新型去氢表雄酮C3酯类衍生物的合成及体外抗肿瘤活性评价
刘荣春, 任 博, 郭 聪, 汤江江
(西北农林科技大学 化学与药学院,陕西 杨凌 712100)
甾体是一类天然化合物,具有不同的生物学特性[1-4],并且对各种疾病都有一定的治疗作用,包括:肾上腺功能不全[5]、自身免疫性疾病[6]、心血管疾病[7]、真菌和微生物感染[8]。此外,许多不同的甾体衍生物均被报道具有抗癌作用[9-12]。去氢表雄酮(DHEA)也称为脱氢表雄酮,是人类身上最丰富的循环类固醇激素[13],DHEA及其硫酸酯还具有多种潜在的生物效应,如抗衰老作用[14-15]、抗炎[16-17]、免疫调节[18]、抗病毒[19]、抗抑郁药[20]和抗癌作用[21]。

Scheme 1
众所周知,具有共轭体系和负电子特性的芳环被广泛用于先导化合物的结构修饰,它可以与目标蛋白发生π-π相互作用并增加与目标的亲和力。研究发现芳香环中不同类型的取代基与电荷分布、化合物的性质和抗肿瘤效力密切相关[22-23],尤其是卤素基团,据报道它们显著增强了生物活性并改善母体的代谢稳定性[24-25]。Vosooghi等[26]已经证明在去氢表雄酮的C16位置进行修饰可以增强其抗肿瘤活性,Garrido等[27]研究表明,在去氢表雄酮的C3位置酯化后,可改进其亲脂性并表现出更强的穿透细胞膜能力,从而显示出更强的生物学效应。
基于此,合成了22个新的去氢表雄酮酯类衍生物,包括11个去氢表雄酮C3的酯类衍生物和11个(E)-16-(2-氯亚苄基)的C3酯类衍生物。进一步研究的化合物的体外抗肿瘤活性,并发现一些具有更好抗肿瘤活性的甾体化合物,为新型甾体抗肿瘤药物的研发提供参考。
1 实验部分
1.1 仪器与试剂
X-4型熔点仪;Bruker AV-400/500 MHz型超导核磁共振仪(TMS为内标); LCQ Advantage MAX 型质谱仪。
所用溶剂均为分析纯。
1.2 合成
(1)3的合成
依次称取12.0 g(6.94 mmol),二氯苯甲醛1.1 g(7.64 mmol)和NaOH 3.5 g(87.5 mmol)溶解于40 mL甲醇中,室温搅拌1 h(TLC检测)。将反应混合物倒入冰水中,过滤最终的沉淀物。用冷水洗涤,减压干燥,并在甲醇中结晶得白色固体3。
(2)2a~2k,4a~2k的合成
依次称取10.29 g(1 mmol)、 EDCI 0.58 g(3 mmol)、 DMAP 0.06 g(0.5 mmol)和Et3N 0.3 g(3 mmol)溶解于5 mL无水二氯甲烷后,再加入相应的酸(2 mmol),室温搅拌4 h(TLC检测)。减压除去溶剂,残留物经硅胶柱层析(洗脱剂:石油醚/乙酸乙酯=20/1,V/V)纯化得相应的酯2a~2l;使用3作为试剂,通过相同的方法制备4a~4k。
2a: 白色固体,收率90%, m.p.200~202 ℃;1H NMR(500 MHz, CDCl3)δ: 7.67(d,J=16.0 Hz, 1H), 7.55~7.49(m, 2H), 7.38(s, 3H), 6.42(d,J=16.0 Hz, 1H), 5.48~5.40(m, 1H), 4.75(dt,J=11.1 Hz, 6.1 Hz, 1H), 2.51~2.36(m, 3H), 2.09(dt,J=19.0 Hz, 9.1 Hz, 2H), 2.00~1.83(m, 4H), 1.66(m, 5H), 1.58~1.44(m, 2H), 1.35~1.25(m, 2H), 1.24~1.17(m, 1H), 1.08(s, 3H), 0.89(s, 3H);13C NMR(101 MHz, CDCl3)δ: 221.25, 166.50, 144.64, 140.04, 134.56, 130.33, 128.99, 128.16, 122.07, 118.67, 73.94, 51.80, 50.24, 47.65, 38.30, 37.08, 36.88, 35.98, 31.58, 31.51, 30.91, 27.93, 22.01, 20.45, 19.51, 13.68; ESI-MSm/z: Calcd for C28H34O3{[2M+Na]+} 859.50, found 859.01。
2b: 白色固体,收率90%, m.p.215~217 ℃;1H NMR(500 MHz, CDCl3)δ: 7.65(d,J=16.4 Hz, 1H), 7.42(d,J=8.0 Hz, 2H), 7.19(d,J=7.9 Hz, 2H), 6.38(d,J=16.0 Hz, 1H), 5.43(s, 1H), 4.74(dq,J=10.9 Hz, 5.6 Hz, 5.1 Hz, 1H), 2.51~2.41(m, 2H), 2.37(s, 3H), 2.15~2.06(m, 2H), 1.94(m,3H), 1.88~1.83(m, 1H), 1.68 m, 5H), 1.58~1.45(m, 2H), 1.35~1.28(m, 2H), 1.27~1.17(m, 2H), 1.08(s, 3H), 0.89(s, 3H);13C NMR(125 MHz, CDCl3)δ: 166.69, 144.65, 140.73, 140.12, 131.87, 129.73, 128.16, 122.03, 117.61, 73.84, 51.84, 50.29, 47.66, 38.34, 37.11, 36.91, 35.99, 31.62, 31.55, 30.93, 27.96, 22.02, 21.60, 20.47, 19.52, 13.69; ESI-MSm/z: Calcd for C29H36O3{[2M+Na]+} 887.54, found 887.78。
2c: 白色固体,收率50%, m.p.203~205 ℃;1H NMR(500 MHz, CDCl3)δ: 7.62(d,J=15.9 Hz, 1H), 7.47(d,J=8.7 Hz, 2H), 6.89(d,J=8.7 Hz, 2H), 6.29(d,J=15.9 Hz, 1H), 5.43(d,J=4.9 Hz, 1H), 4.73(tt,J=10.9 Hz, 4.9 Hz, 1H), 3.83(s, 3H), 2.44(m, 3H), 2.14~2.03(m, 2H), 1.93(m, 3H), 1.87~1.82(m, 1H), 1.71~1.63(m, 5H), 1.58~1.45(m, 2H), 1.34~1.15(m, 4H), 1.07(s, 3H), 0.88(s, 3H);13C NMR(125 MHz, CDCl3)δ: 166.83, 161.45, 144.31, 140.15, 129.80, 127.35, 122.00, 116.19, 114.44, 73.74, 55.49, 51.83, 50.29, 47.65, 38.35, 37.11, 36.90, 35.97, 31.61, 31.54, 30.92, 27.97, 22.01, 20.46, 19.50, 13.67; ESI-MSm/z: Calcd for C29H36O4{[2M+Na]+} 919.52, found 919.75。
2d: 白色固体,收率85%, m.p.158~160 ℃;1H NMR(500 MHz, CDCl3)δ: 7.58(d,J=16.0 Hz, 1H), 7.34~7.29(m, 1H), 7.24(d,J=7.1 Hz, 1H), 7.18(d,J=9.6 Hz, 1H), 7.07~7.01(m, 1H), 6.38(d,J=16.0 Hz, 1H), 5.41(d,J=5.0 Hz, 1H), 4.76~4.67(m, 1H), 2.47~2.33(m, 3H), 2.13~2.01(m, 2H), 1.96~1.80(m, 4H), 1.65(m 5H), 1.56~1.42(m, 2H), 1.27(m, 2H), 1.16(m, 1H), 1.04(s, 3H), 0.86(s, 3H);13C NMR(125 MHz, CDCl3)δ: 166.01, 162.05, 143.11, 139.91, 136.75, 130.48, 124.07, 122.05, 120.06, 117.17, 114.37, 74.07, 51.74, 50.19, 47.56, 38.18, 36.98, 36.80, 35.87, 31.51, 31.45, 30.83, 27.82, 21.91, 20.37, 19.40, 13.58; ESI-MSm/z: Calcd for C28H33O3F {[2M+Na]+} 895.52, found 895.39。
2e: 白色固体,收率85%, m.p.208~210 ℃;1H NMR(500 MHz, CDCl3)δ: 7.63(d,J=16.0 Hz, 1H), 7.53~7.47(m, 3H), 7.07(t,J=8.3 Hz, 2H), 6.34(d,J=16.0 Hz, 1H), 5.44(s, 1H), 4.74(dt,J=10.9 Hz, 5.8 Hz, 1H), 2.44(m, 3H), 2.09(dt,J=18.9 Hz, 9.1 Hz, 2H), 1.99~1.83(m, 4H), 1.66(m, 5H), 1.51(m, 2H), 1.30(m, 2H), 1.25~1.16(m, 1H), 1.07(s, 3H), 0.89(s, 3H);13C NMR(125 MHz, CDCl3)δ: 166.36, 163.00, 143.32, 140.09, 130.92, 130.05, 129.98, 122.11, 118.55, 116.24, 116.07, 74.04, 51.88, 50.34, 47.66, 38.34, 37.12, 36.92, 35.97, 31.65, 31.58, 30.94, 27.96, 22.02, 20.49, 19.51, 13.69; ESI-MSm/z: Calcd for C28H33O3F {[2M+Na]+} 895.48, found 895.82。
2f: 白色固体,收率82%, m.p.158~160 ℃;1H NMR(500 MHz, CDCl3)δ: 7.70~7.59(m, 5H), 6.49(d,J=16.0 Hz, 1H), 5.48~5.40(m, 1H), 4.76(dt,J=10.8 Hz, 5.6 Hz, 1H), 2.50~2.37(m, 3H), 2.09(dt,J=18.9 Hz, 9.1 Hz, 2H), 1.99~1.82(m, 4H), 1.73~1.62(m, 4H), 1.59~1.45(m, 2H), 1.25(m, 3H), 1.08(s, 3H), 0.89(s, 3H);13C NMR(125 MHz, CDCl3)δ: 165.90, 142.74, 139.99, 138.04, 131.98, 128.28, 126.00, 125.97, 125.05, 122.22, 121.38, 74.35, 51.88, 50.34, 47.66, 38.30, 37.10, 36.92, 35.97, 31.65, 31.58, 30.95, 27.94, 22.02, 20.49, 19.50, 13.69; ESI-MSm/z: Calcd for C29H33O3F3{[2M+Na]+}995.48, found 995.17。
2g: 白色固体,收率85%, m.p.182~184 ℃;1H NMR(500 MHz, CDCl3)δ: 8.07(d,J=16.0 Hz, 1H), 7.61(dd,J=7.5 Hz, 1.5 Hz, 1H), 7.43~7.39(m, 1H), 7.32~7.25(m, 2H), 6.42(d,J=16.0 Hz, 1H), 5.44(d,J=4.8 Hz, 1H), 4.76(tt,J=10.9 Hz, 5.0 Hz, 1H), 2.45(m, 3H), 2.09(dt,J=19.1 Hz, 9.0 Hz, 2H), 2.00~1.89(m, 3H), 1.86(d,J=12.8 Hz, 1H), 1.67(m, 5H), 1.51(m, 2H), 1.30(m, 2H), 1.21(m, 1H), 1.08(s, 3H), 0.89(s, 3H);13C NMR(125 MHz, CDCl3)δ: 166.11, 140.53, 140.15, 135.15, 133.02, 131.19, 130.40, 127.86, 127.29, 122.22, 121.51, 74.31, 51.95, 50.39, 47.76, 38.38, 37.19, 37.01, 36.08, 31.72, 31.65, 31.03, 28.02, 22.12, 20.57, 19.62, 13.79; ESI-MSm/z: Calcd for C28H33O3Cl{[2M+Na]+} 927.42, found 927.83。
2h: 白色固体,收率85%, m.p.173~175 ℃;1H NMR(500 MHz, CDCl3)δ: 7.59(d,J=16.0 Hz, 1H), 7.50(s, 1H), 7.38(d,J=7.2 Hz, 1H), 7.36~7.29(m, 2H), 6.41(d,J=16.0 Hz, 1H), 5.43(d,J=5.0 Hz, 1H), 4.74(tt,J=10.6 Hz, 5.0 Hz, 1H), 2.44(m, 3H), 2.08(m, 2H), 1.99~1.83(m, 4H), 1.72~1.62(m, 5H), 1.59~1.44(m, 2H), 1.29(m, 2H), 1.25~1.15(m, 1H), 1.07(s, 3H), 0.89(s, 3H);13C NMR(125 MHz, CDCl3)δ: 166.06, 142.99, 139.99, 136.45, 135.03, 130.25, 130.18, 127.90, 126.33, 122.15, 120.23, 74.18, 51.84, 50.29, 47.65, 38.28, 37.08, 36.89, 35.97, 31.61, 31.54, 30.92, 27.91, 22.01, 20.46, 19.50, 13.68; ESI-MSm/z: Calcd for C28H33O3Cl{[2M+Na]+}927.42, found 927.91。
2i: 白色固体,收率85%, m.p.185~187 ℃;1H NMR(500 MHz, CDCl3)δ: 7.62(d,J=16.0 Hz, 1H), 7.45(d,J=8.5 Hz, 2H), 7.36(d,J=8.5 Hz, 2H), 6.39(d,J=16.0 Hz, 1H), 5.44(d,J=5.0 Hz, 1H), 4.79~4.71(m, 1H), 2.50~2.37(m, 3H), 2.10(dt,J=19.0 Hz, 9.1 Hz, 2H), 1.94(m, 3H), 1.86(m, 1H), 1.73~1.63(m, 5H), 1.60~1.53(m, 1H), 1.49(m, 1H), 1.31(m, 2H), 1.25~1.16(m, 1H), 1.08(s, 3H), 0.89(s, 3H);13C NMR(125 MHz, CDCl3)δ: 166.49, 143.43, 140.27, 136.48, 133.37, 129.57, 129.54, 122.39, 119.59, 74.36, 52.09, 50.54, 47.91, 38.55, 37.34, 37.15, 36.22, 31.86, 31.80, 31.18, 28.18, 22.27, 20.72, 19.75, 13.93; ESI-MSm/z: Calcd for C28H33O3Cl{ [2M+Na]+}927.42, found 927.54。
2j: 白色固体,收率83%, m.p.191~193 ℃;1H NMR(500 MHz, CDCl3)δ: 7.59(d,J=16.0 Hz, 1H), 7.51(d,J=8.4 Hz, 2H), 7.38(d,J=8.4 Hz,2H), 6.40(d,J=16.0 Hz, 1H), 5.43(d,J=5.0 Hz, 1H), 4.74(tt,J=10.6 Hz, 5.0 Hz, 1H), 2.50~2.35(m, 3H), 2.09(dt,J=19.1 Hz, 9.1 Hz, 2H), 1.99~1.82(m, 4H), 1.72~1.64(m, 4H), 1.52(m, 2H), 1.35~1.23(m, 3H), 1.23~1.15(m, 1H), 1.07(s, 3H), 0.89(s, 3H);13C NMR(125 MHz, CDCl3)δ: 166.22, 143.24, 140.01, 133.54, 132.26(2C), 129.54(2C), 124.57, 122.14, 119.45, 74.13, 51.84, 50.29, 47.65, 38.30, 37.09, 36.90, 35.97, 31.61, 31.55, 30.93, 27.93, 22.02, 20.47, 19.50, 13.68; ESI-MSm/z: Calcd for C28H33O3Br{ [2M+Na]+}1015.32, found 1015.56。
2k: 白色固体,收率70%, m.p.149~151 ℃;1H NMR(500 MHz, CDCl3)δ: 7.31~7.26(m, 2H), 7.20(d,J=6.6 Hz, 3H), 5.40(d,J=5.1 Hz, 1H), 4.61(tt,J=10.9 Hz, 5.3 Hz, 1H), 2.95(t,J=7.8 Hz, 2H), 2.61(t,J=7.8 Hz, 2H), 2.46(dd,J=19.3 Hz, 8.7 Hz, 1H), 2.35~2.24(m, 2H), 2.14~2.04(m, 2H), 1.95(m, 1H), 1.89~1.80(m, 3H), 1.70~1.59(m, 4H), 1.55(m, 2H), 1.48(m, 1H), 1.29(m, 3H), 1.18~1.10(m, 1H), 1.04(s, 3H), 0.88(s, 3H);13C NMR(125 MHz, CDCl3)δ: 172.44, 140.69, 140.06, 128.57(2C), 128.45(2C), 126.34, 121.99, 73.89, 51.84, 50.27, 47.65, 38.18, 37.06, 36.87, 36.34, 35.98, 31.61, 31.55, 31.18, 30.91, 27.81, 22.02, 20.46, 19.48, 13.68; ESI-MSm/z: Calcd for C28H36O3{[2M+Na]+}863.54, found 863.01。
4a: 白色固体,收率85%, m.p.205~207 ℃;1H NMR(500 MHz, CDCl3)δ: 7.78(s, 1H), 7.67(d,J=16.0 Hz, 1H), 7.56~7.49(m, 3H), 7.45~7.41(m, 1H), 7.39~7.35(m, 3H), 7.33~7.27(m, 2H), 6.42(d,J=16.0 Hz, 1H), 5.43(d,J=3.9 Hz, 1H), 4.75(tt,J=10.5 Hz, 4.8 Hz, 1H), 2.75(dd,J=15.9 Hz, 5.7 Hz, 1H), 2.48~2.37(m, 3H), 2.18~2.11(m, 1H), 2.03~1.90(m, 3H), 1.82~1.53(m, 5H), 1.48~1.32(m, 2H), 1.27~1.17(m, 2H), 1.11(s, 3H), 1.00(s, 3H);13C NMR(125 MHz, CDCl3)δ: 208.78, 166.37, 144.55, 140.10, 138.17, 135.77, 134.50, 133.82, 130.24, 130.06, 129.91, 129.22, 128.90, 128.07, 126.60, 121.82, 118.61, 73.84, 50.23, 49.74, 47.54, 38.24, 36.94, 36.88, 31.56, 31.19, 30.95, 29.18, 27.85, 20.40, 19.47, 14.21; ESI-MSm/z: Calcd for C35H37O3Cl{[2M+Na]+}1103.48, found 1103.86。
4b: 白色固体,收率85%, m.p.197~199 ℃;1H NMR(500 MHz, CDCl3)δ: 7.78(s, 1H), 7.65(d,J=16.0 Hz, 1H), 7.56~7.51(m, 1H), 7.42(t,J=8.6 Hz, 3H), 7.30(q,J=6.3 Hz, 5.4 Hz, 2H), 7.18(d,J=7.8 Hz, 2H), 6.38(d,J=16.0 Hz, 1H), 5.43(d,J=4.0 Hz, 1H), 4.74(tt,J=10.5 Hz, 4.8 Hz, 1H), 2.75(dd,J=15.9 Hz, 5.9 Hz, 1H), 2.48~2.38(m, 3H), 2.36(s, 3H), 2.19~2.10(m, 1H), 2.03~1.90(m, 3H), 1.81~1.53(m, 5H), 1.48~1.32(m, 2H), 1.27~1.16(m, 2H), 1.11(s, 3H), 1.00(s, 3H);13C NMR(125 MHz,CDCl3)δ: 208.86, 166.64, 144.63, 140.71, 140.21, 138.25, 135.84, 133.89, 131.84, 130.13, 129.98, 129.71, 129.29, 128.13, 126.67, 121.85, 117.57, 73.79, 50.31, 49.81, 47.61, 38.32, 37.02, 36.95, 31.63, 31.26, 31.02, 29.25, 27.93, 21.57, 20.46, 19.54, 14.28; ESI-MSm/z: Calcd for C36H39O3Cl{[2M+Na]+}1131.52, found 1131.73。
4c: 白色固体,收率50%, m.p.209~211 ℃;1H NMR(500 MHz, CDCl3)δ: 7.78(s, 1H), 7.63(d,J=15.9 Hz, 1H), 7.56~7.51(m, 1H), 7.51~7.41(m, 3H), 7.29(dt,J=6.2 Hz, 2.8 Hz, 2H), 6.90(d,J=8.7 Hz, 2H), 6.29(d,J=15.9 Hz, 1H), 5.43(d,J=4.6 Hz, 1H), 4.74(dt,J=11.1 Hz, 5.9 Hz, 1H), 3.83(s, 3H), 2.75(dd,J=15.9 Hz, 5.3 Hz, 1H), 2.47~2.35(m, 3H), 2.20~2.11(m, 1H), 2.03~1.89(m, 3H), 1.84~1.53(m, 5H), 1.48~1.33(m, 2H), 1.28~1.17(m, 2H), 1.11(s, 3H), 1.00(s, 3H);13C NMR(125 MHz, CDCl3)δ: 208.94, 166.83, 161.46, 144.33, 140.30, 138.29, 135.89, 133.95, 130.17, 130.16, 130.00, 129.81, 129.36, 127.36, 126.69, 121.85, 116.20, 114.45, 73.74, 55.51, 50.36, 49.85, 47.66, 38.38, 37.07, 37.00, 31.67, 31.31, 31.06, 29.29, 27.98, 20.50, 19.57, 14.31; ESI-MSm/z: Calcd for C36H39O4Cl{[2M+Na]+}1163.50, found:1163.91。
4d: 白色固体,收率75%, m.p.203~205 ℃;1H NMR(500 MHz, CDCl3)δ: 7.78(s, 1H), 7.61(d,J=16.0 Hz, 1H), 7.56~7.50(m, 1H), 7.45~7.41(m, 1H), 7.37~7.32(m, 1H), 7.31~7.26(m, 3H), 7.21(d,J=9.5 Hz, 1H), 7.07(dt,J=8.0 Hz, 4.1 Hz, 1H), 6.41(d,J=16.0 Hz, 1H), 5.43(d,J=3.9 Hz, 1H), 4.74(tt,J=10.4 Hz, 4.8 Hz, 1H), 2.75(dd,J=15.8 Hz, 5.1 Hz, 1H), 2.47~2.37(m, 3H), 2.14(d,J=17.0 Hz, 1H), 2.03~1.89(m, 3H), 1.81~1.74(m, 1H), 1.74~1.63(m, 3H), 1.62~1.53(m, 1H), 1.47~1.33(m, 2H), 1.26~1.18(m, 2H), 1.11(s, 3H), 1.00(s, 3H);13C NMR(125 MHz, CDCl3)δ: 208.83, 166.05, 162.12, 143.20, 140.10, 138.24, 136.82, 135.85, 133.90, 130.50, 130.14, 129.98, 129.31, 126.67, 124.14, 121.97, 120.13, 117.24, 114.44, 74.12, 50.32, 49.82, 47.61, 38.28, 37.00, 36.95, 31.64, 31.27, 31.03, 29.25, 27.90, 20.47, 19.53, 14.28; ESI-MSm/z: Calcd for C35H36O3FCl{[2M+Na]+}1139.46, found 1139.35。
4e: 白色固体,收率75%, m.p.197~199 ℃;1H NMR(500 MHz, CDCl3)δ: 7.78(s, 1H), 7.62(d,J=16.0 Hz, 1H), 7.55~7.47(m, 3H), 7.46~7.40(m, 1H), 7.32~7.27(m, 2H), 7.06(t,J=8.5 Hz, 2H), 6.34(d,J=16.0 Hz, 1H), 5.42(d,J=4.0 Hz, 1H), 4.74(tt,J=10.4 Hz, 4.7 Hz, 1H), 2.75(dd,J=15.8 Hz, 5.8 Hz, 1H), 2.48~2.36(m, 3H), 2.19~2.10(m, 1H), 2.02~1.89(m, 3H), 1.81~1.53(m, 5H), 1.48~1.32(m, 2H), 1.28~1.21(m, 2H), 1.10(s, 3H), 1.00(s, 3H);13C NMR(125 MHz, CDCl3)δ:208.85, 166.32, 143.31, 140.15, 138.25, 135.86, 133.91, 130.86, 130.83, 130.14, 130.03, 129.98, 129.96, 129.33, 126.67, 121.93, 118.48, 118.46, 116.22, 116.04, 73.98, 50.32, 49.82, 47.62, 38.31, 37.01, 36.96, 31.64, 31.28, 31.03, 29.26, 27.92, 20.48, 19.54, 14.28. ESI-MSm/z: Calcd for C35H36O3FCl{[2M+Na]+}1139.46, found 1139.54。
4f: 白色固体,收率70%, m.p.164~166 ℃;1H NMR(400 MHz, CDCl3)δ: 7.78(s, 1H), 7.71~7.59(m, 5H), 7.56~7.51(m, 1H), 7.46~7.40(m, 1H), 7.33~.27(m, 2H), 6.49(d,J=16.0 Hz, 1H), 5.47~5.41(m, 1H), 4.76(tt,J=10.3 Hz, 5.4 Hz, 1H), 2.76(dd,J=15.8 Hz, 5.1 Hz, 1H), 2.48~2.37(m, 3H), 2.20~2.11(m, 1H), 2.05~1.89(m, 3H), 1.86~1.52(m, 6H), 1.49~1.32(m, 2H), 1.29~1.17(m, 1H), 1.11(s, 3H), 1.00(s, 3H);13C NMR(101 MHz, CDCl3)δ: 208.87, 165.88, 142.74, 140.05, 138.24, 137.97, 135.87, 133.90, 131.96, 130.16, 129.99, 129.35, 128.26, 126.68, 125.94, 122.05, 121.30, 74.29, 50.32, 49.83, 47.63, 38.27, 37.00, 36.96, 31.64, 31.27, 31.03, 29.27, 27.90, 20.48, 19.54, 14.29; ESI-MSm/z: Calcd for C35H36O3FCl{[2M+Na]+}1139.46, found 1139.51。
4g: 白色固体,收率75%, m.p.98~100 ℃;1H NMR(500 MHz, CDCl3)δ: 8.08(d,J=16.0 Hz, 1H), 7.79(s, 1H), 7.62(d,J=6.9 Hz, 1H), 7.57~7.52(m, 1H), 7.47~7.39(m, 2H), 7.34~7.27(m, 4H), 6.42(d,J=16.0 Hz, 1H), 5.48~5.40(m, 1H), 4.76(tt,J=10.5 Hz, 4.7 Hz, 1H), 2.76(dd,J=15.8 Hz, 5.6 Hz, 1H), 2.50~2.38(m, 3H), 2.16(d,J=16.9 Hz, 1H), 2.05~1.90(m, 3H), 1.84~1.54(m, 5H), 1.49~1.33(m, 2H), 1.29~1.18(m, 2H), 1.12(s, 3H), 1.01(s, 3H);13C NMR(125 MHz, CDCl3)δ: 208.85, 165.94, 140.38, 140.13, 138.24, 135.84, 135.00, 133.89, 132.86, 131.07, 130.26, 130.13, 129.97, 129.30, 127.73, 127.16, 126.67, 121.93, 121.36, 74.15, 50.30, 49.81, 47.61, 38.27, 37.00, 36.95, 31.64, 31.27, 31.03, 29.25, 27.89, 20.47, 19.54, 14.28; ESI-MSm/z: Calcd for C35H36O3Cl2{[2M+Na]+}1171.40, found 1171.37。
4h: 白色固体,收率75%, m.p.172~174 ℃;1H NMR(500 MHz, CDCl3)δ: 7.78(s, 1H), 7.59(d,J=16.0 Hz, 1H), 7.55~7.48(m, 2H), 7.45~7.41(m, 1H), 7.38(d,J=7.1 Hz, 1H), 7.35~7.27(m, 4H), 6.42(d,J=16.0 Hz, 1H), 5.43(d,J=4.2 Hz, 1H), 4.74(tt,J=10.5 Hz, 4.9 Hz, 1H), 2.75(dd,J=15.9 Hz, 5.5 Hz, 1H), 2.42(td,J=11.2 Hz, 9.8 Hz, 5.4 Hz, 3H), 2.14(d,J=16.9 Hz, 1H), 2.03~1.89(m, 3H), 1.83~1.53(m, 5H), 1.48~1.32(m, 2H), 1.28~1.16(m, 2H), 1.11(s, 3H), 1.00(s, 3H);13C NMR(125 MHz, CDCl3)δ: 208.84, 166.01, 142.97, 140.10, 138.24, 136.43, 135.86, 135.01, 133.91, 130.23, 130.15, 129.98, 129.33, 127.87, 126.67, 126.31, 121.97, 120.21, 74.14, 50.32, 49.82, 47.62, 38.28, 37.00, 36.96, 31.64, 31.27, 31.03, 29.26, 27.90, 20.48, 19.54, 14.29; ESI-MSm/z: Calcd for C35H36O3Cl2{[2M+Na]+}1171.40, found 1171.14。
4i: 白色固体,收率75%, m.p.201~203 ℃;1H NMR(500 MHz, CDCl3)δ: 7.78(s, 1H), 7.61(d,J=16.0 Hz, 1H), 7.55~7.51(m, 1H), 7.44(t,J=6.2 Hz, 3H), 7.35(d,J=8.4 Hz, 2H), 7.32~7.27(m, 2H), 6.39(d,J=16.0 Hz, 1H), 5.43(d,J=3.6 Hz, 1H), 4.74(tt,J=10.5 Hz, 4.8 Hz, 1H), 2.75(dd,J=15.8 Hz, 5.5 Hz, 1H), 2.46~2.38(m, 3H), 2.18~2.11(m, 1H), 2.03~1.90(m, 3H), 1.83~1.53(m, 5H), 1.48~1.33(m, 2H), 1.27~1.17(m, 2H), 1.11(s, 3H), 1.00(s, 3H);13C NMR(125 MHz, CDCl3)δ: 208.85, 166.19, 143.16, 140.13, 138.25, 136.22, 135.87, 133.92, 133.10, 130.15, 129.98, 129.34, 129.28, 126.67, 121.97, 119.31, 74.07, 50.33, 49.83, 47.63, 38.30, 37.01, 36.97, 31.65, 31.28, 31.04, 29.82, 29.27, 27.92, 20.49, 19.54, 14.29; ESI-MSm/z: Calcd for C35H36O3Cl2{[2M+Na]+}1171.20, found 1171.84。
4j: 白色固体,收率75%, m.p.199~201 ℃;1H NMR(400 MHz, CDCl3)δ: 7.78(s, 1H), 7.65~7.57(m, 1H), 7.56~7.48(m, 3H), 7.43(d,J=7.3 Hz, 1H), 7.38(d,J=8.0 Hz, 2H), 7.32~7.25(m, 2H), 6.41(d,J=16.0 Hz, 1H), 5.43(s, 1H), 4.74(dt,J=10.3 Hz, 5.3 Hz, 1H), 2.76(dd,J=15.8 Hz, 5.7 Hz, 1H), 2.48~2.37(m, 3H), 2.15(d,J=17.0 Hz, 1H), 2.05~1.89(m, 3H), 1.84~1.52(m, 5H), 1.48~1.32(m, 2H), 1.29~1.19(m, 2H), 1.11(s, 3H), 1.00(s, 3H);13C NMR(101 MHz, CDCl3)δ: 208.83, 166.15, 143.20, 140.07, 138.21, 135.84, 133.87, 133.49, 132.21, 130.13, 129.96, 129.50, 129.30, 126.66, 124.53, 121.95, 119.39, 74.06, 50.28, 49.79, 47.59, 38.27, 36.98, 36.93, 31.61, 31.24, 31.00, 29.24, 27.89, 20.45, 19.52, 14.27; ESI-MSm/z: Calcd for C35H36O3BrCl{[2M+Na]+}1259.30, found 1259.93。
4k: 白色固体,收率60%, m.p.161~163 ℃;1H NMR(400 MHz,CDCl3)δ: 7.78(s, 1H), 7.53(d,J=5.8 Hz, 1H), 7.43(d,J=7.0 Hz, 1H), 7.33~7.24(m, 4H), 7.20(d,J=6.9 Hz, 3H), 5.39(s, 1H), 4.66~4.56(m, 1H), 2.94(t,J=7.7 Hz, 2H), 2.74(dd,J=15.9 Hz, 5.8 Hz, 1H), 2.60(t,J=7.7 Hz, 2H), 2.47~2.24(m, 3H), 2.13(d,J=17.1 Hz, 1H), 1.99(d,J=12.5 Hz, 1H), 1.92~1.51(m, 7H), 1.47~1.20(m, 3H), 1.20~1.11(m, 1H), 1.07(s, 3H), 0.99(s, 3H);13C NMR(101 MHz, CDCl3)δ: 172.37, 140.64, 140.13, 138.22, 135.83, 133.87, 130.12, 129.96, 129.28, 128.54, 128.41, 126.66, 126.30, 121.80, 73.82, 50.26, 49.78, 47.59, 38.15, 36.95, 36.89, 36.30, 31.61, 31.23, 31.13, 30.98, 29.24, 27.76, 20.43, 19.49, 14.26; ESI-MSm/z: Calcd for C35H39O3Cl{[2M+Na]+}1107.52, found 1107.89。
1.3 生物活性
(1) 细胞系和细胞培养
Huh-7和MCF-7细胞在高糖的DMEM培养基中37 ℃和5% CO2下生长。每2 d更换一次培养基,用0.05%胰蛋白酶(含0.025% EDTA溶液)处理后传代细胞。
(2) 体外抗肿瘤活性
使用SRB测定法测定化合物的细胞毒性。将3×103个细胞/孔接种到96孔培养板中,每孔加入100 μL细胞悬液,在CO2培养箱中于37 ℃孵育24 h。然后,将所有合成的化合物添加到细胞中,再孵育48 h。药物处理48 h后,将细胞用10%(wt/V)的三氯乙酸固定并染色30 min,然后通过用1%(V/V)的乙酸反复洗涤去除多余染料。将结合蛋白的染料溶于pH 10.5的10 mM Tris碱中,并使用酶标仪在570 nm下测定OD值。阳性对照使用一种临床上用于治疗多种恶性肿瘤的药物依托泊苷(VP-16)。
(3) 细胞周期
将MCF-7细胞以2.5×105/孔种于6孔板中,培养24 h,然后分别用2d、依托泊苷和DMSO作为对照处理细胞24 h,收获细胞后用70%乙醇于-20 ℃固定过夜,PBS洗涤细胞后,在37 ℃下用PI和1 mg/mL RNase染色40 min,最后用流式细胞仪检测细胞周期。
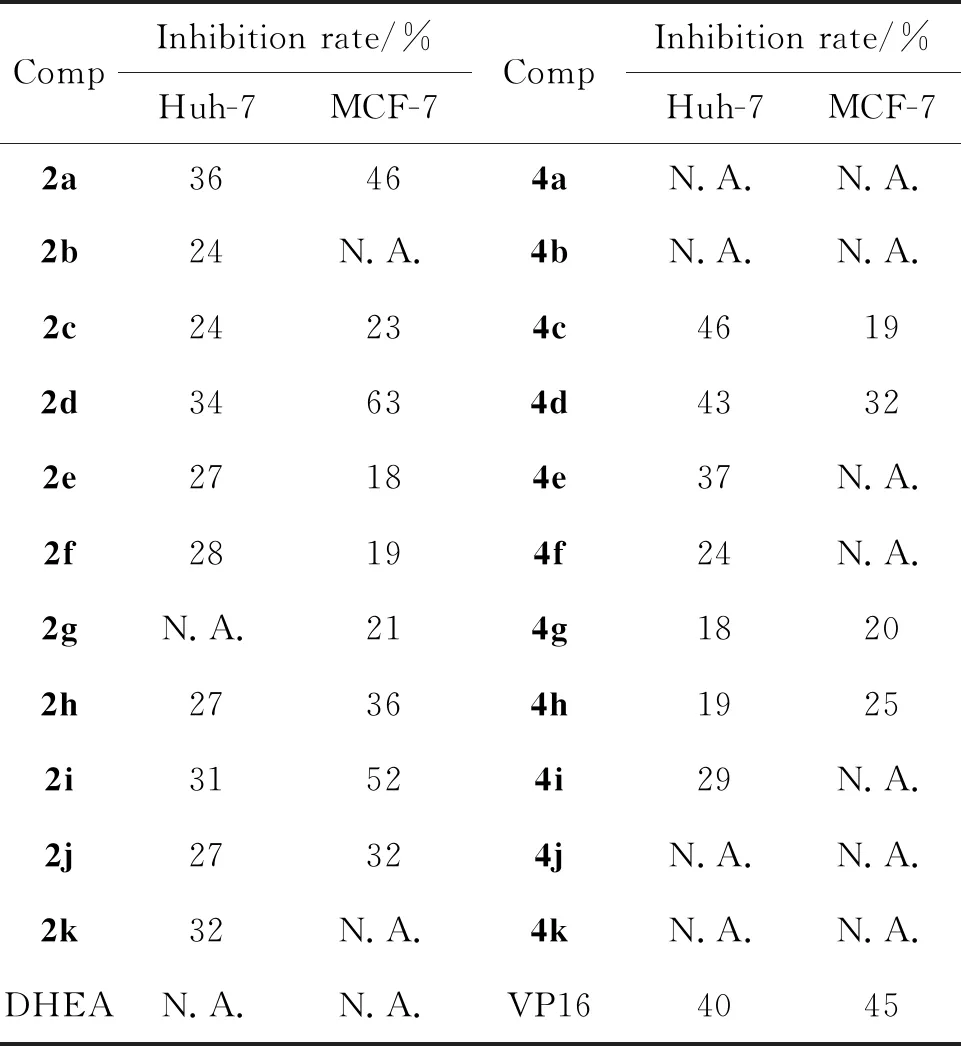
表1 化合物对Huh-7细胞和MCF-7细胞的生长抑制率
2 结果与讨论
2.1 合成
以去氢表雄酮(DHEA)为原料,通过和不同取代基肉桂酸在EDCI,DMAP和Et3N的条件下酯化得到11个新的DHEA酯类衍生物2a~2k,产率为50%~90%;进一步,DHEA与相应的苯甲醛通过羟醛缩合反应得到关键中间产物3,只得到了(E)-异构体的3,可能是因为DHEA与芳香醛发生羟醛缩合反应时,羰基的去屏蔽效应使得乙烯基的trans构型在COC=CH中更有利于结构的稳定;接着,3再与不同的肉桂酸在EDCI,DMAP和Et3N的条件下酯化得到11个新的含有(E)-16-(2-氯亚苄基)的酯类衍生物4a~4k,产率为50%~80%。在酯化反应中加入Et3N可以使反应更高效。
2.2 抗肿瘤活性
(1) 体外抗增殖活性
表1为2a~2k,4a~4k在50 μM浓度下对人乳腺癌细胞MCF-7、人肝癌细胞Huh-7的体外抑制活性。2a~2k,4a~4k均显示有一定的抑制活性,其中2d对MCF-7细胞的抑制率最高,为63%,进一步测定其IC50值为48 μM,4c对Huh-7细胞的抑制率最高,为46%, IC50值为55 μM。该结果表明去氢表雄酮C3酯类衍生物具有一定的抗癌活性。
(2) 细胞周期
为探究这些化合物的活性作用机制,使用FACS Calibur流式细胞仪分析化合物2d(25和50 μM)活性和阳性对照VP-16(10 μM)处理 24 h后的MCF-7细胞周期分布情况。细胞用碘化丙啶(PI)染色后上流式分析,结果如图2A所示。
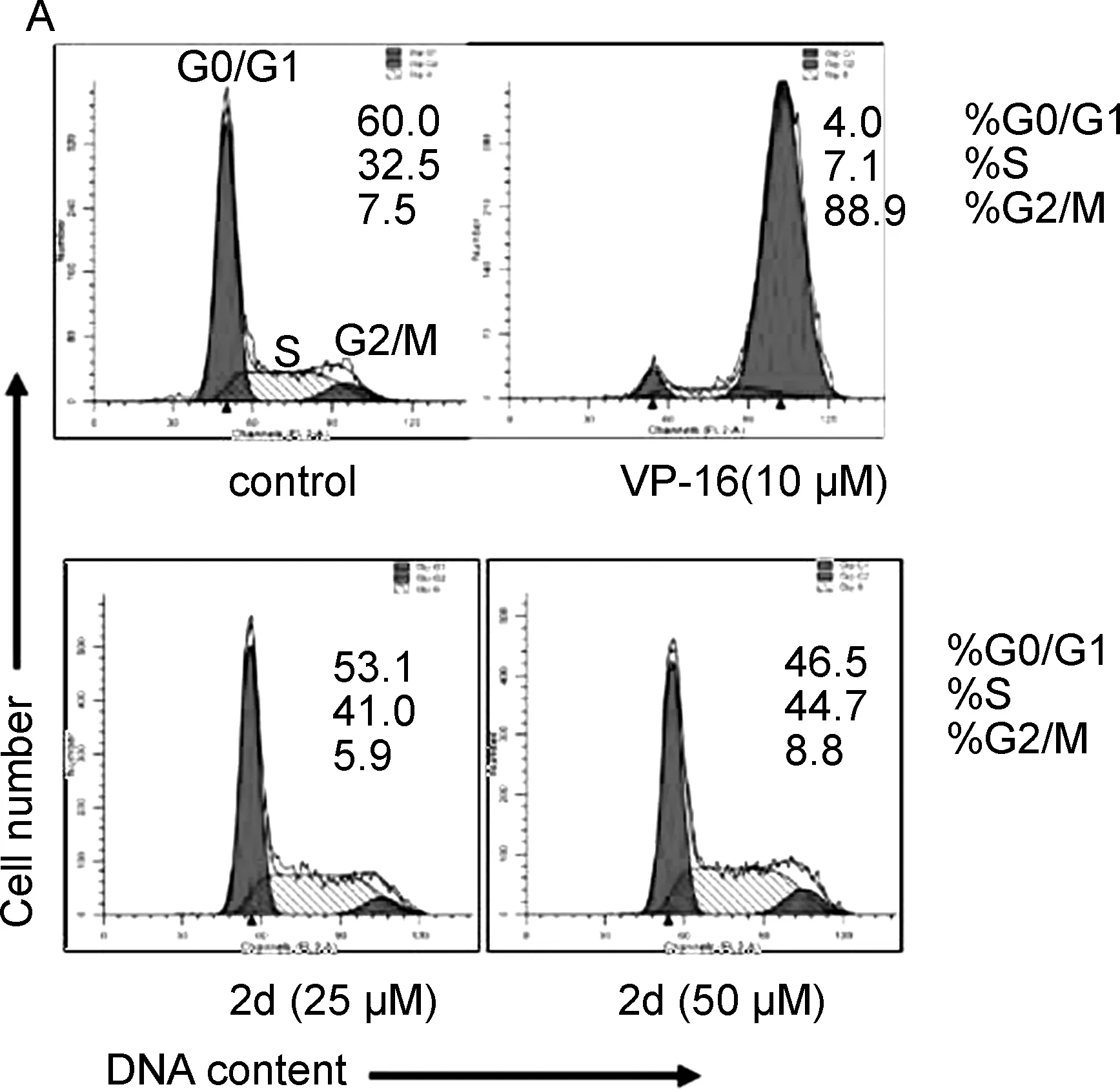
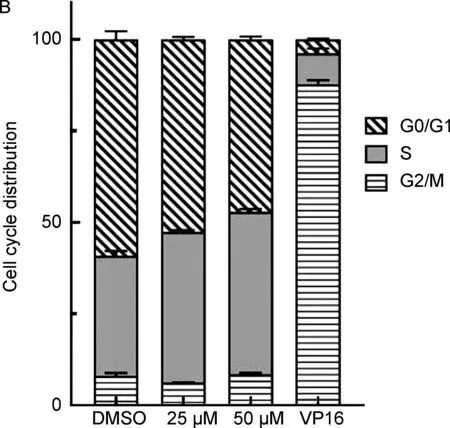
图12d对MCF-7细胞周期阻滞的影响
VP-16处理的MCF-7细胞在G2/M期捕获到了88.9%的细胞,而空白对照只能捕获到7.5%,说明VP-16阻滞MCF-7细胞周期在G2/M期。而当2d处理MCF-7细胞后,G0/G1期的细胞数随2d浓度的增加而减少,而S期的细胞数相应的增多。与空白对照(G0/G1: 60.0%)相比,25 μM处理组 G0/G1期细胞数减少为53.1%, 50 μM处理组 G0/G1期细胞数减少为46.5%,而S期细胞数相应从32.5%增加到了41.0%(25 μM)和44.7%(50 μM)(图1B)。说明去氢表雄酮C3酯类衍生物的抗癌活性与细胞周期阻滞有关。
以去氢表雄酮(DHEA)为原料,在C3位上与不同的酸反应,以较高的产率得到了22个新的酯类衍生物,其结构经核磁和质谱表征均正确。体外抗癌活性实验发现,DHEA对MCF-7和Huh-7细胞均没有明显抑制作用,但是酯类衍生物显示了抑制活性,其中2d对MCF-7细胞的抑制率最高,4c对Huh-7细胞的抑制率最高。进一步的研究发现,2d对MCF-7细胞周期分布、G0/G1期缩短、S期延长均有显著影响。

