(1 000 mg/L) were prepared from the following procedure:1.598 5 g of Pb(NO3)2accurately weighed was thrown into 10 mL of HNO3solution(0.2 %),then the mixed solution was transferred to 1 000 mL volumetric flask after being dissolved thoroughly,and the solutions obtained were stored in polyethylene bottle at 4 ℃.The desired concentrations of Pb2+were prepared by diluting stock solutions with deionized distilled water.
1.2 Biosorption Experiments of Pb2+
The whole chemical used in this study were of analytical reagent grade and were used without further purification and all dilutions were carried out by double deionized water(Milli-Q Millipore 18.2 MΩ/cm conductivity).For all experiments of Pb2+biosorption,1 g of preparedRhodotorulamucilaginosacells(20 g/L) were added to 50 mL of Pb2+solutions in 250 mL erlenmeyer flaskes at 160 r/min in an shaker at 25 ℃ for different adsorption times of 5 min,15 min or 25 min.The initial concentration of Pb2+solutions were 10 mg/L,20 mg/L,30 mg/L,respectively.Before adding biomass,the pH of the Pb(NO3)2solutions measured by pHS-25 pH meter was adjusted to the desired values 3,5,or 7 with 0.1 mol/L NaOH or 0.1 mol/L HCl.After sorption,the biomass was separated by centrifugation at 10 000 r/min for 10 min and the concentration of Pb2+in the supernatant was determined by using inductive coupled plasma(ICP-AES) all-direct-reading spectrometer(LEEMAN Prodidy,USA).Each lead ions adsorption experiment was repeated three times,and the average values were singled out as actual results.
Metal sorption value byRhodotorulamucilaginosawas determined according to Eq.(1)[22].
q=V(ρi-ρe)/1 000m
(1)
Whereq(metal uptake) is the amount of metal ions adsorbed on the biosorbent,mg/g of biomass;Vis the volume of metal solution,mL;ρiandρeare the initial and equilibrium(residual) concentration of metal,mg/L;respectively andmis the dry weight of added biosorbent,g.
1.3 Experimental Designs of Response Surface Methodology
Three main experimental parameters including initial concentration of Pb2+initial value of pH and times were chosen to implement the experimental plans of seventeen points(five central points) on three factors and three levels at the request of Box-Behnken design approach,based on the preliminary single-factor and orthogonal experiments.The setting for factor and level values of variables were shown in Table 1.Then Table 2 depicted the experimental design and results of orthogonal tests and the experimental sequence numbers were taken by software Design-Expert 8.0(Stat-Ease,Inc,Minneapolis,USA) at random.

Table 1 Factors,levels and codes for Box-Behnken design
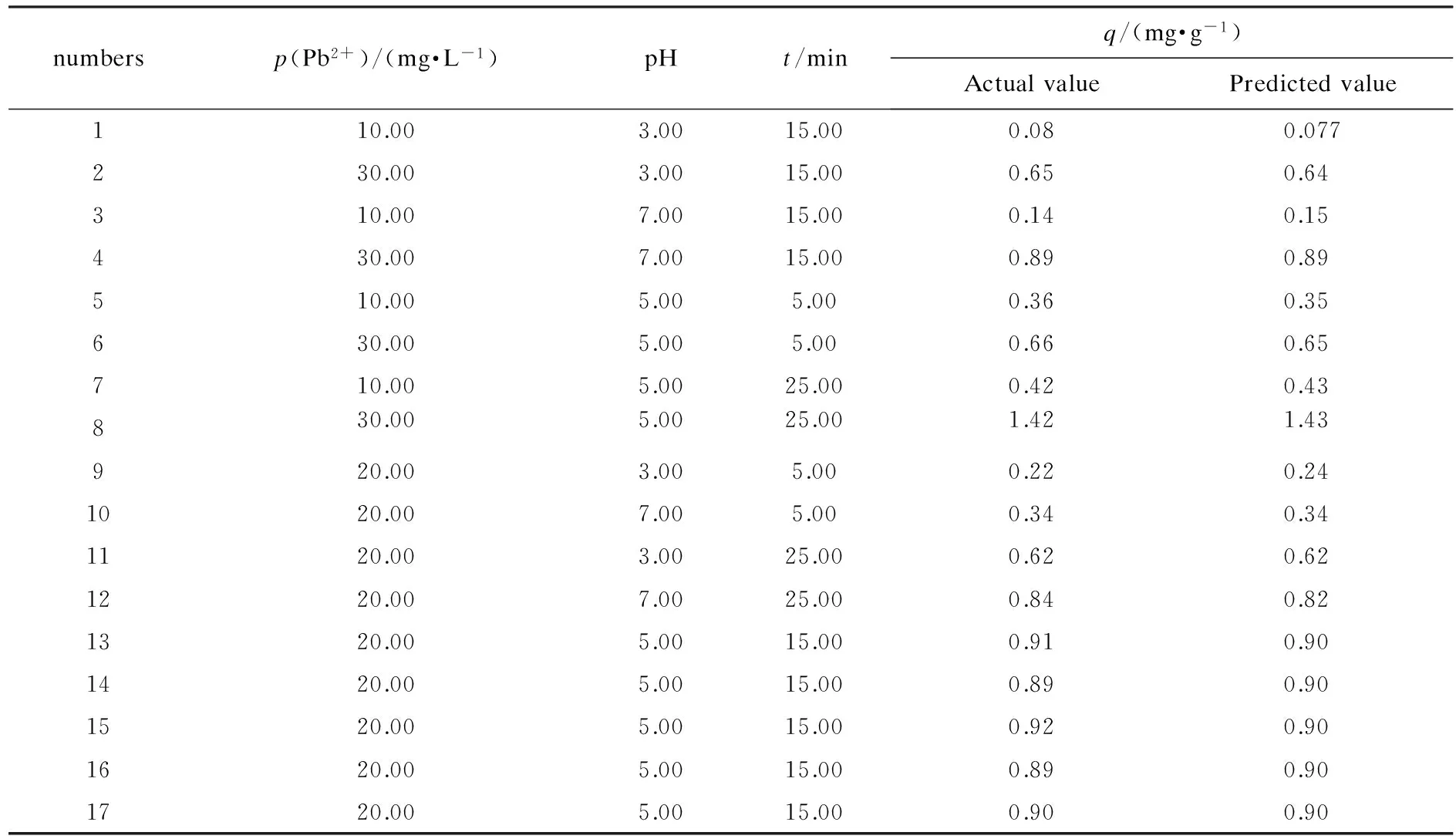
Table 2 Orthogonal experimental design and results
Notes:R2=0.999 1,adjustedR2=0.998 0,predictedR2=0.991 1,C.V.=2.40 %.
1.4 SEM and EDX Analysis
SEM and EDX were performed on the biomass for observation and confirmation of adsorbed elements,which were conducted using a scanning electron microscope(Shimadzu SSX-550,Japan),equipped with an energy dispersive X-ray microanalysis system.Natural and lead-adsorped cells ofRhodotorulamucilaginosawere dried and then covered with Au for later scanning.The accelerated voltage was constant at 15 kV,and the magnification was ×10 000.
1.5 FTIR Spectra Determination
Infrared spectroscopy(IR) is a common method for determining chemical organic functional groups.The Pb2+-unloaded and Pb2+-loaded cells ofRhodotorulamucilaginosawere analyzed by FTIR Fourier infrared spectrometer(Thermo electron corporation,Nicolet 380,USA) as the following procedures:The cells which had been collected and washed twice with deionized water were grinded by agate mortars to certain fineness after drying in an oven at 50 ℃ for 24 h.Then 1 mg of grinded cells and about 150 mg of potassium bromide powder were mixed together for 4~5 min by agate mortars.Finally,the samples obtained were pressed with a pressure machine.
2 Results and Discussion
2.1 Analysis of Rhodotorula Mucilaginosa cells Structure and Elements with EDX
From them,it was revealed that the originalRhodotorulamucilaginosabiomass were integrated cells without transforming shapes or structure.But the micro-capsule whose major component was polysaccharide outside cells were destructed and disappeared after adsorbing lead ions due to formed chemical bonds between lead ions and polysaccharide.And the shapes of cells had also been changed,furthermore,there were a few of floccules emerged on the surface of cells,which indicated that some metallic sediments of lead ions had been formed on the cells′ surface.Maybe the reasons for adsorption of lead ions was that complexation occurred between some radicals and Pb2+on the cells wall or some granular sediments of Pb2+attached to the mycelium surface were formed.
Energy dispersive X-ray(EDX) was used to study the chemical and elemental characteristics of the adsorbent.EDX analysis ofRhodotorulamucilaginosabefore and after Pb2+sorption was demonstrated.The peaks at 0.277 4 keV and 0.524 9 keV were identified as C and O,respectively,which were essential constituents of cell membranes and cell walls.
The peaks of Au at 2.120 5 keV were observed both in the process of before and after Pb2+adsorption because of the pretreatment on spraying gold before SEM analysis,and the peak at 2.342 6 keV also observed following the biosorption of Pb2+onRhodotorulamucilaginosacells was accordance with Pb,which disclosed that the biosorption of Pb2+on theRhodotorulamucilaginosacells occurred indeed.
2.2 Fourier Transforms Infrared Spectroscopy of Rhodotorula Mucilaginosa Cells
The FTIR spectra ofRhodotorulamucilaginosabiomass before and after adsorping lead ions were presented in Fig.1,which reflected the possible interactions between the functional groups ofRhodotorulamucilaginosaand metal ions.From Fig.1,in the spectra of dried unloadRhodotorulamucilaginosabiomass,the strong and wide band at 3 420 cm-1was attributed to stretching vibration of hydroxyl group(—OH),and the peaks observed at 2 921 cm-1and 1 645 cm-1could be assigned to the stretching vibration of —C—H group and carboxyl group(—C==O),in addition,the bands observed at 1 079 cm-1and 1 410 cm-1were also due to C—O stretching vibration belonging to carboxylic acids,alcohols or esters.After the biosorption of Pb(Ⅱ) ions onRhodotorulamucilaginosabiomass,although no apparent new bands and peaks appeared,the deviation of peaks of functional groups has illustrated the mechanism of biosorption onRhodotorulamucilaginosabiosorbent to some extent by the changes of stretching vibration and bending vibration frequency for some groups.After adsorping Pb2+,the stretching and bending vibration of —OH,—C==O,and C—O groups were shifted from 3 420,1 645,1 410,and 1 079 cm-1to 3 416,1 639,1 395 and 1 067 cm-1,respectively,in the spectra of loaded Pb2+Rhodotorulamucilaginosabiomass,which indicated that the functional groups of hydroxyl(—OH),carboxyl(—C==O) and C—O were mainly involved in the biosorption of Pb2+onRhodotorulamucilaginosabiomass,and chemical interactions between the lead ions and functional groups mentioned above occurred on the biomass surface.Furthermore,C—H group of the biomass did not participate in the biosorption of metal ions because no frequency change was observed.

Fig.1 FTIR spectrum of unloaded and Pb2+-loaded
2.3 Discussions on Response Surface Methodology
To learn and investigate the process of biosorption and the process variables better,a more realistic model[9]was built based on the preliminary orthogonal experiments results as shown in Table 2 with Expert-Design 8.0 software[10]under response surface methodology[11].The values of factor and level for variables used to analyze the optimum conditions of higher biosorption efficiency in this experiment[12]at the request of Box-Behnken design as shown in Table 1 and Table 2 were dependent on the former literature reports for biosorption byRhodotorulastrains[13].
The response is available for quadratic model according to the fitting of experimental data by means of polynomial regression analysis.The final quadratic response equation on an empirical relationship between metal ions uptake(q) and tested variables in terms of coded factors obtained by software Design-Expert 8.0 was as follows in Eq.(2).
q=0.90+0.33A+0.08B+0.22C+0.045AB+0.18AC+0.025BC-0.13A2-0.34B2-0.061C2
(2)
whereqis the response,namely equilibrium biosorption capacity,andA,B,Care the coded values of the main impact factors including initial concentrations of Pb2+,initial values of pH,and times.A2,B2,C2are the measures of the square effects of variables initial concentrations of Pb2+,initial values of pH,and times,respectively,then the variablesAB,ACandBCrepresent the interaction effect among initial concentrations of Pb2+,initial values of pH,and times.The central point was repeated for five times.
The results for the analysis of variance on this response surface quadratic model(ANOVA) were demonstrated in Table 3.It is reported that the significance of each coefficient was determined byF-values andP-values,then higherF-value and lowerP-value represent more significance of relevant coefficients[16].From Table 3,it was observed that the coefficients for the main and square effects were highly significant(P<0.000 1) compared to the interaction effects.TheF-value of the model(902.08) with a low probability value(P<0.000 1,less than 0.05) demonstrates a high significance for the regression model,furthermoreA,B,C,AB,AC,BC,A2,B2,C2are significant model terms due to their lowP-values in this case.The “Lack of FitP-value” of 0.248 4(>0.05) implies the Lack of Fit is not significant and the model fits well.The goodness of the fitting of the model was also evaluated by the multiple correlation coefficient(R2) equal to 0.999 1 in this case,which revealed that this regression is statistically significant and only 0.09 % of the total variances is not explained by the model.The “PredR2” of 0.991 1 is in reasonable agreement with the “AdjR2” of 0.998 0.Besides,a relatively lower value of the coefficient of variance(C.V.=2.40 %) reflects a better accuracy and reliability of the biosorption experiments were implemented.

Table 3 ANOVA for the response surface quadratic model in the BBD design
2.4 Effects of Initial Concentrations of Pb2+and pH on the Lead Ions Uptake
The response surface quadratic model and response surface plots obtained by software Design-Expert 8.0 under Box-Behnken design were used to optimize the three main experimental parameters and understand heavy metal removal and lead ions uptake efficiency better.The contour lines and response surface plot ofY=f(A,B,15 min) were shown in Fig.2,which depicted the effect of intial concentrations of Pb2+and intial values of pH on the lead ions uptake and biosorption capacity ofRhodotorulamucilaginosawhen the adsorption time was a constant value of 15 min.The biosorption capacity of Pb2+rised with increase of initial lead ions concentrations ranging from 10 mg/L to 30 mg/L as well as with the initial values of pH up to 5,but has a little decline as the pH value higher than 5,namely,reducing the biosorption efficiency.In addition,its obtained optimum value of pH was more or less 5.It is reported that the increase of metal ions uptake and biosorption efficiency by adding initial metal ions concentration could be contributed to the increase in the driving force of concentration gradient and on the same conditions,the active sites ofRhodotorulamucilaginosabiomass would be occupied by more metal ions once the initial concentrations of metal ions in the solution were higher,then the process of biosorption would also be carried out more sufficiently[23].Meanwhile,both the solubility of metal ions and the binding of metal ions above the cell wall are affected by the value of pH in solution.The precipitation of Pb2+could be formed if the pH value is beyond 5[22],but at low pH on one hand,the biased acidity in solution maybe do damage to the cell wall of biosorbent biomass,on the other hand metal binding sites become positively charged due to the high concentration protons on the cell wall functional groups,then metal cations
and protons compete for binding sites,which leads to lower adsorption of metal ions,so the higher values of metal ions uptake would be obtained at higher initial solution pH due to the increase in availability of binding sites and improvement for the access of metal ions to the metal-binding sites of cell wall.

Fig.2 Contour lines and response surface
The results in this study are also in line with some researches in other literatures,for example,Goksungur studied the removal of cadmium and lead ions byS.cerevisiae[24].They have found that it had high efficiency of biosorption in medium pH and had a maximum values at pH 6 and 5 for cadmium and lead ions,respectively.Han[25]have revealed that biosorption capacity of biomass increased with the pH of solution went up from 2 to 6 for copper and lead ions and in their studied the equilibrium biosorption capacity raised with the increasing of initial metal ions concentration.
2.5 Effects of Initial Concentrations of Pb2+and Adsorption Times on the Lead Ions Uptake
The contour lines and response surface plot ofY=f(A,C,5) were shown in Fig.3,which depicted the effect of intial concentrations of Pb2+and adsorption times on the lead ions uptake and biosorption capacity ofRhodotorulamucilaginosawhen the initial value of pH was a constant value of 5.

Fig.3 Contour lines and response surface
The biosorption capacity of Pb2+still increased with the increase of initial lead ions concentration ranging from 10 mg/L to 30 mg/L as more lead ions surrounded and were adsorbed on the active sites of 20 g/L ofRhodotorulamucilaginosabiomass at higher concentrations of Pb2+.The biosorption capacity and efficiency raised from the beginning of 5 min adsorption times,within the first 20 min,biosorption of Pb2+have achieved nearly 90 % of the total Pb2+adsorption capacity,furthermore,the amount of adsorped lead ions did not vary significantly among adsorption times of 20~25 min,which indicated that the process of Pb2+biosorption almost reached adsorption equilibrium at this stage.It is a tremendous strength that the biosorption of Pb2+could be implemented in rapid and short contact time between biosorbent and metal ions for practical application and development of this technology[26].
2.6 Effects of Initial Values of pH and Adsorption Times on the Lead Ions Uptake
The contour lines and response surface plot ofY=f(B,C,20 mg/L) were shown in Fig.4,which depicted the effect of intial values of pH and adsorption times on the lead ions uptake and biosorption capacity ofRhodotorulamucilaginosawhen the initial concentrations of Pb2+was a constant value of 20 mg/L.The biosorption process trended to steady level after 20 min adsorption,and the maximum biosorption capacity was obta-ined at 25 min of adsorption time.The biosorption capacity and efficiency still increased with the increase in pH from 3 to 5 firstly,then decreased while the value of pH rised from 5 to 7 continuously.Although The optimal pH value for adsorption of metal ions changes with the type of biomass and metal ions,in this case the optimum pH was about 5 according to experimental results.A pH between 4.0 and 8.0 is widely accepted as being optimal for metal sorption for almost all types of biomass[27].
Above all,from Fig.2,Fig.3,and Fig.4,it was acquired that the optimum values of the experimental variables and the corresponding maximum biosorption capacity of lead ions obtained were 1.45 mg/g biomass accompanied with initial lead ions concentration of 30 mg/L,initial solution pH of 5.45 and adsorption time of 25 min,respectively.
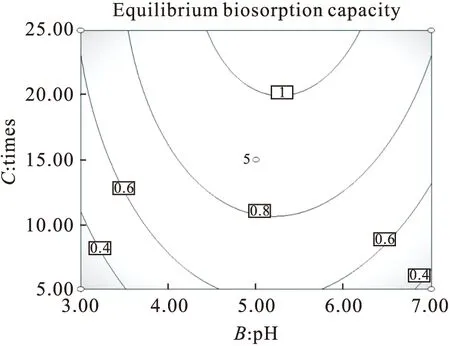
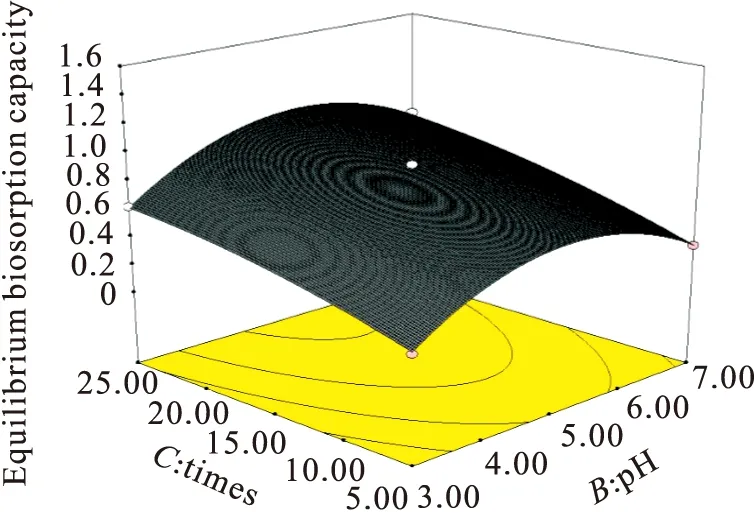
Fig.4 Contour lines and response surface
3 Conclusions
(1) The morphology and elements of unload-Pb2+and loaded-Pb2+Rhodotorulamucilaginosabiomass were observed by SEM and EDX,which indicated that the existing lead ions after adosrption and interaction of biosorption on biomass occurred indeed.Through the analysis of FTIR spectra before and after adsorbing Pb2+,the functional groups of —OH,—C==O,and C—O were mainly involved in the biosorption of Pb2+onRhodotorulamucilaginosabiosorbent.
(2) The quantitative relationship between the lead ions takeup(q) and different levels of three factors was used to work out optimal levels of these parameters by Box-Behnken esign with response surface methodology.The biosorption capacity of Pb2+increased with increasing initial concentrations of Pb2+and adsorption times but it firstly increased and then decreased when the initial pH was rising.The optimum value of lead ions uptake of 1.45 mg/g biomass was carried out with RSM under Design-Expert software on the condition that the three parameters were 30 mg/L of initial Pb2+concentration,5.45 of initial pH and 25 min of adsorption time,respectively.The fit of the model was checked by the determination of the value of the multiple correlation coefficient(R2=0.999 1),which indicated that this regression is statistically significant and only 0.09 % of the total variations was not explained by the model.A relatively lower value of the coefficient of variance(C.V.=2.4 %) obtained from Design-Expert software indicated the experiments were carried out more precisely and reliably and the optimization for the experimental parameters by response surface methodology.
(3) It was concluded thatRhodotorulamucilaginosabiomass could be used as a reasonable,natural and low-cost biosorbents for the removal of heavy metal ions,meanwhile,providing a great boost for the commercial application of biosorption technology in the future.
[1] GADD G M.Biosorption:Critical Review of Scientific Rationale,Environmental Importance and Significance for Pollution Treatment[J].Journal of Chemical Technology and Biotechnology,2009,84(1):13-18.
[2] KIM Y E,JIN A L,JEONG S K,et al.Comparison of Carbon Dioxide Absorption in Aqueous MEA,DEA,TEA,and AMP Solutions[J].Bulletin- Korean Chemical Society,2013,34(3):783-787.
[4] AKAR S T,GORGULU A,ANILAN B,et al.Investigation of the Biosorption Characteristics of Lead(Ⅱ) Ions onto Symphoricarpus Albus:Batch and Dynamic Flow Studies[J].Journal of Hazardous Materials,2009,165(1/2/3):126-133.
[5] BUENO B Y M,TOREM M L,CARVALHO R J D,et al.Fundamental Aspects of Biosorption of Lead(Ⅱ) Ions onto aRhodococcusOpacusStrain for Environmental Applications[J].Minerals Engineering,2011,24(14):1619-1624.
[6] VASUDEVAN P,PADMAVATHY V,DHINGRA S C.Biosorption of Monovalent and Divalent Ions on Baker′s Yeast[J].Bioresource Technology,2002,82(3):285-289.
[7] YIPMANTIN A,MALDONADO H J,LY M,et al.Pb(Ⅱ) and Cd(Ⅱ) Biosorption onChondracanthusChamissoi(a Red Alga)[J].Journal of Hazardous Materials,2011,185(2/3):922-929.
[8] GHORBANI F,YOUNESI H,GHASEMPOURI S M,et al.Application of Response Surface Methodology for Optimization of Cadmium Biosorption in an Aqueous Solution bySaccharomycesCerevisiae[J].Chemical Engineering Journal,2008,145(2):267-275.
[9] CHO D H,CHU K H,KIM E Y.Lead Uptake and Potentiometric Titration Studies with Live and Dried Cells ofRhodotorulaGlutinis[J].World Journal of Microbiology and Biotechnology,2011,27(8):1911-1917.
[10]LI Z J,YUAN H L.Characterization of Cadmium Removal byRhodotorulasp.Y11[J].Applied Microbiology and Biotechnology,2006,73(2):458-463.
[11]LI Z J,YUAN H L,HU X D.Cadmium-resistance in GrowingRhodotorula,sp.Y11[J].Bioresource Technology,2008,99(5):1339-1344.
[12]CHO D H,KIM E Y.Characterization of Pb2+,Biosorption from Aqueous Solution byRhodotorulaGlutinis[J].Bioprocess and Biosystems Engineering,2003,25(5):271-277.
[13]CHO D H,YOO M H,KIM E Y.Biosorption of Lead(Pb2+) from Aqueous Solution byRhodotorulaAurantiaca[J].Journal of Microbiology and Biotechnology,2004,14(2):250-255.
[14]GOMES N C M,ROSA C A,PIMENTEL P F,et al.Uptake of Free and Complexed Silver Ions by Different Strains ofRhodotorulaMucilaginosa[J].Brazilian Journal of Microbiology,2002,33(1):62-66.
[15]SALINAS E,ORELLANO M E D,REZZA I,et al.Removal of Cadmium and Lead from Dilute Aqueous Solutions byRhodotorulaRubra[J].Bioresource Technology,2000,72(2):107-112.
[17]MALDONADE I R,RODRIQUEZ-AMAYA D B,SCAMPARINI A R.Statistical Optimisation of Cell Growth and Carotenoid Production byRhodotorulaMucilaginosa[J].Microbiol,2012,43(1):109-115.
[18]VIJAYALAKSHMI G,SHOBHA B,VANAJAKSHI V,et al.Response Surface Methodology for Optimization of Growth Parameters for the Production of Carotenoids by a Mutant Strain ofRhodotorulaGracilis[J].European Food Research and Technology,2001,213(3):234-239.
[19]CAN M Y,KAYA Y,ALGUR O F.Response Surface Optimization of the Removal of Nickel from Aqueous Solution by Cone Biomass ofPinusSylvestris[J].Bioresource Technology,2006,97(14):1761-1765.
[20]PREETHA B,VIRUTHAGIRI T.Application of Response Surface Methodology for the Biosorption of Copper UsingRhizopusArrhizus[J].Journal of Hazardous Materials,2007,143(1/2):506-510.
[22]SUBBAIAH M V,YUVARAJA G,VIJAYA Y,et al.Equilibrium,Kinetic and Thermodynamic Studies on Biosorption of Pb(Ⅱ) and Cd(Ⅱ) from Aqueous Solution by Fungus(TrametesVersicolor) Biomass[J].Journal of the Taiwan Institute of Chemical Engineers,2011,42(6):965-971.
[23]LUO F,LIU Y H,LI X M,et al.Biosorption of Lead Ion by Chemically-modified Biomass of Marine Brown AlgaeLaminariaJaponica[J].Chemosphere,2006,64(7):1122-1127.

[25]HAN R,LI H,LI Y,et al.Biosorption of Copper and Lead Ions by Waste Beer Yeast[J].Journal of Hazardous Materials,2006,137(3):1569-1576.
[26]RIDVAN S,NALAN Y,ADIL D.Biosorption of Cadmium,Lead,Mercury,and Arsenic Ions by the FungusPenicilliumPurpurogenum[J].Separation Science and Technology,2003,38(9):2039-2053.
[27]MONTAZER-RAHMATI M M,RABBANI P,ABDOLALI A,et al.Kinetics and Equilibrium Studies on Biosorption of Cadmium,Lead,and Nickel Ions from Aqueous Solutions by Intact and Chemically Modified Brown Algae[J].Journal of Hazardous Materials,2011,185(1):401-407.
红酵母生物吸附Pb(Ⅱ)的响应面分析
西瓦·K·M1, 姜彬慧2, 赵 彦2, 雷 蕾3, 王 黎4
(1.澳大利亚伍龙贡大学 土木矿业环境学院可持续生态资源研究中心, 新南威尔士 伍龙贡 2500;2.东北大学 资源与土木工程学院, 辽宁 沈阳 110819; 3.武汉科技大学, 湖北 武汉 430081;4.沈阳化工大学 环境与安全工程学院, 辽宁 沈阳 110142)
通过Box-Behnken设计对Pb2+的生物吸附过程主要参数进行响应曲面分析优化.利用扫描电子显微镜、能量弥散X射线探测器以及傅里叶变换红外光谱,分析Pb2+对生物质表面的生物吸附机理和特点.通过扫描电子显微镜和能量弥散X射线探测器可观察到Rhodotorulamucilaginosa生物质的形态和组成元素,显示生物吸附发生后存在铅离子.在初始条件下,Pb2+的质量浓度为30 mg/L,pH为5.45,利用Design-Expert软件,通过响应面法得到响应面图,可以看出铅离子对生物质的最佳吸收值达到1.45 mg/g,吸收时间为25 min.二次型方差分析(ANOVA)证明该模型的相关性,其变异系数C.V.=2.4 %,表明实验结果的准确性和可靠性.
红酵母; 生物吸附; 铅的去除; 反应曲面法; 废水处理
Foundation items: This work was supported by the NSFC under Grant (51574185),NSP(2015BAB18B01) and BHSTF(2015ACA064)
2095-2198(2016)03-0278-11
10.3969/j.issn.2095-2198.2016.03.019
X712 Document code: A
Received date: 2016-09-09
Biography: SIVAKUMAR K M, born in 1952, Male, Australian, Professor, PhD, engaged in environmental engineering.

