α-Glucosidase inhibitor produced by an endophytic fungus,Xylariaceae sp.QGS 01 from Quercus gilva Blume
Anastasia Wheni Indrianingsih,Sanro Tachiana
a Research Unit for Natural Products Technology,Indonesian Institute of Sciences,Gading,Playen,Gunungkidul,Yogyakarta,55581,Indonesia
b Department of Applied Biosciences,Faculty of Agriculture,Ehime University,3-5-7 Tarumi Matsuyama,Ehime 790-8566,Japan
Abstract Xylariaceae sp.QGS 01,an endophytic fungus isolated from the stem of Quercus gilva Blume showed high α-glucosidase inhibitory activity.α-Glucosidase inhibitor have the role as one of carbohydrate-hydrolyzing enzymes to postpone absorption of glucose in the digestive organs.The α-glucosidase inhibitor constituents were isolated from the ethyl acetate extract of the mycellium of endophytic fungi Xylariaceae sp.QGS 01 using a bioassay-guided fractionation technique.Further separation and purificatio of the active fraction led to the isolation of constituents with strong inhibitory activities against α-glucosidase:8-hydroxy-6,7-dimethoxy-3-methylisocoumarine(1)with inhibitory concentration(IC50)values against α-glucosidase from Saccharomyces cerevisiae of 41.75 μg/mL,while quercetin as the standard had an IC50 value of 4.80 μg/mL.The results of the present study showed that the endophytic fungus Xylariaceae sp.QGS 01 is potentially a rich source of antidiabetic medicine.
Keywords: Endophytic fungi;Quercus qilva Blume;α-Glucosidase inhibitory activity;Xylariaceae sp.;Isocoumarine derivative
1.Introduction
Diabetes mellitus (DM) is a serious global health program which is characterized by high blood glucose levels,which lead to complications such as retinopathy,hypertension,neuropathy and diabetic foot ulcers [1].Type 2 DM caused by defects in insulin secretion or insulin resistance[2]is the most frequently encountered form of DM,accounting for more than 80%of all cases[3].The α-glucosidase enzyme in the intestine is essential for carbohydrate degradation so that the resulted monosaccharides can be absorbed.The inhibition of α-glucosidase enzyme leads to a delay in the digestion ofingested carbohydrates[4].Thus α-glucosidase inhibitors exhibit high promise as therapeutic agents for the treatment of type 2 of DM [5].Most of DM treatments are based on the use of synthetic drugs,which are associated with several side effects [6].Therefore,the development ofinatural compounds as alternatives pharmaceuticals for the treatment of DM without any side effects is urgently needed.Another advantage is that natural compounds may be safely consumed in the daily diet,thereby reducing the risk of DM[7].
Endophytic fungiare the microorganisms that spend all or part of their life cycles within plant tissue without causing harmful effects on the plant [8].Endophytic fungiusually get nutrition and protection from their host plant and promote the growth of the plant by producing certain bioactive substances[9].Endophytic fungiin plants are promising sources of bioactive metabolites.Recent studies show that endophytic fungihave an ability to produce many novel chemicals that could be directly use as drugs or source of bioactive natural products[10].
Several research indicate endophytic fungihave bioactive compounds that could potentially be applied in various applications such as antioxidant[11],antifungal[12],antiviral[13],antibacterial [14]and cyotoxic [15].In our previous study,we found that several bioactive compounds such as catechin,epicatechin,tiliroside,β-sitosterol glucoside and condensed tannins were isolated fromQuercus gilvaBlume andQuercus phillyraeoidesA.Gray [16,17].Catechin and epicatechin isolated fromQ.gilvaBlume were having good antioxidant properties while tiliroside had strong ability as α-glucosidase inhibitor activity;therefore,in this study we conducted isolation of endophytic fungifromQuercus gilvaBlume(Q.gilva).Endophytic fungus QGS 01 fromQ.gilvawas found to have a strong α-glucosidase inhibitory activity.This QGS 01 fungus was identifie as aXylariaceaesp.This present work highlighted the α-glucosidase inhibitory activity of constituents isolated from the mycelium extract ofXylariaceaesp.QGS 01.Extract ofXylariaceaesp.QGS 01 mycelium were obtained using ethyl acetate and the isolation of active constituent was conducted using bioassay-guided fractionation technique.Anin vitroassay of α-glucosidase inhibitory activity was conducted using αglucosidase enzyme obtained fromSaccharomyces cerevisiae(S.cerevisiae) yeast.This assay may be used for preliminary observations in the evaluation of pharmalogical activities and also to verify the medicinal effects of these active constituents isolated from endophytic fungus.
2.Materials and methods
2.1.General instrumentation and chemicals
An analysis using gas chromatography(GC)was conducted on a GC-FID 2014 model (Shimadzu,Japan).The electron ionization mass spectra (EI-MS) ofisolated constituents were recorded on a GC Mass Spectrometer(GC-MS QP 2010 Plus,Shimadzu,Japan).Single crystal analysis ofisolated constituent was recorded on Rigaku Saturn 724 X-Ray Diffractometer.TLC was run on silica gel 60 F254pre-coated plates(Merck 5554)and spots were detected using UV light.
α-Glucosidase [(EC 3.2.1.20)]type I fromS.cerevisiae,p-nitrophenyl α-D-glucopyranoside (p-NPG) and bis(trimethylsilyl) acetamide (BSA) were purchased from Wako Pure Chemicals,Ltd.(Osaka,Japan).Quercetin,palmitic acid,stearic acid,oleic acid,linoleic acid,and linolenic acid were purchased from Sigma-Aldrich Co.,Ltd.(Tokyo,Japan).All solvents used in this study (methanol,ethanol,toluene,pyridine,ethyl acetate,chloroform,hexane,and acetone)were purchased from Wako Pure Chemicals,Ltd.
2.2.Isolation and culture of the endophytic fungus
The stem ofQuercus gilvaBlume was collected from Ehime University Garden,Ehime Prefecture,Japan,in October 2014.Samples were cleaned in tap water and sterilized by consecutive washes in 75% EtOH (1 min),1% NaOCl (2 min),75% EtOH(30 s)and rinsed with sterile distilled H2O three times.The surface sterilized material was cut into 0.4×0.4 cm pieces and the tissues were deposited on a Petri dish containing potato dextrose agar(PDA)medium and incubated at 25°C.The hyphal of was transferred to fresh PDA medium and purifie for three times.
2.3.Molecular identification of fungus
Fungal strains were maintained on PDA and incubated for 7 days.DNA was extracted from these fungibased on the Doyle and Doyle[18]method with a slight modification The extracted DNA was used as a template for PCR to amplify the ITS1-F and ITS4-B regions.Products were then sequenced using two PCR primers and an automated ABI Prism DNA sequence.The result of sequencing was compared with the National Centre for Biotechnology Information(NCBI)GenBank database.A phylogenetic tree was constructed using MEGA software(version 5.2.2).The endophytic fungus was identifie asXylariaceaesp.QGS 01(GenBank accession number:KU764517).
2.4.Fermentation and extraction
The endophytic fungusXylariaceaesp.QGS 01 was inoculated into the Erlenmeyer flask (500 mL)at 25°C for 3 weeks,each flas containing 200 ml potato dextrose broth (PDB).Mycelium and culture broth were separated and extracted with equalvolumeofethylacetateatroomtemperature.Theextractof endophytic fungus was condensed in a rotating evaporator under reduced pressure.The obtained crude extracts of the endophytic fungus(mycelium and culture broth extracts)were screened for the α-glucosidase activity and had the inhibitory concentration(IC50)of 12.50 and 21.70 μg/mL,respectively.The results indicated that the mycelium extract ofXylariaceaesp.QGS 01 had higher activity;therefore further research was carried out on the fungal strainXylariaceaesp.QGS 01.Large scale fermentation ofXylariaceaesp.QGS 01 was conducted with total volume of 30 L which were resulting of 4.12 g of mycelium extract and 1.92 g of culture broth extract.
2.5.Isolation procedures of active constituents
The ethyl acetate extract(4.12 g)was separated using silica column chromatography using solvents with increasing polarity fromn-hexane,chloroform,ethylacetate(EtOAc),andmethanol(MeOH)toobtain6fractions(F1-F6).Allfractions(F1-F6)were screened for α-glucosidase inhibitory activity (Table1),with the F1 fraction exhibiting the highest activity.The active F1 fraction was separated again by silica column chromatography to get 4 fractions(F1.1-F1.4).Compound 1(5.1 mg)was isolated from F1.1 as a colorless needle crystal by using eluent hexaneethyl acetate(4:1)and further purifie by recrystallization using chloroform.
2.6.Single crystal analysis of compound 1
A block of crystal was mounted on a glass fibe.All measurements were made on a Rigaku Saturn 724 X-Ray diffractometer using multi-layer mirror monochromated Mo-Kα radiation.The crystal to detector distance was 44.96 mm.The data were collected at a temperature of-172±1oC to a maximum 2θ value of 63.0o.A total of 1440 oscillation images were collected.Cell constants and an orientation matrix for data collection corresponded to a primitive orthorhombic cell with dimensions:a=7.086(3)Å,b=13.885(5)Å,c=21.390(8)Å and V=2104.5(13)Å3.The data were corrected for Lorentz and polarization effects.The structure was solved by direct methods [19]and expanded using Fourier techniques.The values used for the mass attenuation coefficient were those of Creagh and Hubbell,[20].All calculations were performed using the Crystal Structure crystallographic software package except for refinement which was performed using SHELXL Version 2014.
2.7.GC/GC–MS analysis of fatty acid mixture
Fatty acids mixture was isolated from then-hexane fraction using alkali solution.The methylation of fatty acids was conducted using methanol acidifie with sulphuric acid at 64°C for 30 min to form Fatty Acid Methyl Ester (FAME)for determination of fatty acid content.Chemical analysis was conducted using gas chromatography coupled with mass spectrometry (GC-MS QP-2010) equipped with a SPB-50 column(30 m×0.25 mm ID,0.25 μm fil thickness).The analysis was performed according to Yamamoto et al.,2008 [21];column temperature,235°C,carrier gas helium linear gas velocity,30 cm/sec;split ratio,1/30,ion source temperature,200°C and interface temperature,280°C.The identificatio of chemicals was performed in comparison with database (NIST08 library)and confirme using authentic standard samples.
2.8.Alpha-glucosidase inhibitory assay
The inhibitory activity of α-glucosidase was evaluated as reported by Kim et al.,2005 [22].Samples were dissolved in dimethyl sulfoxide at various concentrations (10 μl) and then treated withp-NPG (250 μL,3 mmol/L) in phosphate buffer solution (490 μL,100 mmol/L,pH 7).The solution was preincubated at 37°C for 5 min.Two hundred and fift microliters of α-glucosidase(0.065 U/mL)was then added and the reaction continued for 15 min.The reaction was stopped by the addition of 1 mL of 0.2 mol/L Na2CO3.The mixtures were measured at 400 nm using a UV-vis spectrophotometer.The percentage inhibition of α-glucosidase inhibitory activity was calculated using Eq.(1).

whereA0istheabsorbanceofthecontrolandA1isabsorbancein the presence of the sample.The inhibitory concentration(IC50)of the samples was calculated using a regression analysis from the graph plotting scavenging activity against concentration.All experiments were carried out in triplicate and the results are expressed as the mean±SD of three determinations.
2.9.Statistical analysis
All assays were conducted in triplicate.Statistical analyses were performed with SPSS 16.0 for an analysis ofivariance(ANOVA) followed by Duncan’s test.Differences at p <0.05 were considered significant

Fig.1. Xylariaceae sp.QGS 01 grown on PDA medium.
3.Results and discussion
3.1.Identification of endophytic fungi
Fig.1.shows the endophytic fungus of QGS 01 isolated from the stem ofQ.gilva.
According to a molecular gene analysis strain QGS 01 was similar(99-100%)toXylariaceaesp.(GenBank accession number:KU764517).A phylogenetic tree ofXylariaceaesp.QGS 01 is shown in Fig.2.
The Xylariaceae is well known ascomycete family that has wide biological diversity and has been found to be the source of many metabolites with novel structures.Several alkaloids and a new pyridine derivative had been isolated from the culture broth of a marine-derived fungusXylariaceaesp.SCSGAF0086[23].This is the firs report of aXylariaceaesp.isolated fromQ.gilva.
3.2.Isolation of α-glucosidase inhibitory active compounds
Between the mycelium and the culture broth extracts,the higher α-glucosidase inhibitory activity was the mycelium of QGS 01 fungus(IC50=12.50 μg/mL),therefore,the mycelium of QGS 01 fungus was further separated to obtain the most active constituents on α-glucosidase inhibitory activity.The ethyl acetate extract of QGS 01 fungus mycelium was fractionated using silica gel chromatography to obtain the F1 fraction as the most active fraction (Table1).The active F1 fraction was separated again by silica column chromatography to get 4 fractions (F1.1-F1.4).Compound 1 was isolated from F1.1(IC50=8.25 μg/mL)as the most active constituents.Its spectral data were compared with reported data and the structure was identifie as isocoumarin derivative(1).

Fig.2.Phylogenetic tree for endophytic fungus QGS 01 based on ITS sequences.
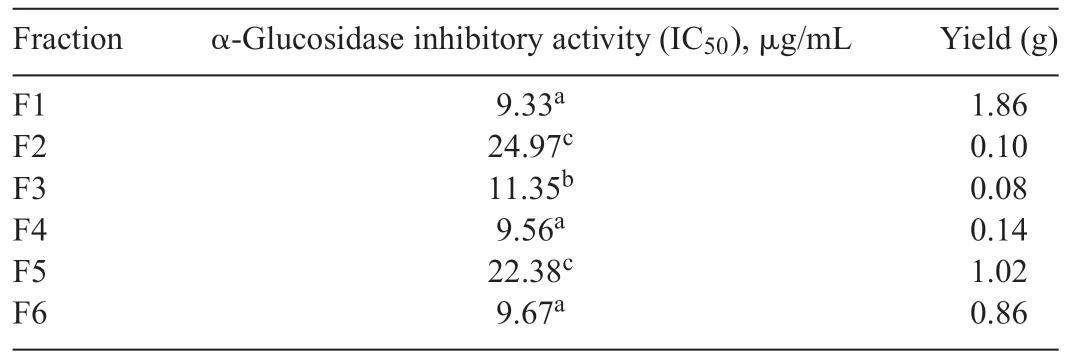
Table1 α-Glucosidase inhibitory activity of Fraction 1 to Fraction 6 of mycelium extract from Xylariaceae sp.QGS 01.
Compound 1:EI-MS,m/z(I,%):236 [M]+(100),221[M-CH3]+(96),193 [M-CH3-CO]+(70);m.p:196-198°C.Crystal data:C12H12O5,Mr=236.19,a colorless needle crystal,a primitive orthorhombic,approximate crystal dimensions 0.100×0.020×0.010 mm3.Space group,a=7.086(3)Å,b=13.885(5)Å,c=21.390(8)Å and V=2104.5(13) Å.Based on this information and search of the literature [24],compound 1 was identifie as 8-hydroxy-6,7-dimethoxy-3-methylisocoumarine.The X-ray diffractogram of 1 is shown in Fig.3.
8-Hydroxy-6,7-dimethoxy-3-methylisocoumarine (6-Omethylreticulol) had been reported as a metabolite of fungusStreptomyces mobaraensis[25]and liverwortWettsteinia schusterana[26].Reticulol and its derivatives had been proved to be a potential antitumor agent.Reticulol showed antitumor activity against melanoma B16F10,it had the ability to inhibit topoisomerase I(Topo I),an enzyme involved in the melanoma metastatis mechanism [27].Dihydroisocoumarins,secondary metabolites produced by Xylariaceae family have also been isolated and identifie [28].However,this is the firs report about compound 1 isolated fromXylariaceaesp.QGS 01 having antidiabetic activity on inhibiting α-glucosidase enzyme.There has been no report yet about isocoumarin derivative isolated fromQ.gilvaBlume as a host plant ofXylariaceae spQGS01.

Table2 α-Glucosidase inhibitory activity of constituents from Xylariaceae sp.QGS 01.
Fatty acid mixture was major constituents ofXylariaceaesp.QGS 01.Analysis using GC-MS and comparison with authentic standards showed that fatty acid mixture had two saturated fatty acids,palmitic acid (C16:0) and stearic acid (C18:0) and three unsaturated fatty acids,namely oleic acid(C18:1),linoleic acid (C18:2) and linolenic acid (C18:3) (Fig.4).The major constituent in fatty acids mixture were linoleic acid (33.7%)followed by oleic acid (29.8%),palmitic acid (24.1%),stearic acid(7.5%)and linolenic acid(3.6%).
ThemassspectraofmethylatedfattyacidsareshowninFig.5.
The α-glucosidase activity of compound 1,fatty acid mixture,and standard are shown in Table2.This resulted suggested that compound 1 was confirme as the most active constituent as α-glucosidase inhibitor with IC50of 41.75 μg/mL,meanwhile the fatty acid mixture from F1.1 also exhibit strong activity with IC50of 5.95 μg/mL.
These results indicated that fatty acids with a double bond showed stronger inhibition than fatty acids without double bond.The chemical structure of the isolated fatty acids fractions are showninFig.6.Itissuggestedthattheexistenceofadoublebond was important factor ofinhibition α-glucosidase acitivity[29].Other fatty acids were also reported to have α-glucosidase activity,such as fatty acids from endophytic fungusColletotrichumsp.[30],fatty acids from sea cucumber[31],and fatty acids from wheat germ[32].

Fig.3.X-ray structure of compound 1.

Fig.4.Gas chromatogram profil of the standard(a)and isolated fatty acids(b):(1)palmitic acid methyl ester,(2)stearic acid methyl ester,(3)oleic acid methyl ester,(4)linoleic acid methyl ester,(5)linolenic acid methyl ester.
α-Glucosidase inhibitor assay were performed as antidiabetic properties capability in the terms of the ability of the constituents to inhibit an intestinal carbohydrate-digesting enzyme,namely α-glucosidase.The α-glucosidase inhibitors delay intestinal carbohydrate absorption by blocking the activity of glucosidase enzyme,and consequently reduce the concentration of postprandial blood glucose and slow down the rise in blood sugar level;therefore,they play a significan role as chemotherapeutic agents for non-insulin-dependent DM.Acarbose,miglitol,and voglibose are some examples of α-glucosidase inhibitors presently being used in the primary treatment of type 2 DM [33].However,since the currently available α-glucosidase inhibitors for clinical use are causing several side effects,safer antidiabetic agent are needed.Effective and safe α-glucosidase inhibitors from nature have been sought in the development of physiological functional food or constituents for antidiabetic therapy[34].Our study showed that constituents from ethyl acetate extract of Xylariaceae sp.QGS 01 are potent inhibitor of αglucosidase activity,and therefore suggests that endophytic fungus Xylariaceae sp.QGS 01 as a rich source ofinatural antidiabetic medicine.This report is the firs report about secondary metabolites (isocoumarine derivative) produced by Xylariaceae sp.having antidiabetic activity on inhibiting αglucosidase enzyme.
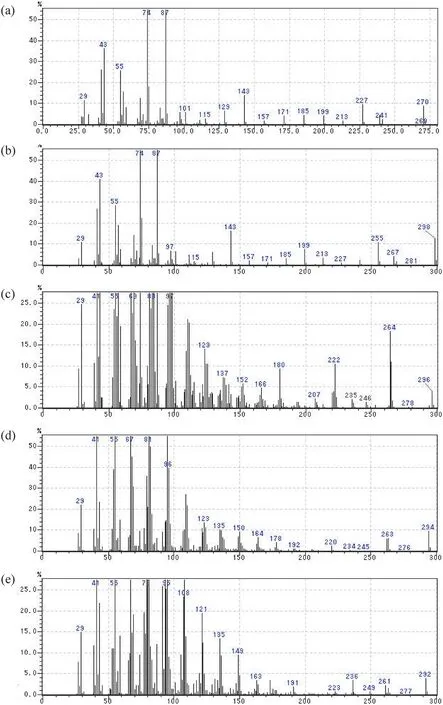
Fig.5.MS spectra of fatty acid derivatives(a)Palmitic acid methyl ester(m/z 270),(b)Stearic acid methyl ester(m/z 298),(c)Oleic acid methyl ester(m/z 296),(d)Linoleic acid methyl ester(m/z 294),and(e)Linolenic acid methyl ester(m/z 292).
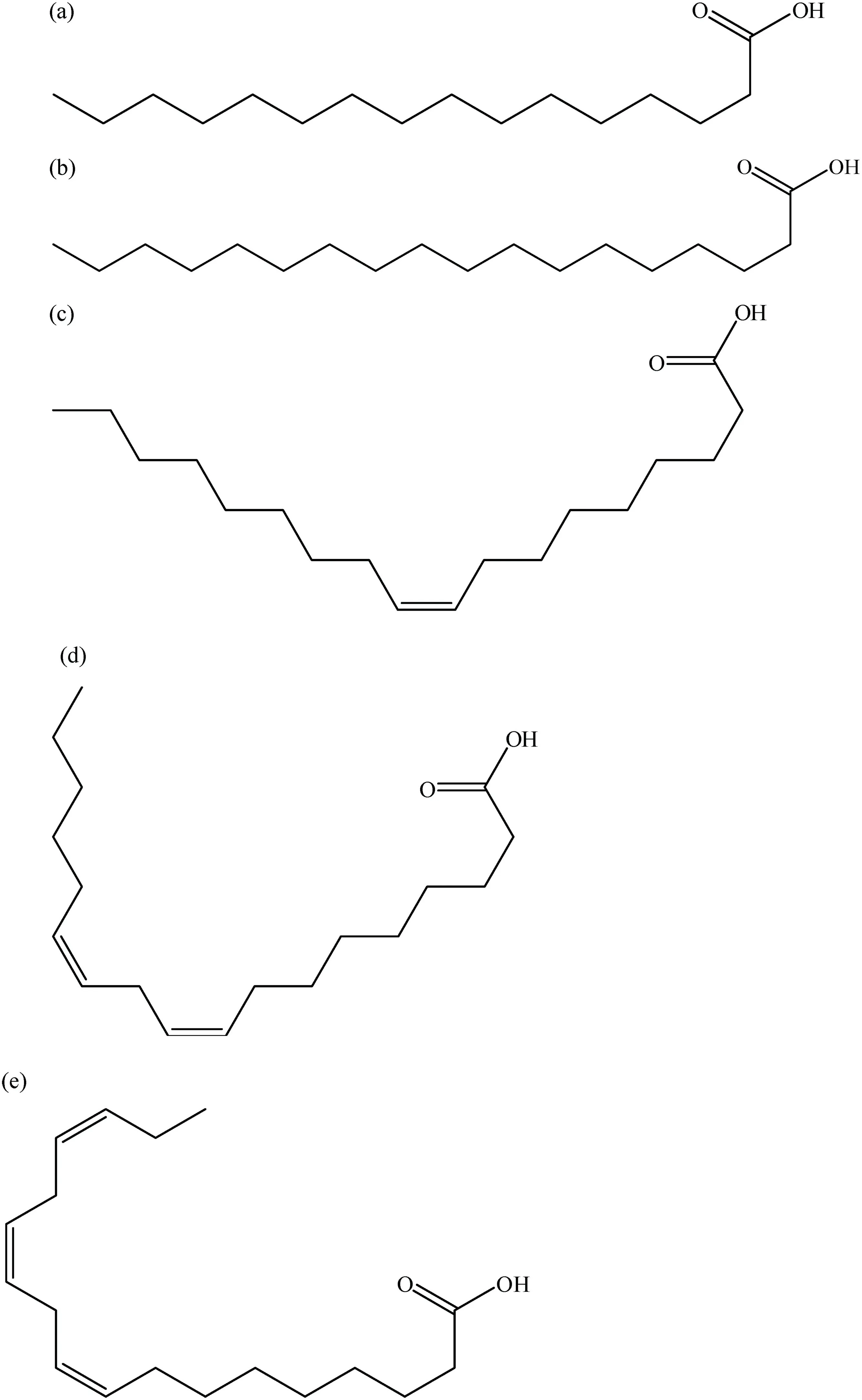
Fig.6.The chemical structure of the isolated fatty acids fractions;saturated fatty acids:(a)palmitic acid(C16:0),(b)stearic acid(C18:0)and unsaturated fatty acids,(c)oleic acid(C18:1),(d)linoleic acid(C18:2),(e)linolenic acid(C18:3).
4.Conclusion
Endophytic fungiXylariaceaesp.QGS 01 was isolated from the stem ofQuercus gilvaBlume and showed high activity of α-glucosidase inhibitory activity.One active constituent:8-hydroxy-6,7-dimethoxy-3-methylisocoumarine(1)was isolated from endophytic fungiXylariaceaesp.QGS 01.Compound 1 had inhibitory concentration(IC50)values against α-glucosidase fromSaccharomyces cerevisiaeof 41.75 μg/mL.
Acknowledgement
The authors are deeply thankful to Dr.Shigeki Mori of the Integrated Center for Sciences,Ehime University for obtaining the data of X-ray crystallography.
- 食品科学与人类健康(英文)的其它文章
- PCR-based methodologies for detection and characterization of Listeria monocytogenes and Listeria ivanovii in foods and environmental sources
- Potential antioxidant and cytoprotective effects of essential oil extracted from Cymbopogon citratus on OxLDL and H2O2 LDL induced Human Peripheral Blood Mononuclear Cells(PBMC)
- Extraordinary tunable dynam ic range of electrochem ical aptasensor for accurate detection of ochratoxin A in food samples
- Dietary fenugreek(Trigonella foenum-graecum)seeds and garlic(Allium sativum)alleviates oxidative stress in experimental myocardial infarction
- About the Beijing Academy of Food Sciences
- GUIDE FOR AUTHORS

