Hyperosmolarity disrupts tight junction via TNF-α/MMP pathway in primary human corneal epithelial cells
INTRODUCTION
Dry eye disease is a developing health problem resulting in serious impacts on general state of health, functional vision, economy and life quality
. The primary pathogenesis of dry eye disease contains, tear film flimsiness, causing and went with by tear hyperosmolarity, driving to epithelial inflammation and damage
. Recent evidences have documented that the levels of pro-inflammatory cytokines and chemokines increase in the tear fluid of dry eye
. In dry eye conditions tear clearance decreases, which can also cause increased concentrations of proinflammatory cytokines;whereas before these studies, dry eye disease was thought to be caused simply by insufficient tear production. Nowadays,dry eye disease is also known as an inflammatory disorder.
Tear film disorders are followed by matrix metalloproteinase(MMP) intervened corneal disease
. Collagenases (MMP-1,MMP-13), gelatinases (MMP-9), and stromelysins (MMP-3) have been recommended to play a critical part within the disruption of corneal epithelium under hyperosmolar model
.In ocular surface, the corneal epithelium forms a barrier to noxious stimuli and prevents foreign material within the outside environment
. Four kinds of junctions have been identified to form this barrier, including tight junction (TJ),desmosomes, adherens junctions, and gap junctions
. The foremost layer is the TJ between adjoining cells. The TJ proteins ZO-1 and Occludin form the very first barrier for the eyeball. Recent studies have found that MMP-9 is able to disrupt one of the proteins, Occludin
. TNF-α has been found to induce the destruction of ZO-1 from the neighboring human corneal epithelial cells (HCECs)
. Disruption of these TJ proteins leads to numerous pathological situations, resulting in infection, or melting of the cornea
.
But the physiological mechanism of the disruption of the TJ proteins, as well as the association between tumor necrosis factor (TNF)-α and MMPs under hyperosmotic dry eye condition, remains unclear.
HCECs are the most broadly used models for studies of dry eye disease
. Hyperosmolar stress is widely applied to these primary cells to mimic dry eye hyperosmotic conditions
. The latest studies showed that dry eye disease models were treated with hyperosmotic saline solution in 500 mOsM/L both
and
. We hypothesized that the TJ may be disrupted in hyperosmolarity dry eye condition by inflammation, and we used nuclear factor-κB(NF-κB) inhibitor and MMP inhibitor to identify the roles of TNF-α and MMPs in this process.
MATERIALS AND METHODS
Fresh corneoscleral tissues of human(<72h after death), donors aged 18-70 years old, after the central cornea had been used for keratoplasty. Primary HCECs (Figure 1A) were cultured on 8-chamber slides for immunofluorescence (IF) staining and in 12-well plates for RNA extraction or enzyme-linked immunosorbent assay(ELISA) for cytokine proteins. Primary corneal epithelial cells migrate from limbus. We used the explants from corneal limbus in supplemented hormonal epidermal medium (SHEM)with 5% fetal bovine serum (FBS) according to the protocol of previous publications
.
Primary HCECs cultured for 14-19d were exchanged with serum-free medium for 24h, then adding 44,69 or 94 mmol/L sodium chloride (NaCl) for hyperosmolar stress group (400, 450 and 500 mOsM) or adding rh-TNF-α(10 ng/mL) as TNF-α group. NF-κB inhibitor (5 μmol/L) was added 1h before that treatment (500 mOsM or TNF-α group)to antagonize the effect of TNF-α. MMP inhibitor GM-6001(20 μmol/L) was also added 1h before that treatment (500 mOsM or TNF-α group) to antagonize the effect of MMP.
水力压裂是个复杂的过程,裂隙通常沿垂直于最小主应力方向扩展,但裂隙形态、扩展方向并不仅取决于某一个因素,如煤体均质性、原生裂隙分布特征、地应力状态等都可能会对压裂效果产生影响[17-18]。在多孔控制定向水力压裂技术中,孔隙水压的分布特征与地应力组合影响下煤体起裂、裂隙扩展规律有待研究。
HCECs treated for 4h were used for mRNA expression.HCECs treated for 24h were used for IF staining or ELISA.
因为故对任意ε>0,存在Nk,使得当n>Nk时, ρ(An(xk),ak)<ε,即1-an(xk)↔ak> <ε,从而an(xk)↔ak> 1-ε。取N=max{N1,…,Nr},则对任意ε>0,存在N,当n>N时,
ε,从而an(xk)↔ak
ε,即1-an(xk)↔ak
综上,针对肝硬化患者,在给予常规治疗及护理的同时,配合实施优质护理干预,可有效提高患者的生活质量,效果较好,值得推广。
GM-6001 (potent and broad spectrum MMP inhibitor) protected cells from destruction of ZO-1 and Occludin by hyperosmolar stress and rh-TNF-α.
The cultured HCECs on 8-chamber slides were exchanged with FBS-free SHEM and were treated with 90 mmol/L of NaCl to make hyperosmolar media (500 mOsM) for 24h, and the untreated media served as control group (312 mOsM). MMP inhibitor GM-6001(20 μmol/L) was added 1h before. Cells on slides were fixed in cold acetone (for TJ proteins ZO-1, Occludin staining),then blocked with 20% goat serum (Abcam, Cambridge, MA,USA). Slides were then incubated at 4℃ overnight with rabbit anti-mouse ZO-1 and Occludin antibodies (Invitrogen, dilution 1:200), then incubated with secondary antibodies and DAPI for one hour.
VRML除了在工程技术、建筑、电子商务和娱乐等领域广泛应用外,也可以应用于高职智慧课堂教学。由VRML实现的模拟仿真课件,与一般的模拟仿真课件相比,能营造更为逼真的环境或场景,人机交互更为自然,更能增强想象力,增加学生的感官刺激,激发学习兴趣。下面列出几个典型的网络教学应用。
Data analysis was done using GraphPad Prism 8.0.2 (California, USA). Student’s
-test was used to infer statistical significance between two groups.
<0.05 were considered to be statistically significant.
RESULTS
To evaluate the subcellular distribution of TJ proteins in dry eye, hyperosmotic media at 500 mOsM was used in HCECs. IF staining was done to evaluate the integrity of major TJ proteins, ZO-1 and Occludin. As shown in Figure 1B,the immunoreactivities staining of these two proteins showed a contiguous network morphology in the untreated (UT) group(SHEM with iso-osmolarity 312 mOsM).
However, these two junction proteins were significantly disrupted when exposed to hyperosmotic media at 500 mOsM after 24h, appearing their inadequate connections and irregular staining of the damaged network between cells.
东海 2号机组是第15台获得规制委重启许可的机组,是首台在 2011年3月的地震和海啸中受损并获准重启的机组,同时也是第三台获准重启的沸水堆机组。另外两台获准重启的沸水堆机组是柏崎·刈羽6号和7号机组。
The corneal epithelial barrier is formed by four kinds of junctions. Among them, the TJ is the major intercellular intersection structure, with ZO-1, Occludin, and others as essential component proteins. Studies showed that the TJ play an imperative role in protecting the ocular surface against adverse external conditions
. Another important role for TJ was the control of cell proliferation and gene expression
.We found that these important TJ proteins were significantly disrupted, and the net-structure stain of TJ proteins was destroyed when exposed to hyperosmotic media after 24h.However, the physiological mechanism of the disruption of the TJ proteins in hyperosmolarity condition is still elusive.


HCECs were treated with either hyperosmolar media (500 mOsM) or rh-TNF-α (10 ng/mL) for 4h or 24h with or without NF-κB inhibitor (5 μmol/L) added 1h before.
4.一旦在得到确诊的基础上,笔者认为采取封锁、隔离、紧急接种、药物治疗等综合措施,是能够有效治愈和控制疫病的流行和传播,为养猪户最大限度减少经济损失,保障规模养猪大户经济利益,促进我区生猪生产健康发展。
When exposed to 500 mOsM or rh-TNF-α, the structure of ZO-1 and Occludin proteins was disrupted. However, 20 μmol/L of GM-6001 pre-added in 500 mOsM medium 1h before,restored most of those disruption. Interestingly, this phenomenon also happened in rh-TNF-α treated medium. When 20 μmol/L GM-6001 pretreated, the disruption of ZO-1 and Occludin was prevented in rh-TNF-α treated HCECs (Figure 4).
The mRNA level of MMP-9 increased to 4.68±0.71 and 3.85±0.74 fold, when exposed to hyperosmolar stress (500 mOsM)and rh-TNF-α (
<0.001,
<0.005), but decreased to 2.08±0.36 fold (
<0.001, 500 mOsM group) and 2.40±0.44 fold(
<0.005, TNF-α group) with addition of NF-κB inhibitor.The MMP-9 protein level increased from 7.43±1.55 pg/mL to 24.86±2.82 and 25.15±3.55 pg/mL (
<0.001,
<0.001) when exposed to hyperosmolar stress (500 mOsM) and rh-TNF-α,but decreased to 9.80±2.10 pg/mL (
<0.0001, 500mOsM group) and 8.95±2.33 pg/mL (
<0.001, TNF-α group) with addition of NF-κB inhibitor (Figure 3C).
The mRNA level of MMP-3 increased to 4.90±0.60 and 4.06±0.42 fold, when exposed to hyperosmolar stress(500 mOsM) and rh-TNF-α (
<0.001,
<0.0001), but decreased to 2.25±0.60 fold (
<0.0001, 500 mOsM group)and 2.00±0.64 fold (
<0.0001, TNF-α group) with addition of NF-κB inhibitor. The MMP-3 protein level increased from 19.54±5.32 pg/mL to 87.61±15.20 and 73.83±12.03 pg/mL(
<0.001,
<0.001) when exposed to hyperosmolar stress(500 mOsM) and rh-TNF-α, but decreased to 39.47±7.35 pg/mL(
<0.01, 500 mOsM group) and 33.16±7.51 pg/mL (
<0.01,TNF-α group) with addition of NF-κB inhibitor (Figure 3D).NF-κB is a key mediator of TNF-α actions. The effect of TNF-α was antagonized by NF-κB inhibitor (NF-κB-I). The levels of MMPs increased significantly when HCECs were exposed to hyperosmolar stress. The expression levels of these MMPs also increased in HCECs treated with rh-TNF-α. The stimulated MMPs by hyperosmolarity and TNF-α were largely suppressed by NF-κB inhibitor.
1.4 统计学处理 利用WHONET 5.4版软件对两组CNS药敏数据进行耐药性分析。采用SPSS 13软件包对两组数据进行统计分析,计数资料选用Z检验,P<0.05为差异有统计学意义。
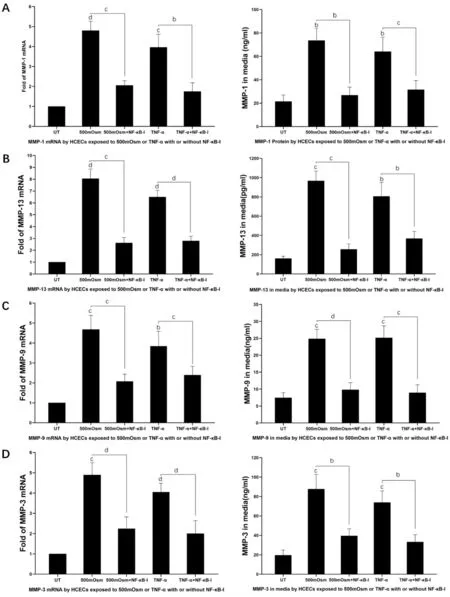
From these results, we infer that TNF-α mediated the mRNA and protein level of MMPs stimulated by hyperosmotic stress.That hyperosmolarity stimulates the level of MMPs through the increased expression of TNF-α.
The cell culture supernatants were collected after different treatments
. ELISA for TNF-α and MMPs (BioLegend, San Diego, CA, USA)were performed by manufacturers’ protocols and previously reported methods
. Absorbance was studied at 450 nm and 570 nm using Infinite M200 microplate reader (Tecan US, Inc.,Morrisville, NC, USA).
教师观察是一种很好的参与,同时也是为了有效发现问题,并提出有效的解决措施。比如,教师的观察内容包括幼儿的有时间、进入语言区的幼儿数量、语言区幼儿具体活动情况以及遇到的困难等方面,哪些图书是深受幼儿喜欢,哪些是幼儿不看的图书,哪些幼儿需要教师的个别指导,等等。

The mRNA level of MMP-1 increased to 4.80±0.47 and 3.96±0.65 fold, respectively, when exposed to hyperosmolar stress (500 mOsM) and rh-TNF-α (
<0.0001,
<0.001).MMP-1 level decreased, with addition of NF-κB inhibitor, to 2.05±0.23 fold (
<0.001, 500 mOsM group) and 1.75±0.44 fold (
<0.001, TNF-α group). The protein level of MMP-1 in HCECs increased from 21.39±5.63 to 73.55±10.46 and 64.03±12.39 ng/mL (
<0.005,
<0.01) when exposed to hyperosmolar stress (500 mOsM) and rh-TNF-α, but decreased to 26.79±6.93 (
<0.005, 500 mOsM group) and 31.51±7.81 ng/mL (
<0.001, TNF-α group) with addition of NF-κB inhibitor (Figure 3A).
The mRNA level of MMP-13 increased to 8.05±0.81 and 6.49±0.58 fold, when exposed to hyperosmolar stress (500 mOsM) and rh-TNF-α, (both
<0.0001), but decreased to 2.62±0.45 fold (
<0.001, 500 mOsM group) and 2.79±0.40 fold (
<0.0001, TNF-α group) with addition of NF-κB inhibitor. The MMP-13 protein level increased from 159.82±25.00 pg/mL to 966.53±101.79 and 806.19±143.41 pg/mL (
<0.001,
<0.005) when exposed to hyperosmolar stress (500 mOsM) and rh-TNF-α, but decreased to 255.18±57.87 pg/mL (
<0.001, 500 mOsM group) and 367.49±74.03 pg/mL (
<0.005, TNF-α group)with addition of NF-κB inhibitor (Figure 3B).
After treatments, total RNA was extracted using extraction kit (RNeasy Micro RNA kit) with protocol. RNA concentrations were measured with NanoDrop® ND-2000 Spectrophotometer. Reverse transcription was performed by RT using Ready-To-Go You-Prime First-Strand Beads.(25℃ for 10min and 37℃ for 60min). Quantitative reverse transcription polymerase chain reaction (RT-qPCR) was performed essentially as described in detail previously
.In short, 50℃ for 2min and 95℃ for 10min, 40 cycles of 95℃ for 15s and 60℃ for 1min. TaqMan gene expression assays of primer were: GAPDH (Hs99999905_m1), MMP-1(Hs00899658_m1), MMP-13 (Hs00942589_m1), MMP-9(Hs00234579_m1), MMP-3 (Hs00233962_m1), TNF-α(Hs00174128_m1).
Taken together, these results suggest that hyperosmolarity disrupts the TJ proteins ZO-1 and Occludin
TNF-α/MMP pathway in HCECs.
DISCUSSION
As assessed by RT-qPCR (Figure 2A), TNF-α mRNA level was osmolarity-dependently upregulated to 4.11±1.67(
<0.05), 11.98±4.19 (
<0.05) and 17.17±5.12 (
<0.01)fold, respectively, when HCECs were exposed to different hyperosmolar media (400, 450 or 500 mOsM) compared with UT group. To quantify the protein levels of TNF-α in HCECs,ELISA was used. TNF-α levels in the supernatants increased significantly from 28.80±10.84 pg/mL in UT group to 189.49±27.12 pg/mL (
<0.0001) in hyperosmolar stress group (500 mOsM; Figure 2B).
那段时光不知道究竟有多长,但青辰记得,自己是被一种“嘎嘎咔咔”的声音惊醒的。醒来时,他发现自己仍然跪伏着,头脸埋在地上,身体在不听使唤地战栗着。
In dry eye conditions proinflammatory cytokines, such as TNF-α increased
. MMPs were proposed to play the important role in the disruption of corneal epithelium under hyperosmolar stress
. But the relationship between TNF-α and MMPs in the process is not clear.
We observed that TNF-α and MMPs both increased by hyperosmolar stress at mRNA and protein level. Interestingly,the stimulatory effect of MMPs was repressed by NF-κB inhibitor under hyperosmolar stress. NF-κB is a key mediator of TNF-α actions
. TNF-α activates the NF-κB signaling pathway in HCECs
. TNF-α production and activation of the NF-κB/IκBα pathway were measured to evaluate inflammatory effects
dry eye disease model
.
To confirm that TNF-α can cause the increase of MMPs, we added rh-TNF-α in medium and found that in rh-TNF-α treated HCECs, MMPs also increased. These stimulated MMPs were suppressed by NF-κB inhibitor. These findings reveal that the hyperosmolar stress stimulated production of MMPs was through increasing TNF-α expression.
Then we tried to determine if the disruption of TJ is in connection with this TNF-α/MMP pathway in HCECs under hyperosmolar stress. When exposed to 500 mOsM or rh-TNF-α treated medium, the integrity structure of ZO-1 and Occludin was disrupted. While, 20 μmol/L GM-6001 (MMP inhibitor) pre-added in 500 mOsM medium one hour before,prevented most of this disruption.
Interestingly, this phenomenon also occurred in rh-TNF-α treated medium. When 20 μmol/L GM-6001 pre-added in rh-TNF-α treated medium, the disruption of ZO-1 and Occludin was reduced, suggesting TJ disruption in hyperosmolar stress and TNF-α was protected by MMP inhibitor GM-6001. Taken together, hypertonicity-induced TNF-α upregulation leads to disruption of TJ proteins through MMP activation.
In conclusion, our findings demonstrate that hyperosmolarity disrupts the TJ proteins ZO-1 and Occludin
TNF-α/MMP pathway in HCECs, further suggesting that drugs targeting MMPs upregulation may be a protective option to the ocular surface in dry eye disease. Although, HCECs are the most broadly used models
for studies of dry eye disease
.Some data from animal models or human patients would be significant. However, animal experiments are proceeding very slowly at present, due to the pandemic of COVID-19. These findings would further validated using an animal model in our future work.
None;
None;
None;
None;
None;
None.
1 Dana R, Meunier J, Markowitz JT, Joseph C, Siffel C. Patient-reported burden of dry eye disease in the United States: results of an online cross-sectional survey.
2020;216:7-17.
2 Siffel C, Hennies N, Joseph C, Lascano V, Horvat P, Scheider M,Ganzera F. Burden of dry eye disease in Germany: a retrospective observational study using German claims data.
2020;98(4):e504-e512.
3 McDonald M, Patel DA, Keith MS, Snedecor SJ. Economic and humanistic burden of dry eye disease in Europe, North America, and Asia: a systematic literature review.
2016;14(2):144-167.
4 Craig JP, Nichols KK, Akpek EK, Caffery B, Dua HS, Joo CK, Liu ZG, Nelson JD, Nichols JJ, Tsubota K, Stapleton F. TFOS DEWS II definition and classification report.
2017;15(3):276-283.
5 Akpek EK, Amescua G, Farid M, Garcia-Ferrer FJ, Lin A, Rhee MK,Varu DM, Musch DC, Dunn SP, Mah FS, American Academy of Ophthalmology Preferred Practice Pattern Cornea and External Disease Panel. Dry eye syndrome preferred practice pattern®.
2019;126(1):P286-P334.
6 Laihia J, Järvinen R, Wylęgała E, Kaarniranta K. Disease aetiologybased design of multifunctional microemulsion eye drops for moderate or severe dry eye: a randomized, quadruple-masked and activecontrolled clinical trial.
2020;98(3):244-254.
7 Kim YH, Oh TW, Park E, Yim NH, Park KI, Cho WK, Ma JY. Antiinflammatory and anti-apoptotic effects of acer palmatum thumb.extract, KIOM-2015EW, in a hyperosmolar-stress-induced
dry eye model.
2018;10(3):E282.
8 Roda M, Corazza I, Bacchi Reggiani ML, Pellegrini M, Taroni L,Giannaccare G, Versura P. Dry eye disease and tear cytokine levels-A meta-analysis.
2020;21(9):E3111.
9 Zhang LL, Su ZT, Zhang ZD, Lin J, Li DQ, Pflugfelder SC. Effects of azithromycin on gene expression profiles of proinflammatory and antiinflammatory mediators in the eyelid margin and conjunctiva of patients with meibomian gland disease.
2015;133(10):1117-1123.
10 Hua X, Su ZT, Deng RZ, Lin J, Li DQ, Pflugfelder SC. Effects of l-carnitine, erythritol and betaine on pro-inflammatory markers in primary human corneal epithelial cells exposed to hyperosmotic stress.
2015;40(7):657-667.
11 Li H, Li JF, Hou CT, Li JJ, Peng H, Wang Q. The effect of astaxanthin on inflammation in hyperosmolarity of experimental dry eye model
and
.
2020;197:108113.
12 Li DQ, Shang TY, Kim HS, Solomon A, Lokeshwar BL, Pflugfelder SC. Regulated expression of collagenases MMP-1,-8, and-13 and stromelysins MMP-3,-10, and-11 by human corneal epithelial cells.
2003;44(7):2928.
13 Deng RZ, Su ZT, Hua X, Zhang ZD, Li DQ, Pflugfelder SC.Osmoprotectants suppress the production and activity of matrix metalloproteinases induced by hyperosmolarity in primary human corneal epithelial cells.
2014;20:1243-1252.
14 Pflugfelder SC, Farley W, Luo LH, Chen LZ, de Paiva CS, Olmos LC, Li DQ, Fini ME. Matrix metalloproteinase-9 knockout confers resistance to corneal epithelial barrier disruption in experimental dry eye.
2005;166(1):61-71.
15 Voss K, Nguyen A, Heur M. Non-infectious and non-hereditary diseases of the corneal epithelium.
2021;202:108316.
16 Suzuki K. Cell-matrix and cell-cell interactions during corneal epithelial wound healing.
2003;22(2):113-133.
17 Kimura K, Teranishi S, Fukuda K, Kawamoto K, Nishida T. Delayed disruption of barrier function in cultured human corneal epithelial cells induced by tumor necrosis factor-alpha in a manner dependent on NFkappaB.
2008;49(2):565-571.
18 Huet E, Vallée B, Delbé J, Mourah S, Prulière-Escabasse V,Tremouilleres M, Kadomatsu K, Doan S, Baudouin C, Menashi S,Gabison EE. EMMPRIN modulates epithelial barrier function through a MMP-mediated occludin cleavage: implications in dry eye disease.
2011;179(3):1278-1286.
19 Liang H, Baudouin C, Daull P, Garrigue JS, Brignole-Baudouin F.Ocular safety of cationic emulsion of cyclosporine in an
corneal wound-healing model and an acute
rabbit model.
2012;18:2195-2204.
20 Warcoin E, Baudouin C, Gard C, Brignole-Baudouin F.
inhibition of NFAT5-mediated induction of CCL2 in hyperosmotic conditions by cyclosporine and dexamethasone on human HeLamodified conjunctiva-derived cells.
2016;11(8):e0159983.
21 Marek V, Mélik-Parsadaniantz S, Villette T, Montoya F, Baudouin C, Brignole-Baudouin F, Denoyer A. Blue light phototoxicity toward human corneal and conjunctival epithelial cells in basal and hyperosmolar conditions.
2018;126:27-40.
22 Magny R, Kessal K, Regazzetti A, Ben Yedder A, Baudouin C, Mélik Parsadaniantz S, Brignole-Baudouin F, Laprévote O,Auzeil N. Lipidomic analysis of epithelial corneal cells following hyperosmolarity and benzalkonium chloride exposure: new insights in dry eye disease.
2020;1865(9):158728.
23 Hua X, Deng RZ, Li J, Chi W, Su ZT, Lin J, Pflugfelder SC, Li DQ. Protective effects of L-carnitine against oxidative injury by hyperosmolarity in human corneal epithelial cells.
2015;56(9):5503-5511.
24 Li JM, Lu R, Zhang Y, Lin J, Hua X, Pflugfelder SC, Li DQ. IL-36α/IL-36RA/IL-38 signaling mediates inflammation and barrier disruption in human corneal epithelial cells under hyperosmotic stress.
2021;22:163-171.
25 Bremond-Gignac D, Copin H, Benkhalifa M. Corneal epithelial stem cells for corneal injury.
2018;18(9):997-1003.
26 Hu JY, Gao N, Zhang Y, Chen X, Li JM, Bian F, Chi W, Liu ZG, de Paiva CS, Pflugfelder SC, Li DQ. IL-33/ST2/IL-9/IL-9R signaling disrupts ocular surface barrier in allergic inflammation.
2020;13(6):919-930.
27 Oh S, McCanna DJ, Subbaraman LN, Jones LW. Cytotoxic and inflammatory effects of contact lens solutions on human corneal epithelial cells
.
2018;41(3):282-289.
28 Wang Y, Zhang J, Yi XJ, Yu FSX. Activation of ERK1/2 MAP kinase pathway induces tight junction disruption in human corneal epithelial cells.
2004;78(1):125-136.
29 Ko JA, Yanai R, Nishida T. Up-regulation of ZO-1 expression and barrier function in cultured human corneal epithelial cells by substance P.
2009;583(12):2148-2153.
30 Wang F, Wang DM, Song M, Zhou Q, Liao RF, Wang Y. MiRNA-155-5p reduces corneal epithelial permeability by remodeling epithelial tight junctions during corneal wound healing.
2020;45(8):904-913.
31 González-Mariscal L, Lechuga S, Garay E. Role of tight junctions in cell proliferation and cancer.
2007;42(1):1-57.
32 Liu ZG. Molecular mechanism of TNF signaling and beyond.
2005;15(1):24-27.
33 Pooladanda V, Thatikonda S, Bale S, Pattnaik B, Sigalapalli DK,Bathini NB, Singh SB, Godugu C. Nimbolide protects against endotoxin-induced acute respiratory distress syndrome by inhibiting TNF-α mediated NF-κB and HDAC-3 nuclear translocation.
2019;10(2):81.
34 Hwang SB, Park JH, Kang SS, Kang DH, Lee JH, Oh SJ, Lee JY, Kim JY, Tchah H. Protective effects of cyclosporine A emulsion versus cyclosporine A cationic emulsion against desiccation stress in human corneal epithelial cells.
2020;39(4):508-513.
 International Journal of Ophthalmology2022年5期
International Journal of Ophthalmology2022年5期
- International Journal of Ophthalmology的其它文章
- Yes-associated protein promotes endothelial-tomesenchymal transition of endothelial cells in choroidal neovascularization fibrosis
- Chordin-like 2 influences the differentiation fate of retinal pigment epithelium cells by dynamically regulating BMP pathway
- Exosome-mediated aptamer S58 reduces fibrosis in a rat glaucoma filtration surgery model
- Topography versus non-topography-guided photorefractive keratectomy with corneal cross-linking variations in keratoconus
- Clinical application of a shape-preserving rapid corneal donor dehydrater
- Comparison of preoperative simulated and postoperative real safety distances using anterior segment OCT in patients with phakic lOL according to iris configuration
