Acute and sub-acute toxicities of hydroalcoholic extract of Allium affine aerial parts in rats
Leila Safaeian, Behzad Zolfaghari, Zahra Haghighatian, Mahmoud Etebari, Tahereh Nasirimoghadam
1Department of Pharmacology and Toxicology, Isfahan Pharmaceutical Sciences Research Center, School of Pharmacy and Pharmaceutical Sciences, Isfahan University of Medical Sciences, Isfahan, Iran
2Department of Pharmacognosy, School of Pharmacy and Pharmaceutical Sciences, Isfahan University of Medical Sciences, Isfahan, Iran
3Department of Pathology, School of Medicine, Lorestan University of Medical Sciences, Khorramabad, Iran
ABSTRACT
KEYWORDS: Allium affine; Acute toxicity test; Sub-acute toxicity test; Serum biochemistry; Histopathology
1. Introduction
Numerous natural products have been recognized to prevent and cure various diseases in the world. Many biological and pharmacological activities have been reported for medicinal plants due to the presence of a variety of bioactive components[1]. However,the safety of many plants is still a concern, as their functions may result in unknown adverse effects and toxicities[2,3].
Allium is one of the most widespread genera of the Amaryllidaceae family with more than 850 species[4]. Several famous species of Allium such as onion (A. cepa), garlic (A. sativum), leek (A.ampeloprasum), and wild garlic (A. ursinum) have been cultivated for food and medicinal purposes since ancient times[5]. A wide range of phytochemicals and various pharmacological actions have been identified in different Allium plants[6,7]. However, there are several reports of some adverse effects such as allergic reactions, altered coagulation, and gastrointestinal complications from garlic as an important Allium vegetable especially during chronic uses or intake of large quantities[8].
Allium affine (A. affine) Ledeb. is a lesser-known species of Allium that grows in west and middle Asian countries. This plant is used as an edible vegetable or condiment in the western and central regions of Iran[9]. Limited pharmacological studies have shown antioxidant,and in vitro anticancer and fibrinolytic activities for A. affine extract[10,11]. Since there is little data about the safety profile of this plant, the present study was conducted to assess the acute and subacute toxicity profile of hydroalcoholic extract of A. affine aerial parts in rats.
2. Materials and methods
2.1. Chemicals
The assay kits for the determination of blood biochemical parameters were prepared from Pars Azmoon Co. (Tehran, Iran).Folin-Ciocalteu's phenol reagent and all other chemicals were obtained from Merck KGaA Co. (Germany).
2.2. Plant material and preparation of extract
The aerial parts of A. affine were obtained from the local sellers in Borujen, Chaharmahal, and Bakhtiari Province (Iran) in April 2020.After authentication, the plant was recorded as a voucher specimen(No. 3403) by the Herbarium of the Department of Pharmacognosy in our faculty. The plant aerial parts (1 200 g) were air-dried and grounded and then extracted with 6 000 mL 70% aqueous ethanol for 72 h, 3 times at room temperature using the maceration method.After filtering and concentrating under vacuum by rotary evaporator at 50 ℃, the obtained extract was freeze-dried and kept in the refrigerator till used for the investigation. The plant extract was dissolved in water for oral gavage in the rats.
2.3. Determination of total phenolic content
The Folin-Ciocalteu method was used for the estimation of the amount of total phenolic compounds in the hydroalcoholic extract of A. affine. In this spectrophotometric test, the extract or standard samples were mixed with sodium bicarbonate (20%) and then with diluted Folin-Ciocalteu reagent. After 120 min incubation at room temperature, the absorbance was measured by a UV-visible spectrophotometer (Bio-Tek, PowerWave XS, USA) at 765 nm using. A standard curve was depicted by different concentrations of gallic acid (0-5 500 μg/mL) and was used for quantitation of total phenolic content in the samples. The results were specified in terms of mg of gallic acid equivalents (GAE)/g of the dried extract[12].
2.4. Experimental animals
Male and female Wistar rats weighting (200±20) g were acquired from the animal house of our faculty. The animals were maintained under standard laboratory settings of temperature (20-25 ℃), with a 12 h light/12 h dark cycle in polypropylene cages with free access to tap water and rat chow diet. The animals were adapted to the laboratory conditions for 7 d before the investigation. For acute and subacute toxicity evaluations, animals were randomly assigned to the control or treatment groups. Randomization was done by using a random number table in which all animals had an equal and independent chance of being selected for the sample group.
2.5. Ethical approval
The experimental procedure was carried out in accordance with the international guidelines for laboratory animal use and care (European Directive 2010/63/EU)[13] and approved by the Institutional Research Ethics Committee of Isfahan University of Medical Sciences (ethical approval ID: IR.MUI.RESEARCH.REC.1399.217).
2.6. Acute toxicity study
The acute toxicity assay was conducted according to the recommendations by Organization for Economic Co-operation and Development (OECD) guidelines (No. 423)[14]. For this test,12 rats (6 female and 6 male) were included, and both of the male and the female rats were divided into the control group and the treatment group, respectively, with 3 rats in each group. A single dose of 2 000 mg/kg of the hydroalcoholic extract of A. affine was administrated orally by an intra-gastric tube to the treatment groups.As the control groups, rats were treated orally with an equal volume of normal saline. Animals were inspected for general behavior and any sign of toxicity or death during the first hour and 2, 4, and 6 h after administration of the extract and thereafter daily up to 14 d. All probable abnormalities in food and water consumption,body weight, physical appearance, behavioral and neurological activities were detected. At the termination of the test, rats were sacrificed with exposure to carbon dioxide and the vital organs were macroscopically observed for gross changes.
2.7. Sub-acute toxicity study
The sub-acute toxicity assay was performed according to No. 407 of OECD guidelines[15]. A total of 20 male and 20 female rats were included. Both of the male and the female rats were divided into the control group, the 125 mg/kg group, the 250 mg/kg group and the 500 mg/kg group, with 5 rats in each group. All the treatment group were orally treated with hydroalcoholic extract of A. affine (125,250, and 500 mg/kg/day, respectively), and the control group with normal saline as the control group for 28 d. The highest dose level of 500 mg/kg was considered as the non-observed-adverse-effect at the lowest dose level (NOAEL) based on the previous data[10].
The rats were observed every day for general health and clinical signs of toxicity and weighed at the beginning of the test and every 4 d during the experimental period. After 4 weeks, the blood samples of overnight-fasted rats were taken from retro-orbital sinus under anesthesia by intraperitoneal injection of ketamine (50 mg/kg) and xylazine (5 mg/kg) in two types of test tubes, containing anticoagulant or without anticoagulant for hematological and biochemical examinations, respectively. Liver, heart, lung, kidney,and spleen tissues were dissected and relative organs weight (ratio of organ weight to the total body weight as a percentage) were estimated. The histopathological procedure was completed for these tissues.
2.7.1. Hematological analysis
The anticoagulated blood samples were used to determine the hematological parameters including white blood cells (WBC)and differential leukocytes (neutrophil, lymphocyte, eosinophil,basophil), red blood cells (RBC), red distribution width (RDW),hematocrit (Hct), hemoglobin (Hb), platelets count, mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH),and mean corpuscular hemoglobin concentration (MCHC) by the automated hematology analyzer.
2.7.2. Biochemical analysisThe blood serum samples were used to evaluate the biochemical parameters including fasting blood glucose, urea, uric acid,creatinine, total cholesterol, triglyceride (TG), high-density lipoprotein cholesterol (HDL), low-density lipoprotein cholesterol(LDL), alkaline phosphatase (ALP), alanine aminotransferase (ALT),aspartate aminotransferase (AST), and total protein by commercial kits.
2.7.3. Histopathological assay
For histopathological evaluations, liver, heart, lung, kidney, and spleen tissues were fixed in a solution of 10% buffered formalin.After embedding in a paraffin block, tissues were cut into 5 μm thickness sections and stained with hematoxylin and eosin (H&E)for examination by optical microscopy.
2.8. Statistical analysis
Statistical analyses were performed using SPSS software version 25.0. Results were expressed as mean±standard deviation (SD).The normality of the distribution of the variables was checked using the Kolmogorov-Smirnov test. For this mean, one-way analysis of variance (ANOVA) followed by Tukey post-hoc test. Levene's test was used to verify the homogeneity of variance components between experimental treatments. The significant level of this study was set at α=0.05.
3. Results
3.1. Extractive value and total phenolic content of A. affine
The yield of A. affine extraction was 37.5% (w/w). The amount of total phenolic compounds was measured as (19.5±2.5) mg GAE/g of dried hydroalcoholic extract of the plant using the Folin-Ciocalteu method and a standard curve of gallic acid(y=0.001x+0.008,R2=0.989).
3.2. Acute toxicity study of A. affine extract
In the acute toxicological test, animals receiving the fixed dose of 2 000 mg/kg from hydroalcoholic extract of A. affine did not show any abnormality or clinical sign of toxicity during the 14-day observational period. All rats were alive and no mortality occurred until the end of the test time. The general appearances of rats were normal and no significant alterations in hair, skin, eyes,mucus membrane, respiratory rate, behavioral, neurological, and autonomic profiles were detected. The food and water consumption of the treated animals were similar to the control groups during the test period. The body weights gains were normal in both female and male rats (Figure 1).
3.3. Sub-acute toxicity study of A. affine extract
3.3.1. Effect on general behaviors and mortality
In sub-acute toxicity assay, oral administration of A. affine hydroalcoholic extract at the doses of 125, 250, and 500 mg/kg did not cause any visible sign of toxicity or alteration in general behaviors in female and male rats. No mortality was found during a 28-day exposure to different doses of A. affine extract.
3.3.2. Effect on body weight
The bodyweight gaining was normal and similar to the control group in both sexes of rats which were treated with various doses of A. affine for 4 weeks (Figure 2).
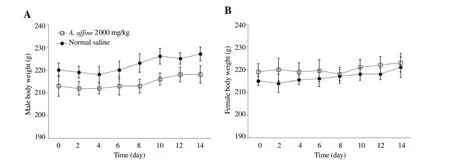
Figure 1. Effect of hydroalcoholic extract of A. affine on mean body weight in male and female rats in acute toxicity study. A: Male rats; B: Female rats.Values are expressed as means±SD, n=3.
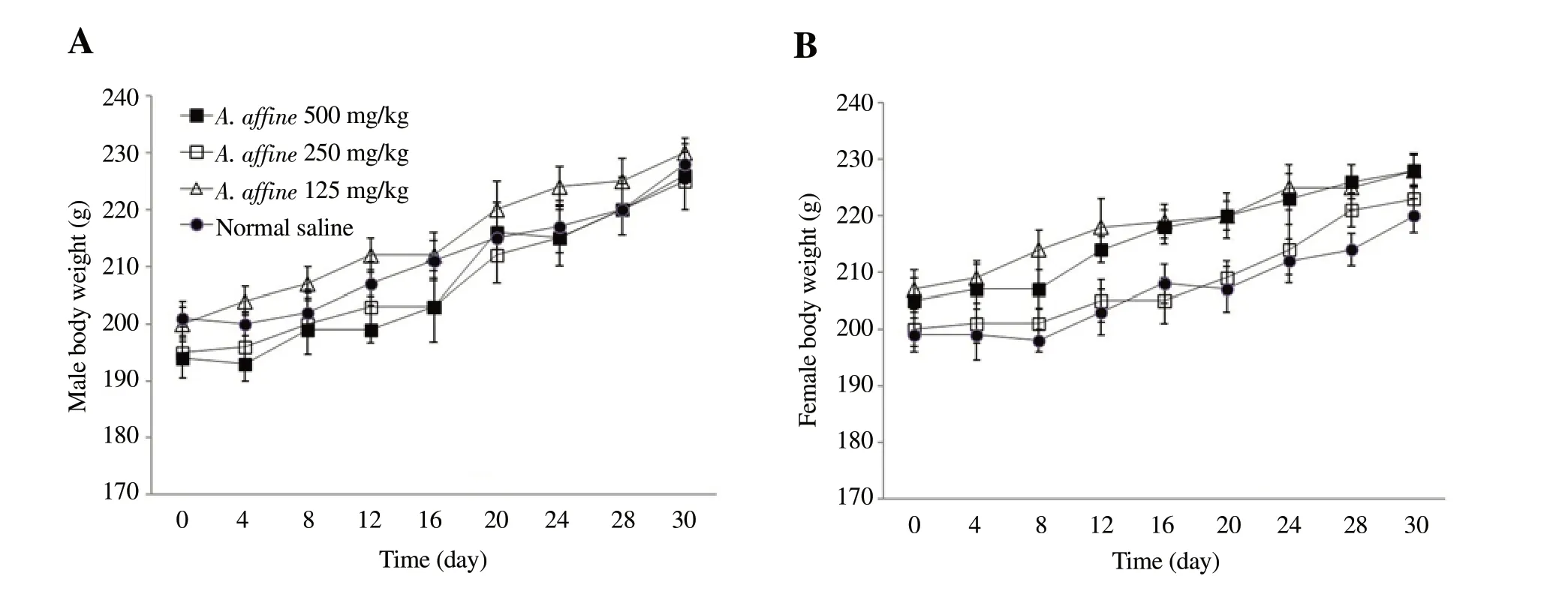
Figure 2. Effect of hydroalcoholic extract of A. affine on mean body weight in male and female rats in sub-acute toxicity study. A: Male rats; B: Female rats.Values are expressed as means ± SD, n=5.
3.3.3. Effect on the relative weight of vital organs
Assessment of the relative weights of heart, kidney, and liver tissues showed no statistically significant difference between treatment and control groups (Table 1). However, there was a significant increase in the relative weight of the spleen in male and female rats (P=0.002) and also in the relative weight of lung in male animals (P=0.007) after administration of the highest dose of the extract (500 mg/kg) for 28 d in the sub-acute study (Table 1).
3.3.4. Effect on hematological parameters
The effect of sub-acute exposure to the A. affine extract on hematological factors has been presented in Table 2. The total WBC count was significantly raised at the dose of 500 mg/kg of extract (P=0.024) in male rats as compared to the normal control group. The neutrophils count was also increased at the dose of 500 mg/kg (P=0.005) in female rats. All other hematological parameters exhibited normal levels within the physiological range after the 28-day experimental period in both sexes of animals(Table 2).
3.3.5. Effect on biochemical parametersAs presented in Table 3, biochemical examination of the sub-acute study displayed a prominent decrease in serum blood sugar level at the doses of 125 and 250 mg/kg of A. affine extract (P<0.005)compared to the control group in male rats. A significant decline in serum concentration of triglycerides was observed at the dose of 500 mg/kg of extract in male rats (P=0.016) and the dose of 250 mg/kg in female rats (P=0.041). The serum LDL level was also decreased at the dose of 250 mg/kg of extract in male rats(P=0.031). However, a significant increase was observed in serum creatinine level at the doses of 250 and 500 mg/kg of A. affine extract (P<0.01) in female rats compared to the control group(P=0.005 and P=0.001, respectively). There were also notable raises in AST and ALT activities as the hepatic indices after exposure to the high dose of A. affine extract in female rats (P=0.045 and P=0.025, respectively). In male rats, AST activity was increased at the dose of 500 mg/kg of extract (P=0.002), and ALT activity was elevated after exposure to the doses of 250 and 500 mg/kg(P=0.005 and P<0.001, respectively) compared to the control group. Other biochemical factors showed normal values (Table 3).
3.3.6. Effect on histopathology of vital organs
Histopathological inspection of heart and kidney tissues of male and female rats treated with 500 mg/kg of A. affine extract during sub-acute toxicity study presented normal architectures without any pathological alteration as compared with control groups (Figure 3A-Figure 3D and Figure 4A-Figure 4D).
Evaluation of liver tissues showed slight portal inflammation with infiltration of inflammatory cells after administration of 500 mg/kg of A. affine extract in male and female rats compared with normal architecture in control groups of both sexes of animals (Figure 3E and Figure 3F, Figure 4E and Figure 4F).
Assessment of lung tissues revealed moderate pneumocyte hyperplasia, congestion, and peri-bronchial inflammation in some areas after exposure to the 500 mg/kg of A. affine extract in maleand female animals as compared with normal controls (Figure 3G and Figure 3H, Figure 4G and Figure 4H).
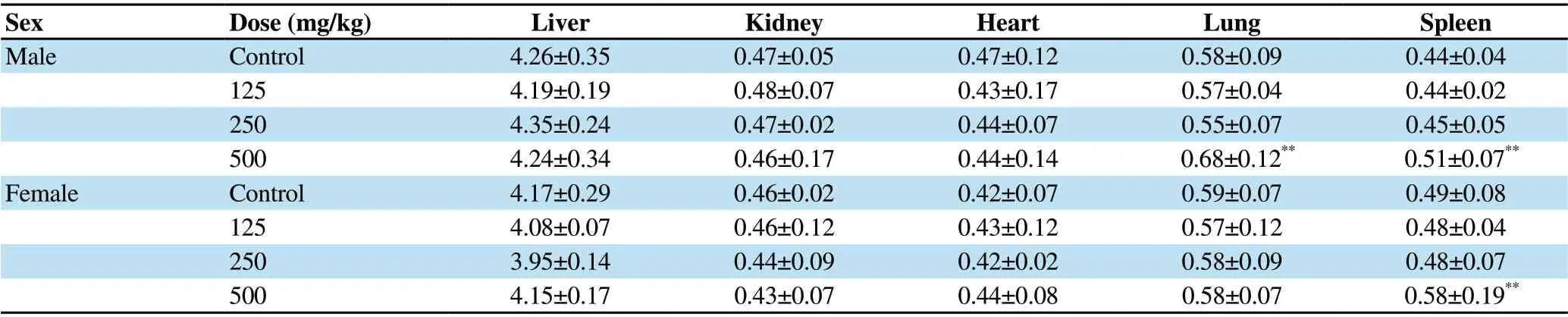
Table 1. Effect of sub-acute administration of A. affine extract on relative organ weight in female and male rats (g% of body weight).

Table 2. Effect of sub-acute administration of different doses (mg/kg) of A. affine extract on hematological parameters in female and male rats.
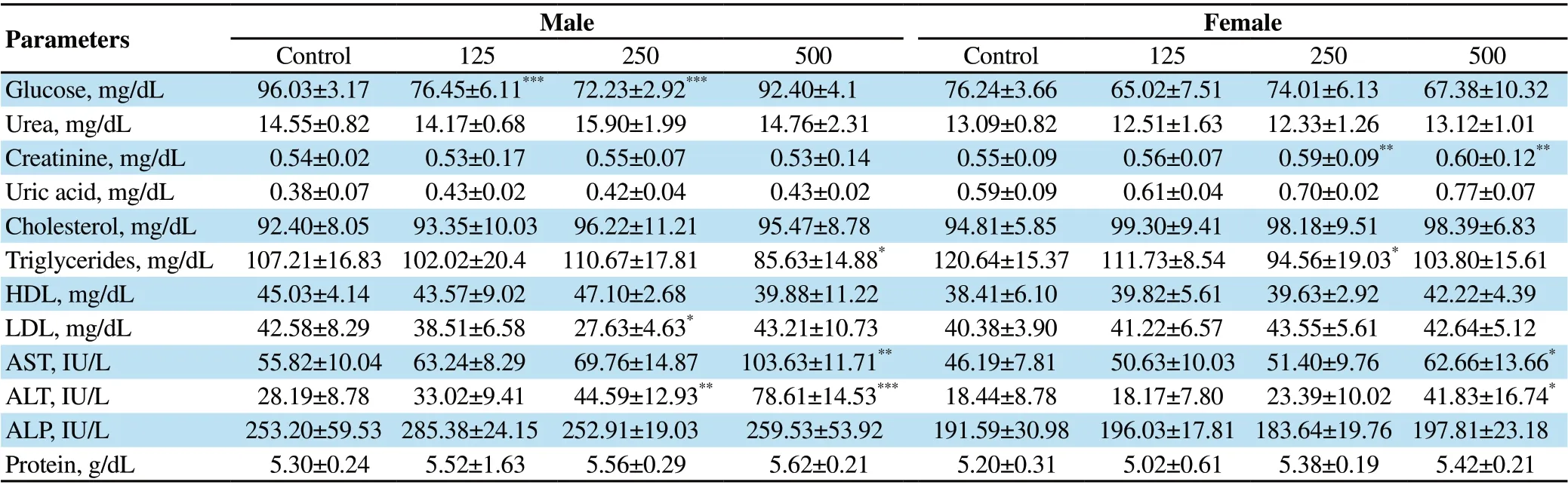
Table 3. Effect of sub-acute administration of different doses (mg/kg) of A. affine extract on biochemical parameters in female and male rats.

Figure 3. Photomicrograph of heart, kidney, liver, lung, and spleen tissues of the control and the treated male rats with 500 mg/kg of A. affine hydroalcoholic extract in sub-acute toxicity study (H&E, ×400 and ×1 000). Circle shows infiltration of inflammatory cells in F and H, and histiocytosis in J; Heart, kidney,liver, lung, and spleen tissues of the control rats: A, C, E, G, I, respectively; Heart, kidney, liver, lung, and spleen tissues of the treated rats: B, D, F, H, J,respectively.

Figure 4. Photomicrograph of heart, kidney, liver, lung, and spleen tissues of the control and the treated female rats with 500 mg/kg of A. affine hydroalcoholic extract in sub-acute toxicity study (H&E, ×400 and ×1 000). Circle shows infiltration of inflammatory cells in F and H, and histiocytosis in J; Heart, kidney,liver, lung, and spleen tissues of the control rats: A, C, E, G, I, respectively; Heart, kidney, liver, lung, and spleen tissues of the treated rats: B, D, F, H, J,respectively.
Histological examination of spleen tissues showed mild histiocytosis and lymphoid follicular activation in male and female rats receiving 500 mg/kg of A. affine extract compared with normal tissues in control groups (Figure 3I and Figure 3J, Figure 4I and Figure 4J).
4. Discussion
A. affine is a sub endemic plant of western and central areas of Iran.Although this plant has some nutritional and medicinal uses, there is a scarce toxicological report about its safety profile. Therefore,the current study evaluated the acute and sub-acute toxicity of oral administration of the hydroalcoholic extract of A. affine aerial parts in rats.
Evaluation of total phenolic content showed (19.50 ± 2.5) mg GAE/g of dried A. affine extract. Large variabilities have been found in the phenols and flavonoids content and antioxidant properties of various Allium plants in different investigations[16]. Antioxidant activity of A. affine has been reported through scavenging of DPPH radical,increasing the ferric reducing antioxidant power, and reducing the hydroperoxides level in a recent study[10].
In this investigation, a single dose of 2 000 mg/kg was administered in rats for assessment of acute toxicity based on the fixed-dose protocol as described in OECD guidelines[14]. No abnormality and no mortality were observed during a 14-days toxicity assay period representing that the median lethal dose (LD50) of A. affine extract exceeds 2 000 mg/kg for both sexes of rats.
In sub-acute toxicity assay, animals treated with different doses of A. affine extract exhibited no obvious sign of toxicity with normal body weight gaining indicating that this Allium plant had no harmful effect on animal appetite and growth, and did not affect food intake.
Assessment of the weight of vital organs showed significantly the enlargement in the size and relative weight of spleen in both sexes of rats after 4 weeks treatment with 500 mg/kg of the extract. There are similar reports about the effect of some Allium plants on the spleen in other investigations. Kuda et al. reported that the addition of 2% garlic (A. sativum) to the mice diet for 4 weeks increased the relative weight of the spleen[17]. Splenomegaly may be induced by blood disorders or liver illnesses or by over-activity of the spleen for eliminating the blood cells and clearing the blood. In the study of Odiase et al., administration of garlic bulb extract for 35 d in rats resulted in some changes in spleen histology including a rise in myeloid-erythroid cells number and stimulation of splenic sinus histiocytes and lymphoid follicles suggesting the immunostimulatory actions of A. sativum[18].
In our study, there was also a significant increase in the relative weight of lungs in male rats. Gender differences have been reported in the severity of some organ's injury and sensitivity to the lethal effects of specific toxic substances[19]. However, Alnaqeeb et al.have described dose-dependent pulmonary toxicity after oral and intraperitoneal treatment with A. sativum in female Sprague-Dawley rats[20].
In the hematological examination, A. affine extract had no adverse effect on most hematological parameters during the 28 d of study.There was an only prominent increase in total WBC and neutrophils count at the highest dose of extract in male and female rats,respectively. Olaniyan et al. detected significant elevations in total WBC, lymphocyte, neutrophils, and also in red blood cell count in rats treated with A. sativum extract[21]. Some investigations have described the stimulatory effects of some Allium vegetables on total and differential WBC counts indicating the immunoregulatory capabilities of these plants. Although, no major change was observed in other hematological parameters in our study while there are controversial reports about the effects of some Allium plants on RBC count, Hb, MCV, and MCH in other animal species[21-23]. A. sativum,A. cepa, A. porum, and A. schoenoprasum have shown the toxic effect on RBC with inducing hemolytic anemia and formation of Heinz bodies in erythrocytes in some animals including horses, sheep,cattle, cats, and dogs[24,25].
Biochemical analysis of blood samples revealed a significant increase in serum creatinine level as a glomerular marker at the higher doses of A. affine extract in female rats indicating its effect on kidney function after sub-acute administration. Abdel Gadir et al. reported elevation of serum urea level, another marker of renal function after addition of 6% garlic or onion to the rats' food diet for 28 d[26].
Our results also displayed significant raises in AST and ALT activities after exposure to the higher doses of A. affine extract in both sexes of rats. In the study of Oko et al., notable elevation in the activities of AST, ALT, and ALP enzymes was observed in rats after 14 d treatment with doses of 400 and 600 mg/kg of A. sativum leaves extract[27]. Huzaifa and co-workers also reported the toxic effect of high doses of garlic aqueous extract (400 and 550 mg/kg) on liver function after 3 weeks of treatment in rats[28].
Interestingly, administration of A. affine extract could reduce serum blood glucose, triglycerides, and LDL levels in our sub-acute study.These findings propose the possible therapeutic potential of this plant in hyperglycemia and hyperlipidemia. Some bioactive agents such as flavonoids and organosulfur compounds may be responsible for hypoglycemic and hypolipidemic activities in different Allium plants[29].
In histopathological examination, slight to moderate histopathological alterations were found in liver, lung, and spleen organs of both sexes of animals after 28 d exposure to the high dose of A. affine extract. In the microscopic evaluation of livers,slight portal inflammation and infiltration of inflammatory cells were observed. Some previous studies have shown dose-related hepatotoxicity including liver degenerative changes and nonspecific focal injury in hepatocytes after intake of high dose of A. sativum for a sub-acute period in rats[20,26]. There are case reports of acute toxicities after ingestion of a large amount of A. ursinum in tortoises with gross biochemical, hematological and histopathological changes[30]. Moreover, toxic damages in the liver, spleen, and kidney tissues have been reported in geese feeding A. Ascalonicum[31].Considering the histopathological alterations and elevation of ALT and AST activities in our findings, the liver tissue could be considered as a target organ for sub-acute toxicity of A. affine extract.
In the microscopic inspection of lung tissues, treatment with a high dose of A. affine extract caused moderate congestion, pneumocyte hyperplasia, and peri-bronchial inflammation. In the study of Alnaqeeb et al., oral treatment of rats with A. sativum caused slight thickening of alveolar walls along with increasing the numbers of RBCs and WBCs in some alveoli. While they observed significant alveolar thickness, edema, and disruption after intraperitoneal administration of a high dose of garlic[20]. Therefore, the lung as a critical tissue for the elimination of volatile substances could be mentioned as the other target organ for sub-acute toxicity of A. affine.
Another histopathological finding was mild histiocytosis and lymphoid follicular activation in spleen tissue in rats receiving the high dose of A. affine. Recent studies have reported similar histological changes in the spleen after administration of A. sativum extract suggesting the immunoregulatory activities for Allium vegetables[17,18].
The presence of various phytochemicals including flavonoids,sulfuric compounds, a steroidal saponin, and sapogenins like diosgenin, tigogenin, and ruscogenin in A. affine may be accountable for various effects of this plant on different tissues and organs[32].However, further investigations are needed for elucidation of the details of the metabolism and elimination of bioactive components of A. affine extract and their toxicity profile.
According to our results obtained through acute and sub-acute toxicity studies, A. affine hydroalcoholic extract could be considered safe at doses of lower than 250 mg/kg in rats. However, regarding some biochemical and pathological changes, caution needs to be taken in excessive and prolonged uses of this plant.
Conflict of interest statement
The authors report no conflict of interest.
Funding
This study was financially supported by Vice-Chancellery for Research and Technology, Isfahan University of Medical Sciences(Grant No. 399187).
Authors'contributions
L.S. was responsible for the research plan, designing the animal studies, analyzing and interpretation of data and editing the manuscript; B.Z. planned the herbal experiments; Z.H. planned the histopathological experiments; M.E. was involved in toxicology counseling and manuscript review; T.N. was involved in literature search, animal treatments, data acquisition and preparation of the manuscript.
 Journal of Acute Disease2022年1期
Journal of Acute Disease2022年1期
- Journal of Acute Disease的其它文章
- Incidence of adverse reactions to COVID-19 vaccination: A metaanalysis of randomized controlled trials
- Health literacy, behavioral and psychosocial characteristics in coronary artery patients: A hospital-based study in Turkey
- Arrhythmia and its risk factors post myocardial infarction: A prospective study
