刚性不对称三氮唑衍生物的Cd(Ⅱ)配合物的制备、晶体结构及其荧光性质
徐周庆 王晓宁 李慧军*, 贾 磊*, 赵惠子 李守杰 马录芳 张培玲
(1河南理工大学物理化学学院,焦作454000) (2洛阳师范学院,洛阳471022) (3河南理工大学电气学院,焦作454000)
刚性不对称三氮唑衍生物的Cd(Ⅱ)配合物的制备、晶体结构及其荧光性质
徐周庆1王晓宁1李慧军*,1贾磊*,1赵惠子1李守杰1马录芳*,2张培玲3
(1河南理工大学物理化学学院,焦作454000) (2洛阳师范学院,洛阳471022) (3河南理工大学电气学院,焦作454000)
用水热法合成了基于刚性不对称三氮唑衍生物配体H2ptp(H2ptp=4-(5-(1H-pyrazol-4-yl)-4H-1,2,4-triazol-3-yl)pyridine)的2个Cd(Ⅱ)配合物{[Cd2(Hptp)2(OAc)2]·7H2O}n(1)和{[Cd(Hptp)Cl(H2O)]·H2O}n(2),并通过元素分析、差热分析、粉末X射线衍射和单晶X射线衍射进行表征。配合物1具有一个梯状的一维结构,在π…π堆积作用下这些一维梯链形成了超分子二维平面。在氢键的连接作用下,客体水分子形成了独特的以双五元水簇为结构单元的水链,这些水链填充在相邻的二维平面之间并通过氢键把二维平面连接成了三维超分子网络。配合物2具有一个新型的二维双层结构,分子间氢键把这些二维平面连成了三维结构。另外,测试了2个配合物的荧光性质。
金属-有机框架;荧光;三氮唑衍生物
0 Introduction
In recent decades,the rational design and construction of novelmetal-organic frameworks(MOFs)has achieved chemist′s great attentions not only for their intriguing variety of architectures and topologies,but also for stems from their potential applications in gas storage,luminescence,magnetism,and catalysis[1-3]. Though numerous MOFs have been achieved,it still remains a long-term challenge to rationally and predictably prepare desired crystals[4-5].Generally,the resulting framework is mainly influenced by several factors,including the structural characteristics of ligands,the coordinated modes of centre metal ions, the solvent system,the counter-anions,and so on[6-10]. In particular,the organic linkers plays crucial role in the construction of the targeted MOFs,because the ligand could adopt different conformations in the crystallization and further leads to structural isomerism. On this point,the suitable ligands selection is considerable significant to regulate and control the topology of coordination frameworks[11-12].
As is well known,the triazole ring possessing aromaticity and variable coordination modes can afford multiple coordinating sites for linking closely situated metal ions to form complex with novel structure[13-14].In this context,a new type of triazole derivatives with the 3-and 5-sites substituted by two different azaheterocycles may be a good candidate because the multiple N atoms in this kind of ligands are more in favor of construction of MOFs with interesting structure and attractive properties.Taking these into account,2-(3-(1H-pyrazol-4-yl)-1H-1,2,4-triazol-5-yl)pyridine(H2ptp),an anisomerous triazole derivative,which contains one potential bidentate chelating site and three monodentate ones,was used as ligand to construct MOFs.Consequently,two novel Cd(Ⅱ)coordination polymers,{[Cd2(Hptp)2(OAc)2]· 7H2O}n(1)and{[Cd(Hptp)Cl(H2O)]·H2O}n(2)had been obtained.These compounds are characterized by elemental analysis,IR spectra,and X-ray crystallography.In addition,their photoluminescent properties have also been investigated.
1 Experimental
1.1M aterials and measurements
All chemicals were commercially purchased and used without further purification.Elemental analyses for carbon,hydrogen and nitrogen were performed on a Thermo Science Flash 2000 element analyzer.FTIR spectra were obtained in KBr disks on a Perkin Elmer Spectrum One FTIR spectrophotometer in 4 000~450 cm-1spectral range.Solid-state(diffuse reflectance) electronic spectra were measured as polycrystalline samples on a Perkin Elmer Lambda2S spectrophotometer,within the range 400~1 100 nm.The powder X-ray diffraction(PXRD)analyses were performed with a Bruker AXSD8 Discover instrument(Cu Kα radiation,λ=0.154 184 nm)over the 2θrange of 5°~50°at room temperature.Thermogravimetric analyses (TGA)were performed using a TG-DTA 2010SMAC apparatus between 30 and 500℃in N2atmosphere with heating rate of 10℃·min-1.The photoluminescent properties were measured on an F-4500 FL Spectrophotometer.
1.2Preparations of the comp lexes 1 and 2
{[Cd2(Hptp)2(OAc)2]·7H2O}n(1).AmixtureofH2ptp (0.05 mmol,10.60 mg),Cd(OAc)2·2H2O(0.10 mmol, 26.65 mg),absolute ethanol(5 mL)and H2O(5 mL) was placed in a Teflon-lined stainless steel vessel(25 mL),heated to 120℃for 3 days,and then cooled to room temperature at a rate of 5℃·h-1.Yellow block crystals of 1 were obtained and picked out,washed with distilled water and dried in air.Yield:16.5 mg, 56%(based on Cd).Elemental analysis Calcd.for C24H34Cd2N12O11(%):C 32.55,H 3.18,N 18.98;Found (%):C 32.15,H 3.08,N 18.87.IR(KBr,cm-1):3 244, 1 610,1 550,1 435,1 355,958,754.
{[Cd(Hptp)Cl(H2O)]·H2O}n(2).Complex 2 was synthesized in the similar way as that described for 1, except that Cd(OAc)2·2H2O was replaced by CdCl2· 2H2O(0.1 mmol,20.13 mg).Colourless block crystals of 2 were obtained and picked out,washed with distilled water and dried in air.Yield:15.3 mg,63% (based on H2ptp).Elemental analysis Calcd.for C10H11CdClN6O2(%):C 30.39,H 2.80,N 21.27;Found(%):C30.21,H 2.68,N 21.18.IR(KBr,cm-1):3 184,1 629, 1 463,1 435,1 384,947,873.
1.3X-ray crystallography
X-ray Single-crystal diffraction analyses of 1 and 2 were carried out on a Bruker SMART APEXⅡCCD diffractometer equipped with a graphite monochromated Mo Kαradiation(λ=0.071 073 nm)by using φ-ωscan techniqueat room temperature.The structures were solved via directmethods and successive Fourier difference synthesis and refined by the full-matrix least-squares method on F2with anisotropic thermal parameters for all non-H atoms(SHELXL-97)[15-16].The empirical absorption corrections were applied by the SADABS program[17].The H-atoms of carbon were assigned with common isotropic displacement factors and included in the final refinement by the use of geometrical restraints.H-atoms of water molecules were first located by the Fourier maps and then refined by the riding mode.The crystallographic data for complexes 1 and 2 are listed in Table1.Moreover, the selected bond lengths and bond angles are listed in Table2and Table3.
CCDC:1429655,1;1415769,2.

Table1 Crystal data and structure refinement details for com p lexes 1~2
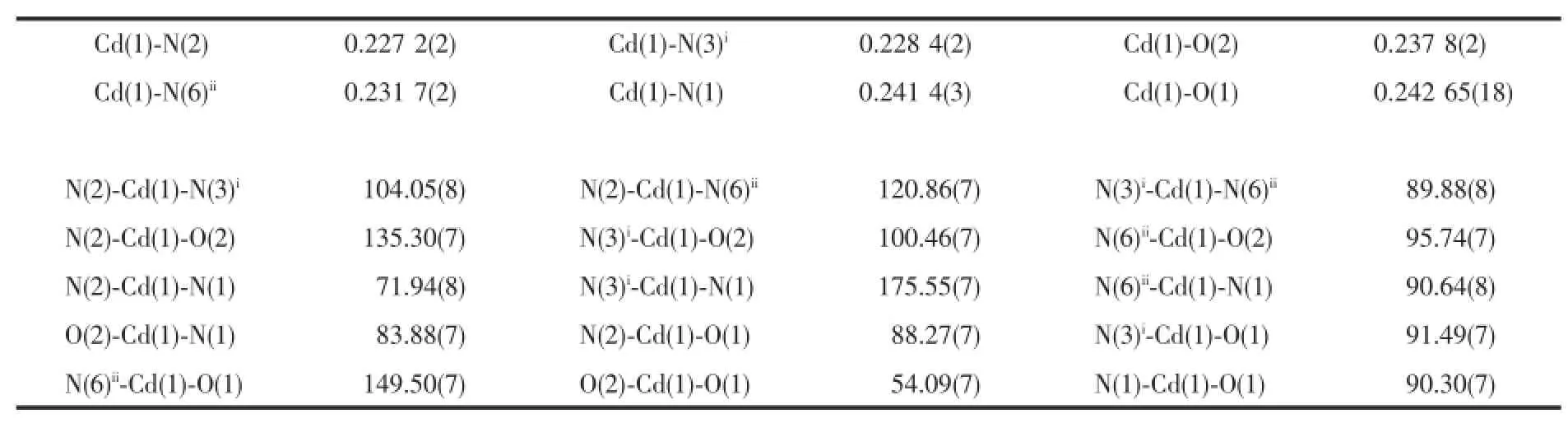
Table2 Selected bonds(nm)and angles(°)for 1

Tab le 3 Selected bonds(nm)and angles(°)for 2
2 Results and discussion
2.1Crystal structures of complexes 1~2
The X-ray diffraction analysis reveals thatcomplex 1 crystallizes in the triclinic system,space group P1. There are one Cd(Ⅱ)ion,one Hptp-ligand,one coordinated acetate anion and three and a half free water molecules in the asymmetric unit of 1.As shown in Fig.1a,the Cd center is six-coordinated by two O atoms(O1 and O2)from one acetate anion and four N atoms(N1,N2,N3iand N6ii)from three Hptp-ligands to form a distorted octahedral geometry.The apical positions are occupied by two nitrogen atom(N1, N3A)from two Hptp-ligands with the N1-Cd1-N3iangle of 175.6(5)°.The ligand is partial deprotonation and coordinates to three Cd(Ⅱ)ions by one chelating fashion through N1 and N2 atomsand twomonodentate linkages via N3,N6 atoms.As shown in Fig.1b,two asymmetric units give rise to a dinuclear secondary building unitwhich connected with each other to form a 1D ladder chain.Aromaticπ…πstacking interaction between adjacent benzene rings connected thechains to form a 2D supramolecular layer along the b axis(Cg1…Cg2 0.339 8 nm)(Fig.1c).
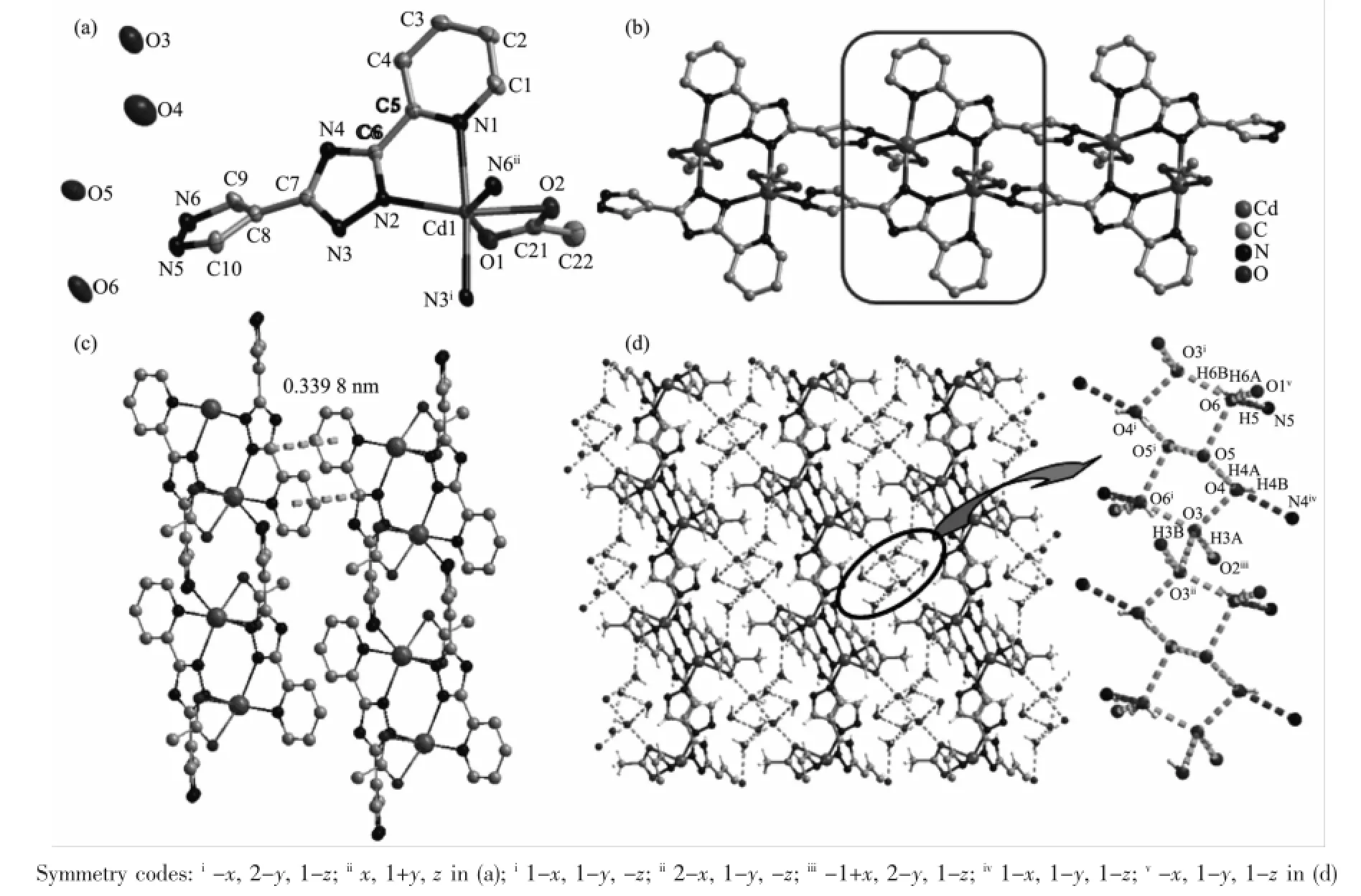
Fig.1(a)ORTEP drawing of 1 with 30%thermal ellipsoids;(b)[Cd2(Hptp)2(OAc)2]dinuclear unit and the 1D ladder chain of 1; (c)π…πstacking interactions in 1;(d)3D supramolecular architecture and the one dimensionalwater chains in 1
As we all know,hydrogen bonding interactions are usually important in the construction of supramolecular architectures.Exactly,double pentanuclear water cluster was formed by intermolecular O-H…O hydrogen bonding between the lattice watermolecules located into the cavity of two adjacent layers in complex 1.The adjacent water clusters are further expended to a water chain by hydrogen bonds of O3-H3B…O3ii(Fig.1d).Moreover,the water chain is fixed to the structure of 1 by the strong hydrogen bonds of O4-H4B…N4iv,N5-H5…O6 and O3-H3B…O2iii(Fig.1d).These hydrogen bonding interactions not only make the skeleton of the structure more stable,but also link the 2D network to form a 3D supramolecular architecture(Fig.1d).
Structural analysis show that 2 crystallizes in the monoclinic system,space group C2/c.As shown in Fig.2a,the asymmetric unit of 2 contains one Cd(Ⅱ)ion,one chloride anion,one Hptp-,one coordinated water molecule as well as one free water molecule. Each Cd(Ⅱ)ion is in a distorted octahedralenvironment with three nitrogen atoms from two Hptp-ligands,one oxygen atom from a coordinated water molecule,and two bridging chlorine anions.The apical positions are occupied by O1 and Cl1(Cd1-O1 0.233 2(3),Cd1-Cl1 0.275 6(2)nm).The equatorial plane is completed byN1,N2,N6 and Cl1 with the mean deviations of 0.126 1 nm from the plane(Cd1-N1 0.237 2(3),Cd1-N2 0.226 7(3),Cd1-Cl1 0.214 8(15),Cd1-N60.228 5(3) nm).The Hptp-ligand coordinates to two Cd(Ⅱ)ions by one chelating fashion through N1 and N2 atoms and onemonodentate linkage via N6 atom.As depicted in Fig.2b,the adjacent Cd(Ⅱ)ions are bridged by Hptp-ligands to form a 1D zigzag chain.Adjacent zigzag chains in one plane are connected by the bridging chlorine anions to form a novel 2D double layer(Fig.2c).
Fig.2(a)ORTEP drawing of 2 with 30%thermal ellipsoids;(b)Zigzag chains in 2;(c)2D double layer in 2; (d)Hydrogen bonds in 2;(e)3D supramolecular architecture connected by hydrogen bonds in 2
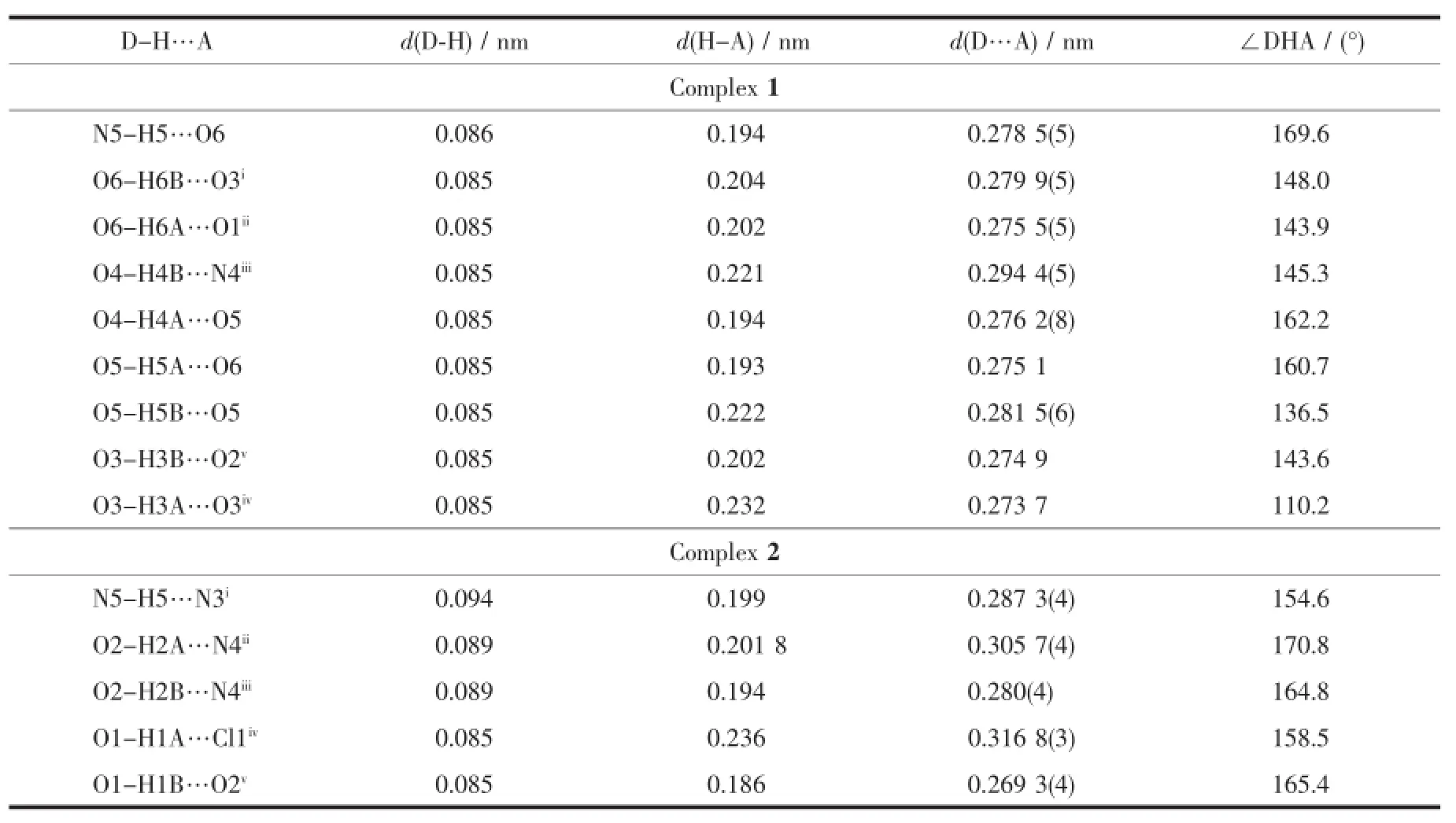
Table4 Hydrogen bond parameters in 1 and 2
The hydrogen bonds also play an important role in the construction of the supramolecular architectures of 2.The lattice water molecules(O2)are trapped inside the gap of the 2D double layer by three kinds of powerful hydrogen bonds:O2-H2A…N4,O2-H2B…N4iand O1-H1B…O2(Fig.2d and Table4).The 2D layers are further expanded to a 3D network by O1-H1A…Cl1 hydrogen bonds and O1-H1B···O2 hydrogen bonds(Fig.2e).
2.2Powder X-ray diffraction analysis
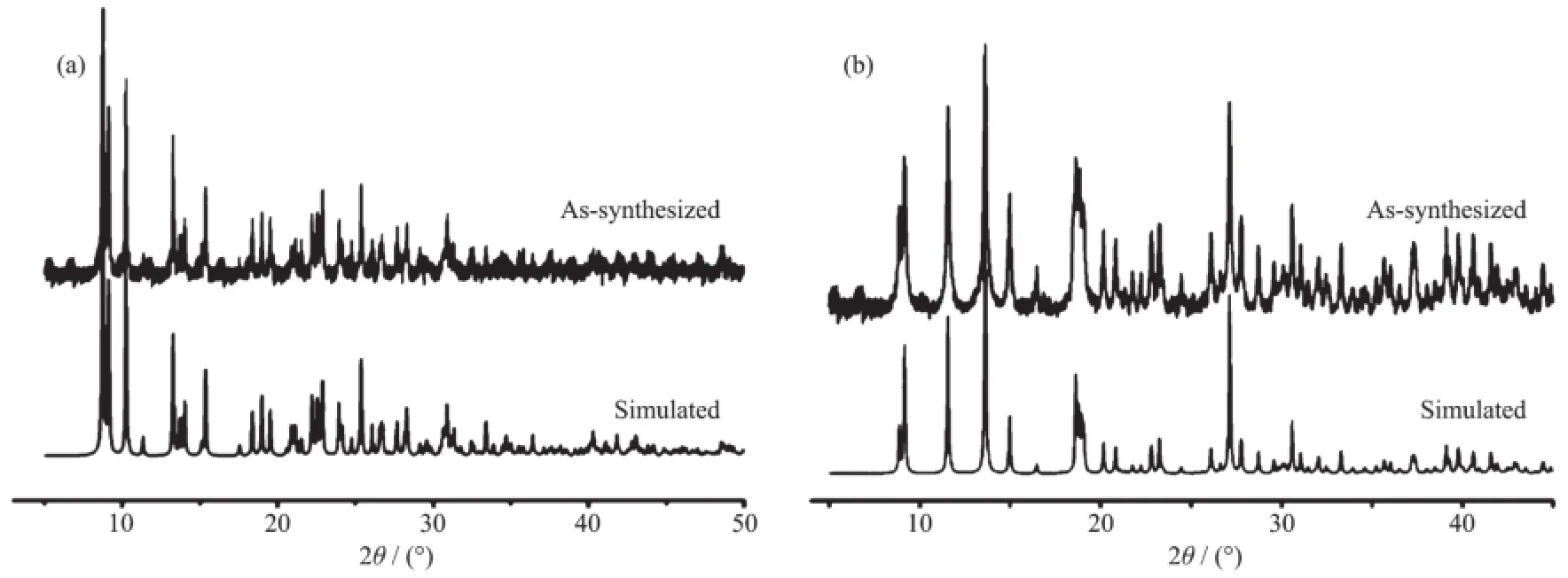
Fig.3 Powder XRD pattern for 1(a)and 2(b)
To check the phase purity of the products, powder X-ray diffraction(PXRD)experiments have been carried out for these complexes(Fig.3).The peakpositions of the experimental and simulated PXRD patterns are in good agreement with each other, indicating that the crystal structures are truly representative of the bulk crystal products.The differences in intensitymay be owing to the preferred orientation of the crystal samples.
2.3Thermal analysis
Thermogravimetric analysis(TGA)was carried out for complex 1 and 2 and the results are shown in Fig.4.For 1,the weight loss of about 14.5%before 260℃corresponds to the release of four lattice water molecules(Calcd.15.98%).The high release temperature confirms the existence of strong hydrogenbonding interactions between water molecules and acetate anions in 1.The TGA of 2 displays that the lattice and the coordinated water molecules are released in the same temperature range(110~205℃) (Found 9.06%;Calcd.9.23%),whichmay be attributed to the strong hydrogen bonds of O2(O2-H2A…N4, O2-H2A…N4iand O1-H1B…O2).Then the Clanions were loosed in the 320~440℃range(Found 17.85%;Calcd.18.46%)and subsequently the complex 2 decomposed.
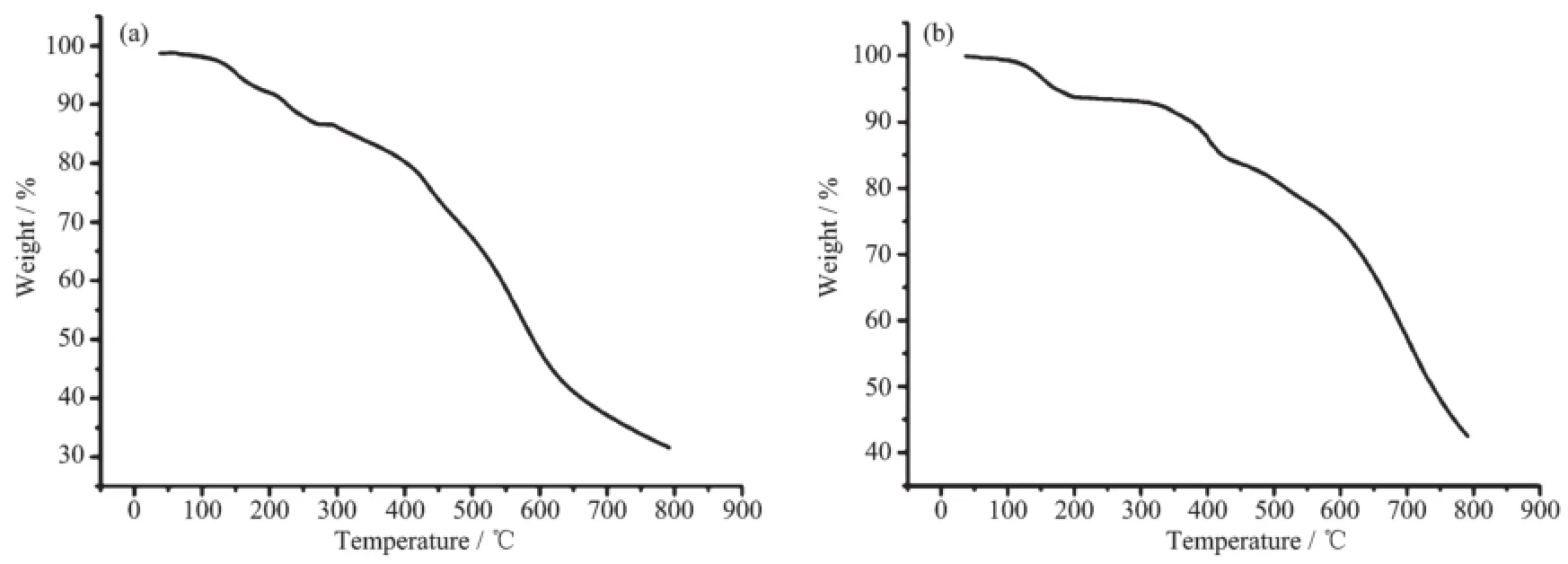
Fig.4 TGA diagram for 1(a)and 2(b)
2.4Photolum inescence properties
Coordination polymers composed of d10metal centres and organic ligands have been investigated to exploit their fluorescence property because of their potential applications as luminescent materials, especially those involving Cd(Ⅱ)ions as coordination centres[18-19].Although the synthesis of the desired luminescentmaterials is still a challenge in this area, it is undoubted that appropriately incorporating conjugated organic ligands and anionic components into a coordination polymeric system is an efficient method toadjust luminescentpropertiesof thematerials, such as excitation/emission wavelength,intensity, lifetime,and so forth.In this study,luminescence properties of all compounds and the free ligand have been explored at room temperature in the solid state because they are virtually insoluble in most common solvents such as acetone,methanol,chloroform, benzene,water,and so forth.Upon excitation at ca. 386 nm,the H2ptp ligand shows emissions at ca.467 nm(Fig.5),which is assigned toπ→π*transitions. However,the strongest emissions occur at 441 nm for 1,426 nm for2 upon excitation at385 nm,respectively, showing a significant blue shift compared with the free ligand.The emissions of complexes 1~2 can beattributed to the ligand-to-metal charge transfer[20-21]. The different coordination behaviors of Hptp-and different counter anions in the framework are probably responsible for the shift difference of the emission bands in 1~2.These observations suggest that these compounds can serve as candidates for potential photoactivematerials.
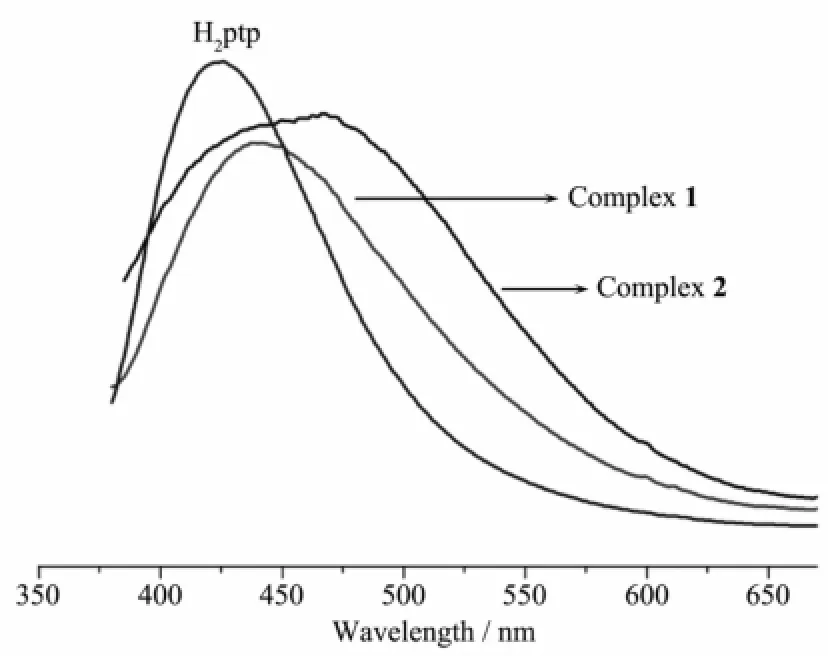
Fig.5 Solid state luminescence spectra for H2ptp,1 and 2 at room temperature
3 Conclusions
By assembling of Cd(Ⅱ)salt and the new asymmetric rigid triazole derivatives,H2pbt,two new Cd(Ⅱ)coordination polymers has been obtained. Complex 1 shows one dimensional(1D)ladder chain, which is further extended to a two dimensional(2D) supramolecular layer viaπ…πstacking interactions. The 2D layers are further linked by hydrogen bonding to form a three dimensional(3D)supramolecular network.Complex 2 exhibits a unique 2D double layered motif which is further expanded to a 3D supramolecular structure through interlayerπ…π stacking interactions and hydrogen bindings.In addition,thephotoluminescencepropertiesinvestigation suggests that these compounds can serve as candidates for potential photoactivematerials.
References:
[1]Du L T,Lu Z Y,Zheng K Y,et al.J.Am.Chem.Soc.,2013, 135:562-565
[2]Zhao B,Cheng P,Chen X Y,et al.J.Am.Chem.Soc.,2004, 126:3012-3013
[3]Liu T F,ZhangW J,Sun W H,et al.Inorg.Chem.,2011,50: 5242-5248
[4]XIE Qing-Fan(解庆范),GAO Ping-Zhang(高平章),CHEN Yan-Min(陈延民).Chinese J.Inorg.Chem.(无机化学学报), 2014,30:2382-2388
[5]Xu Z,Meng W,Li H,et al.Inorg.Chem.,2014,53:3260-3262
[6]Xiao J,Liu B Y,Wei G.Inorg.Chem.,2011,50:11032-11038
[7]Nagarkar SS,Das R,Poddar P,et al.Inorg.Chem.,2012,51: 8317-8321
[8]LIU Hui-Yan(刘会艳),WANG Hai-Ying(王海营),NIU De-Zhong(牛德仲),et al.Chinese J.Inorg.Chem.(无机化学学报),2007,23:611-614
[9]Xu Z,Wang Q,Li H,Meng W,et al.Chem.Commun.,2012, 48:5736-5378
[10]Hou D C,Jiang G Y,Zhao Z.Inorg.Chem.Commun.,2013, 29:148-151
[11]Shi X,Wang X,Li L,et al.Cryst.Growth Des.,2010,10: 2490-2500
[12]Li H X,Wu H Z,ZhangW H,et al.Chem.Commun.,2007, 47:5052-5054
[13]WANG Hui(王慧),GAN Guo-Qing(甘国庆),QU Yang(瞿阳),et al.Chinese J.Inorg.Chem.(无机化学学报),2012, 28:1217-1221
[14]Cheng JJ,Chang Y T,Wu C J,et al.CrystEngComm,2012, 14:537-543
[15]Sheldrick G M.SHELXS-97,Programs for X-ray Crystal Structure Solution,University of Göttingen,Göttingen, Germany,1997.
[16]Sheldrick G M.SHELXL-97,Programs for X-ray Crystal Structure Refinement,University of Göttingen,Göttingen, Germany,1997.
[17]SADABS,Bruker AXS Inc.,Madison,WI,2004.
[18]Xu ZQ,Mao X J,Jia L L,etal.J.Mol.Struct.,2015,1102: 86-90
[19]Li H,Cai H,Xu Z,et al.Inorg.Chem.Commun.,2015,55: 1-4
[20]Li X P,Zhang JY,Pan M,et al.Inorg.Chem.,2007,46: 4617-4625
[21]Jin X H,Sun JK,Cai LX,etal.Chem.Commun.,2011,47: 2667-2669
Syntheses,Crystal Structures and Lum inescent Properties of Two Cd(Ⅱ)Comp lexes Based on 4-(5-(1H-Pyrazol-4-yl)-4H-1,2,4-triazol-3-yl)pyridine
XU Zhou-Qing1WANG Xiao-Ning1LIHui-Jun*,1JIA Lei*,1ZHAOHui-Zi1LIShou-Jie1MA Lu-Fang*,2ZHANG Pei-Ling3
(1Department of Physics and Chemistry,Henan Polytechnic University,Jiaozuo,Henan 454000,China) (2College of Chem istry and Chemical Engineering,Luoyang Normal University,Luoyang,Henan 471022,China) (3School of Electrical Engineering and Automation,Henan Polytechnic University,Jiaozuo,Henan 454000,China)
Two new Cd(Ⅱ)coordination polymers,namely{[Cd2(Hptp)2(OAc)2]·7H2O}n(1)and{[Cd(Hptp)Cl(H2O)]· H2O}n(2),have been obtained through hydrothermal reaction of a new asymmetric rigid triazole derivatives H2ptp (H2ptp=4-(5-(1H-pyrazol-4-yl)-4H-1,2,4-triazol-3-yl)pyridine)and cadmium salts.Complex 1 shows one dimensional(1D)ladder chain.Adjacent chains are further extended to a two dimensional(2D)supramolecular layer viaπ…πstacking interactions.Water chains comprised of double pentanuclear water cluster formed by hydrogen bonds were presented between the two layers.Complex 2 exhibits a unique 2D double layered motif which is further expanded to a 3D supramolecular structure through hydrogen bonding.Furthermore,the photoluminescence properties of these two complexes have been investigated in the solid state.CCDC:1429655, 1;1415769,2.
metal-organic frameworks;photoluminescence;triazole derivative
O614.24+2
A
1001-4861(2016)07-1223-08
10.11862/CJIC.2016.150
2015-12-14。收修改稿日期:2016-05-03。
国家自然科学基金(No.21404033,21401046)、河南省科技攻关项目(No.152102210314)、河南省高等学校重点科研项目(No.16A150010)和河南理工大学博士基金(No.72103/001/103)资助。
*通信联系人。E-mail:lihuijunxgy@hpu.edu.cn,mazhuxp@126.com,jlxj@hpu.edu.cn

