扯根菜化学成分及其保肝活性
郝丽亚, 李冰洁, 王中林, 郑新华
(平顶山学院医学院,河南 平顶山 467000)
扯根菜PenthorumchinensePursh.为虎耳草科扯根菜属植物的全草[1],分布在广西、云南、湖南等地,具有利水除湿、平肝解毒、祛瘀止痛的功效,临床用于治疗肝炎、黄疸、经闭等[2],以其为主要药材的中成药及保健品治疗病毒性肝炎、肝硬化、脂肪肝等疾病疗效显著[3-4],但因需求量大,导致野生资源被严重破坏[5]。该植物化学成分为黄酮、有机酸、木脂素等[6],具有保肝护肝、抗病毒、抗炎、抗肿瘤、利尿等[7],可有效拮抗肝星状细胞生长分化,降低大鼠血清转化生长因子-β1和I型胶原的水平[8],可降低醉酒小鼠血液中乙醇含量[9]。本实验研究扯根菜化学成分及其保肝活性,以期为该植物后续开发提供参考。
1 材料
Agilent 1290-microTOF Q II型高分辨质谱仪(美国Agilent公司);SYS-600型核磁共振波谱仪(德国Bruker公司);BSA124S-CW型分析天平(德国Sartorius公司);JJ124BC型电子天平(常熟市双杰测试仪器厂);Sephadex LH-20葡聚糖凝胶(瑞典Amersham Pharmacia公司);HH-S型恒温水浴锅(郑州长城科工贸有限公司);柱色谱硅胶(青岛海洋化工厂);Rotavapor R-205型旋转蒸发仪(瑞士Buchi公司);氘代试剂DMSO-d6(德国Sigma-aldrich公司);人肝正常细胞HL-7702(山东玉宝生物科技有限公司);APAP培养基(北京安诺伦生物科技有限公司)。所用试剂均为分析纯。
扯根菜采自河南平顶山市,经平顶山学院郑新华教授鉴定为扯根菜PenthorumchinensePursh.的全草。
2 提取与分离
取扯根菜全草18.4 kg,分别用90%、50%乙醇浸渍提取,得浸膏1.7 kg,溶于水中,分别用石油醚、丙酮、正丁醇萃取,得石油醚层浸膏(Fr.A)、丙酮层浸膏(Fr.B)、正丁醇层浸膏(Fr.C)。
取Fr.A 127.4 g,经硅胶柱分离,石油醚-丙酮 (70∶30~50∶50~30∶70)梯度洗脱,得8个流分 Fr.A1~Fr.A8。Fr.A3 (6.8 g)经硅胶柱分离,石油醚-乙酸乙酯 (80∶20~20∶80)梯度洗脱,得7个流分Fr.A3-1~Fr.A3-7,Fr.A3-3 (173.4 mg)经硅胶柱分离,石油醚-乙酸乙酯 (40∶60)洗脱,得化合物1(22 mg);Fr.A3-5 (173.4 mg)经Sephadex LH-20分离,甲醇洗脱,得化合物13(21 mg);Fr.A3-4 (173.4 mg)经硅胶柱分离,石油醚-乙酸乙酯(40∶60)洗脱,得化合物10(18 mg)、12(27 mg)。Fr.A5 (5.2 g)经硅胶柱分离,石油醚-乙酸乙酯(70∶30~30∶70)梯度洗脱,得6个流分Fr.A5-1~Fr.A5-6,Fr.A5-2 (151.3 mg)经硅胶柱分离,石油醚-乙酸乙酯(30∶70)洗脱,得化合物6(17 mg);Fr.A5-3(109.2 mg)经Sephadex LH-20分离,甲醇洗脱,得化合物20(21 mg);Fr.A5-5 (105.4 mg)经硅胶柱分离,石油醚-乙酸乙酯(30∶70)洗脱,得化合物8(29 mg)。Fr.A6 (4.3 g)经硅胶柱分离,二氯甲烷-甲醇(60∶40~40∶60)洗脱,得4个流分Fr.A6-1~Fr.A6-4,Fr.A6-2 (98.2 mg)经Sephadex LH-20分离,甲醇洗脱,得化合物7(18 mg);Fr.A6-4 (83.9 mg)经硅胶柱分离,二氯甲烷-甲醇(20∶80)洗脱,得化合物9(21 mg)。
取Fr.B 97.3 g,经硅胶柱分离,丙酮-甲醇(80∶20~50∶50~20∶80)洗脱,得9个流分Fr.B1~Fr.B9。Fr.B2 (5.7 g)经硅胶柱分离,二氯甲烷-甲醇(80∶20~20∶80)梯度洗脱,得9个流分Fr.B2-1~Fr.B2-9,Fr.B2-3 (161.8 mg)经硅胶柱分离,二氯甲烷-甲醇(30∶70)洗脱,得化合物11(19 mg);Fr.B2-6 (117.2 mg)经Sephadex LH-20分离,甲醇洗脱,得化合物3(31 mg);Fr.B2-5 (102.7 mg)经硅胶柱分离,二氯甲烷-甲醇(20∶80)洗脱,得化合物4(22 mg)、17(18 mg);Fr.B2-8 (93.7 mg)经Sephadex LH-20分离,甲醇洗脱,得化合物19(21 mg)。Fr.B4 (4.9 g)经硅胶柱分离,二氯甲烷-甲醇(80∶20~10∶90)梯度洗脱,得7个流分Fr.B4-1~Fr.B4-7,Fr.B4-2 (125.3 mg)经硅胶柱分离,二氯甲烷-甲醇(20∶80)洗脱,得化合物15(23 mg);Fr.B4-4 (92.8 mg)经Sephadex LH-20分离,甲醇洗脱,得化合物5(24 mg);Fr.B4-6 (83.2 mg)经Sephadex LH-20分离,得化合物16(28 mg)。
取Fr.C 103.5 g,经硅胶柱分离,丙酮-甲醇(90∶10~50∶50~10∶90)洗脱,得7个流分Fr.C1~Fr.C7。Fr.C2 (7.3 g)经硅胶柱分离,乙酸乙酯-甲醇 (80∶20~20∶80)梯度洗脱,得8个流分Fr.C2-1~Fr.C2-8,Fr.C2-4 (85.6 mg)经硅胶柱分离,乙酸乙酯-甲醇(30∶70)洗脱,得化合物2(26 mg);Fr.C2-6 (102.9 mg)经Sephadex LH-20分离,得化合物21(17 mg)。Fr.C4 (8.5 g)经硅胶柱分离,乙酸乙酯-甲醇(70∶30~30∶70)洗脱,得6个流分Fr.C4-1~Fr.C4-6),Fr.C4-2 (135.2 mg)经硅胶柱分离,乙酸乙酯-甲醇(30∶70)洗脱,得化合物14(18 mg);Fr.C4-3(95.2 mg)经硅胶柱分离,乙酸乙酯-甲醇(20∶80)洗脱,得化合物18(23 mg)。
3 结构鉴定
化合物1:黄色粉末。ESI-MSm/z:441.2 [M+Na]+。1H-NMR (600 MHz,acetone-d6)δ:12.51 (1H,s,4′-OH),10.13 (1H,s,5-OH),8.02 (2H,d,J=8.2 Hz,H-2′,6′),7.02 (2H,d,J=8.2 Hz,H-3′,5′),6.81 (1H,d,J=2.2 Hz,H-8),6.42 (1H,d,J=8.2 Hz,H-6),5.57 (1H,d,J=8.2 Hz,1″),4.23 (1H,dd,J=8.2,2.2 Hz,H-2″),3.95 (1H,d,J=8.2 Hz,H-4a″),3.64 (1H,d,J=8.2 Hz,H-4b″),3.35 (2H,d,J=8.2 Hz,H-5″);13C-NMR (150 MHz,acetone-d6)δ:81.2 (C-1),93.7 (C-2),135.2 (C-3),173.6 (C-4),105.1 (C-4a),159.3 (C-5),97.6 (C-6),161.7 (C-7),93.8 (C-8),156.4 (C-8a),120.9 (C-1′),128.3 (C-2′),114.5 (C-3′),114.5 (C-4′),158.6 (C-5′),130.2 (C-6′),106.3 (C-1″),77.5 (C-2″),77.9 (C-3″),73.9 (C-4″),62.1 (C-5″)。以上数据与文献[10]报道的山柰酚-7-O-β-D-芹菜苷基本一致。
化合物2:黄色粉末。ESI-MSm/z:441.3 [M+Na]+。1H-NMR (600 MHz,acetone-d6)δ:6.81 (1H,s,H-8),6.42 (1H,s,H-6),5.59 (1H,s,H-1),3.58 (1H,dd,J=8.2,2.2 Hz,H-5a″),3.51 (1H,m,H-5b″);13C-NMR (150 MHz,acetone-d6)δ:107.1 (C-1),81.4 (C-2),77.1 (C-3),86.1 (C-4),60.3 (C-5)。以上数据与文献[11]报道的山柰酚-7-O-α-L-呋喃阿拉伯糖基本一致。
化合物3:黄色结晶。ESI-MSm/z:309.1 [M+Na]+。1H-NMR (600 MHz,acetone-d6)δ:12.52 (1H,s,5-OH),7.87 (2H,m,H-2′,6′),6.81 (2H,m,H-3′,5′),6.36 (1H,d,J=8.2 Hz,H-8),6.22 (1H,d,J=8.2 Hz,H-6);13C-NMR (150 MHz,acetone-d6)δ:103.5 (C-1),146.9 (C-2),135.6 (C-3),175.3 (C-4),102.6 (C-4a),155.3 (C-5),95.6 (C-6),163.2 (C-7),92.1 (C-8),157.3 (C-8a),117.6 (C-1′),127.6 (C-2′),113.6 (C-3′),157.3 (C-4′),113.9 (C-5′),131.9 (C-6′)。以上数据与文献[12]报道的山柰酚基本一致。
化合物4:黄色粉末。ESI-MSm/z:376.4 [M+Na]+。1H-NMR (600 MHz,acetone-d6)δ:7.91 (2H,d,J=9.6 Hz,H-2′,6′),7.02 (2H,d,J=9.6 Hz,H-3′,5′),6.63 (1H,s,H-3),6.51 (1H,s,H-8),5.19 (1H,m,H-2″),3.27 (2H,m,H-1″),1.81 (3H,s,4″-CH3),1.52 (3H,s,5″-CH3);13C-NMR (150 MHz,acetone-d6)δ:132.4 (C-1),164.2 (C-2),104.3 (C-3),183.3 (C-4),104.3 (C-4a),163.1 (C-5),112.3 (C-6),165.1 (C-7),93.6 (C-8),158.4 (C-8a),122.6 (C-1′),130.2 (C-2′),116.3 (C-3′),159.1 (C-4′),116.6 (C-5′),130.2 (C-6′),21.7 (C-1″),122.6 (C-2″),133.4 (C-3″),18.3 (C-4″),25.2 (C-5″)。以上数据与文献[13]报道的3-methylbut-2-enyl apigen基本一致。
化合物5:棕色粉末。ESI-MSm/z:441.2 [M+Na]+。1H-NMR (600 MHz,acetone-d6)δ:7.79 (1H,s,H-5),7.71 (2H,m,H-2′,6′),7.02 (1H,s,H-8),6.86 (1H,d,J=8.2 Hz,H-5′),6.54 (1H,s,H-3),5.41 (2H,m,H-2″,2‴),3.41 (4H,m,H-1″,1‴),1.82 (6H,s,5″,4‴-CH3),1.79 (3H,s,5‴-CH3),1.71 (3H,s,4″-CH3);13C-NMR (150 MHz,acetone-d6)δ:121.6 (C-1),159.3 (C-2),105.2 (C-3),179.3 (C-4),117.2 (C-4a),125.6 (C-5),131.2 (C-6),163.5 (C-7),103.2 (C-8),157.2 (C-8a),124.2 (C-1′),128.1 (C-2′),129.3 (C-3′),159.7 (C-4′),l 15.3 (C-5′),30.1 (C-l″),123.1 (C-2″),135.3 (C-3″),19.1 (C-4″),25.3 (C-5″),30.1 (C-1‴),122.9 (C-2‴),134.3 (C-3‴),26.1 (C-4‴),18.3 (C-5‴)。以上数据与文献[13]报道的enyllicoflavone A基本一致。
化合物6:棕色粉末。ESI-MSm/z:331.2 [M+Na]+。1H-NMR (600 MHz,acetone-d6)δ:6.91 (1H,d,J=9.6 Hz,H-5),6.73 (2H,m,H-2′,6′),3.91 (3H,s,4′-OCH3),3.83 (6H,s,1,3″-OCH3),1.69-2.91(2,3,4,5,1′,2′-CH2);13C-NMR (150 MHz,acetone-d6)δ:175.3 (C-1),32.6 (C-2),30.1 (C-3),31.3 (C-4),35.8 (C-5),144.1 (C-1′),119.2 (C-2′),132.3 (C-3′),146.1 (C-4′),111.3 (C-5′),115.2 (C-6′),25.1 (C-1″),34.1 (C-2″),181.2 (C-3″),56.1 (4′-OCH3),52.1 (3″-OCH3)。以上数据与文献[14]报道的methyl-5-[4-methoxy-3-[2-(methoxycarbonyl)ethyl]-phenyl]pentanoate基本一致。
化合物7:黄色晶体。ESI-MSm/z:339.2 [M+Na]+。1H-NMR (600 MHz,acetone-d6)δ:12.15 (1H,s,1-OH),7.51 (1H,d,J=8.2 Hz,H-6),7.26 (1H,d,J=8.2 Hz,H-5),6.37 (1H,s,H-2),4.12 (3H,s,3-OCH3),4.02 (3H,s,4-OCH3),3.78 (3H,s,8-OCH3);13C-NMR (150 MHz,acetone-d6)δ:160.8 (C-1),95.1 (C-2),160.2 (C-3),127.9 (C-4),145.2 (C-4a),151.2 (C-4b),113.6 (C-5),123.2 (C-6),150.1 (C-7),147.5 (C-8),116.1 (C-8a),104.4 (C-8b),57.3 (3-OCH3),62.3 (4-OCH3),63.1 (8-OCH3)。以上数据与文献[15]报道的1,7-二羟基-3,4,8-三甲氧基呫吨酮基本一致。
化合物8:黄色晶体。1H-NMR (600 MHz,acetone-d6)δ:12.16 (1H,s,1-OH),10.76 (1H,s,8-OH),7.41 (1H,d,J=9.6 Hz,6-H), 6.83 (1H,d,J=9.6 Hz,7-H),6.61 (1H,d,J=9.6 Hz,4-H),6.41 (1H,d,J=6.3 Hz,2-H),4.02 (3H,s,3-OCH3),3.91 (3H,s,5-OCH3);13C-NMR (150 MHz,acetone-d6)δ:162.1 (C-1),99.3 (C-2),168.1 (C-3),94.6 (C-4),138.3 (C-5),119.3 (C-6),111.4 (C-7),156.7 (C-8),185.3 (C-9),158.4 (C-4a),147.1 (C-4b),109.6 (C-8a),104.6 (C-8b),58.3 (3-OCH3),55.6 (5-OCH3)。以上数据与文献[15]报道的1,8-二羟基-3,5-二甲氧基呫吨酮基本一致。
化合物9:黄色粉末。ESI-MSm/z:325.1 [M+Na]+。1H-NMR (600 MHz,acetone-d6)δ:6.41(1H,d,J=8.2 Hz,H-8),6.23 (1H,d,J=8.2 Hz,H-6),7.83 (1H,d,J=8.2 Hz,H-2′),7.73 (1H,dd,J=8.2,2.2 Hz,H-6′),6.91(1H,d,J=2.2 Hz,H-5′);13C-NMR (150 MHz,acetone-d6)δ:113.4 (C-1),152.7 (C-2),136.9 (C-3),181.3 (C-4),157.3 (C-5),103.5 (C-6),164.3 (C-7),96.1 (C-8),159.1 (C-9),103.6 (C-10),118.3 (C-1′),121.6 (C-2′),146.4 (C-3′),147.6 (C-4′),115.2 (C-5′),122.8 (C-6′)。以上数据与文献[16]报道的槲皮素基本一致。
化合物10:黄色针晶。ESI-MSm/z:291.2 [M+Na]+。1H-NMR (600 MHz,acetone-d6)δ:7.85 (1H,d,J=9.6 Hz,H-5),7.49 (2H,d,J=9.6 Hz,H-2′,6′),7.13 (2H,d,J=9.6 Hz,H-3′,5′),7.02 (2H,dd,J=9.6,6.3 Hz,H-6),6.91 (1H,d,J=9.6 Hz,H-8);13C-NMR (150 MHz,acetone-d6)δ:142.1 (C-1),150.6 (C-2),121.6 (C-3),175.6 (C-4),126.9 (C-5),116.1 (C-6),167.8 (C-7),101.3 (C-8),158.3 (C-9),102.3 (C-10),122.6 (C-1′),129.3 (C-2′),112.3 (C-3′),161.3 (C-4′),112.1 (C-5′),129.5 (C-6′),55.3 (6′-OCH3)。以上数据与文献[17]报道的芒柄花基本一致。
化合物11:灰色针晶。ESI-MSm/z:293.1 [M+Na]+。1H-NMR (600 MHz,acetone-d6)δ:7.41 (2H,d,J=9.6 Hz,H-2′,6′),6.91 (2H,d,J=9.6 Hz,H-3′,5′),6.43 (1H,s,H-8),6.35 (1H,d,J=6.3Hz,H-6),3.03 (1H,s,H-5);13C-NMR (150 MHz,acetone-d6)δ:131.6 (C-1),154.3 (C-2),120.3 (C-3),179.1 (C-4),158.1 (C-5),102.6 (C-6),165.4 (C-7),94.1 (C-8),156.1 (C-9),103.9 (C-10),121.8 (C-1′),129.(C-2′),116.1 (C-3′),163.6 (C-4′),116.1 (C-5′),131.6 (C-6′)。以上数据与文献[18]报道的染料木素基本一致。
化合物12:白色针晶。ESI-MSm/z:323.1 [M+Na]+。1H-NMR (600 MHz,acetone-d6)δ:8.06 (1H,d,J=8.2 Hz,H-2),7.41 (2H,d,J=8.2 Hz,H-2′,6′),6.91 (2H,d,J=8.2 Hz,H-3′,5′),6.51 (1H,s,H-8);13C-NMR (150 MHz,acetone-d6)δ:127.1 (C-1),155.1 (C-2),122.9 (C-3),83.1 (C-4),157.6 (C-5),133.9 (C-6),156.9 (C-7),96.1 (C-8),154.9 (C-9),105.1 (C-10),125.1 (C-1′),132.6 (C-2′),115.3 (C-3′),159.3 (C-4′),115.4 (C-5′),130.7 (C-6′),59.8 (6-OCH3)。以上数据与文献[19]报道的tectorigenin基本一致。
化合物13:白色粉末。ESI-MSm/z:277.1 [M+Na]+。1H-NMR (600 MHz,acetone-d6)δ:8.03 (1H,d,J=9.6 Hz,H-5),7.41 (2H,d,J=9.6 Hz,H-2′,6′),6.87 (1H,m,H-8),6.81 (2H,d,J=9.6 Hz,H-3′,5′),6.79 (1H,d,J=6.3 Hz,H-6);13C-NMR (150 MHz,acetone-d6)δ:129.1 (C-1),154.3 (C-2),123.6 (C-3),176.8 (C-4),129.4 (C-5),116.5 (C-6),163.5 (C-7),101.3 (C-8),115.3 (C-9),104.2 (C-10),122.8 (C-1′),129.3 (C-2′),114.2 (C-3′),158.4 (C-4′),114.5 (C-5′),129.4 (C-6′)。以上数据与文献[20]报道的daidzein基本一致。
化合物14:白色粉末。ESI-MSm/z:471.3 [M+Na]+。1H-NMR (600 MHz,acetone-d6)δ:7.51 (2H,d,H-2′,H-6′),6.93 (2H,d,H-3′,H-5′),6.33 (2H,m,H-6,8),5.39 (1H,dd,J=9.6,6.3 Hz,H-2),4.92 (1H,dd,J=9.6,6.3 Hz,H-1″),4.12 (1H,d,J=9.6 Hz,H-5″),3.58 (1H,t,J=9.6,H-2″),3.49 (2H,m,H-3″,4″),3.16 (1H,dd,J=9.6,6.3 Hz,H-3a),2.81 (1H,d,J=9.6,6.3 Hz,H-3b);13C-NMR (150 MHz,acetone-d6)δ:103.6 (C-1),81.4 (C-2),43.2 (C-3),197.3 (C-4),106.2 (C-4a),167.3 (C-5),98.2 (C-6),165.1 (C-7),97.2 (C-8),165.2 (C-8a),131.2 (C-1′),131.4 (C-2′),117.1 (C-3′),160.6 (C-4′),117.8 (C-5′),130.5 (C-6′),102.3 (C-1″),96.3 (C-2″),73.6 (C-3″),73.1 (C-4″),70.5 (C-5″),173.6 (C-6″)。以上数据与文献[21]报道的柚皮素-7-O-β-D-葡糖醛酸基本一致。
化合物15:白色粉末。ESI-MSm/z:484.1 [M+Na]+。1H-NMR (600 MHz,acetone-d6)δ:7.02 (1H,brs,H-2′),6.91 (1H,brs,H-6′),6.75 (1H,brs,H-5′),6.23 (1H,s,H-6),6.12 (1H,s,H-8),5.26 (1H,dd,J=9.6,6.3 Hz,H-2),5.11 (1H,d,J=9.6 Hz,H-1″),3.32 (1H,m,H-4″),3.22 (1H,dd,J=9.6,6.3 Hz,H-3a),3.14 (2H,m,H-2″,H3″),1.84 (1H,dd,J=9.6,6.3 Hz,H-3b);13C-NMR (150 MHz,acetone-d6)δ:101.3 (C-1),79.7 (C-2),44.3 (C-3),199.1 (C-4),105.5 (C-4a),165.1 (C-5),97.7 (C-6),166.8 (C-7),98.1 (C-8),164.3 (C-8a),131.6 (C-1′),131.7 (C-2′),147.6 (C-3′),147.3 (C-4′),116.3 (C-5′),131.2 (C-6′),100.1 (C-1″),76.1 (C-2″),74.3 (C-3″),72.8 (C-4″),72.9 (C-5″),172.1 (C-6″)。以上数据与文献[21]报道的圣草酚-7-O-葡萄糖醛酸基本一致。
化合物16:白色油状物。ESI-MSm/z:301.2 [M+Na]+。1H-NMR (600 MHz,acetone-d6)δ:7.81 (2H,dd,J=8.2,2.2 Hz,H-3,6),6.48 (2H,dd,J=8.2,2.2 Hz,H-4,5),4.28 (4H,t,J=8.2 Hz,H-l′),1.83 (4H,m,H-2′),1.51 (4H,m,H-3′),1.02 (6H,t,J=7.5 Hz,H-4);13C-NMR (150 MHz,acetone-d6)δ:131.9 (C-1),131.9 (C-2),129.5 (C-3),128.3 (C-4),128.3 (C-5),129.5 (C-6),64.2 (C-l′),31.6 (C-2′),20.7 (C-3′),14.1 (C-4′),168.1 (7-OOC),168.1 (7′-OOC)。以上数据与文献[22]报道的邻苯二甲酸二丁酯基本一致。
化合物17:白色油状物。ESI-MSm/z:413.4 [M+Na]+。1H-NMR (600 MHz,acetone-d6)δ:7.75 (2H,dd,J=8.2,2.2 Hz,H-3,6),7.61 (2H,dd,J=8.2,2.2 Hz,H-4,5),4.18(4H,m,H-1′),0.97 (6H,t,J=8.2 Hz,H-2″),0.87 (6H,t,J=8.2 Hz,H-6′);13C-NMR (150 MHz,acetone-d6)δ:131.5 (C-l),131.5 (C-2),129.1 (C-3),128.6 (C-4),128.6 (C-5),129.1 (C-6),69.3 (C-l′),39.1 (C-2′),31.6 (C-3′),30.5 (C-4′),22.9 (C-5′),13.9 (C-6′),24.1 (C-1″),10.9 (C-2″),165.1 (7-OOC),165.1 (7′-OOC)。以上数据与文献[23]报道的邻苯二甲酸二 (2-乙基)己酯基本一致。
化合物18:白色粉末。ESI-MSm/z:401.2 [M+Na]+。1H-NMR (600 MHz,acetone-d6)δ:6.97 (1H,d,J=8.2,H-2),6.93 (1H,d,J=2.2,H-5′),6.91 (1H,d,J=2.2,H-2′),6.82 (1H,dd,J=8.2,2.2,H-6),6.73 (1H,d,J=2.2,H-5),6.71 (1H,dd,J=8.2,2.2,H-6′),4.73 (1H,d,J=8.2,H-4),4.28 (1H,m,H-7),3.91 (1H,dd,J=8.2,2.2,H-8),3.81 (1H,dd,J=8.2,2.2,H-4′),3.76 (3H,s,3-OCH3),3.47 (2H,t,J=8.2,H-9′),3.28 (3H,s,3′-OCH3),2.58 (2H,t,J=8.2,H-7′),1.78 (2H,m,H-8′);13C-NMR (150 MHz,acetone-d6)δ:133.9 (C-1),114.6 (C-2),148.5 (C-3),47.9 (C-4),120.3 (C-5),122.6 (C-6),75.3 (C-7),87.2 (C-8),61.3 (C-9),131.2 (C-1′),131.2 (C-2′),117.2 (C-3′),160.1 (C-4′),117.1 (C-5′),130.5 (C-6′),139.1 (C-1″),121.3 (C-2″),153.9 (C-3″),147.6 (C-4″),116.2 (C-5″),122.1 (C-6″),33.2 (C-7″),32.8 (C-8″),63.4 (C-9″),56.7 (3-OCH3),46.4 (3′-OCH3)。以上数据与文献[24]报道的4,7,9,9′-tetrahydroxy-3,3′-dimethoxy-8-O-4′-neolignan基本一致。
化合物19:黄色油状物。ESI-MSm/z:260.1 [M+Na]+。1H-NMR (600 MHz,acetone-d6)δ:5.81 (1H,s,H-1),1.37 (3H,s,H-12),1.20 (3H,s,H-13),1.14 (3H,s,H-15),0.97 (3H,d,J=8.2 Hz,H-14);13C-NMR (150 MHz,acetone-d6)δ:122.8(C-1),199.3 (C-2),40.8 (C-3),41.3 (C-4),41.9 (C-5),40.2 (C-6),44.1 (C-7),28.1 (C-8),33.1 (C-9),174.1 (C-10),72.4 (C-11),26.1 (C-12),24.8 (C-13),14.1 (C-14),16.1 (C-15)。以上数据与文献[25]报道的11-hydroxy-valenc-1(10)-en -2-one基本一致。
化合物20:白色油状物。ESI-MSm/z:223.3 [M+Na]+。1H-NMR (600 MHz,acetone-d6)δ:7.12 (1H,d,J=8.2 Hz,H-2),6.93 (1H,d,J=2.2 Hz,H-5),7.03 (1H,dd,J=8.2,2.2 Hz,H-6),5.91 (1H,dt,J=8.2,2.2 Hz,H-8′),5.52 (1H,dd,J=8.2,2.2 Hz,H-9′),5.37 (1H,dd,J=8.2,2.2 Hz,H-17′),5.29 (1H,dd,J=8.2,2.2 Hz,H-16′);13C-NMR (150 MHz,acetone-d6)δ:129.4 (C-1),109.3 (C-2),147.1 (C-3),146.8 (C-4),113.2 (C-5),118.5 (C-6),99.6 (C-7),148.2 (C-1′),134.6 (C-2′),26.7 (C-3′),29.7 (C-4′),29.4 (C-5′),30.1 (C-6′),25.9 (C-7′),33.4 (C-8′),81.2 (C-9′),71.3 (C-10′),78.5 (C-11′),127.4 (C-12′),138.6 (C-13′),28.7 (C-14′),19.3 (C-15′),33.1 (C-16′),20.6 (C-17′),15.1 (C-18′),56.3 (3-OCH3),51.2 (1′-OCH3)。以上数据与文献[26]报道的solacetal C基本一致。
化合物21:白色固体。ESI-MSm/z:231.1 [M+Na]+。1H-NMR (600 MHz,acetone-d6)δ:6.97 (1H,d,J=8.2,H-2),6.64 (1H,d,J=2.2,H-5),6.53 (1H,dd,J=8.2,2.2,H-6),7.36 (1H,d,J=8.2,H-7),6.41 (1H,d,J=8.2,H-8),4.21 (2H,q,J=8.2,H-9);13C-NMR (150 MHz,acetone-d6)δ:126.1 (C-1),114.3 (C-2),1147.2 (C-3),150.1 (C-4),117.1 (C-5),123.5 (C-6),147.1 (C-7),1115.9 (C-8),170.2 (C-9),60.8 (C-1′),15.3 (C-2′)。以上数据与文献[27]报道的咖啡酸乙酯基本一致。
4 保肝活性研究
取冻存于-80 ℃的人肝正常细胞株HL-7702适量,置于37 ℃水浴锅中,使液体融化,移至含10%胎牛血清的1640培养基内,振摇悬浮,离心6 min,去除上清液,再加入含10%胎牛血清的1640培养基,移至培养箱中培养48 h。采用胰蛋白酶消化后,将细胞密度稀释至8×104/mL,以每孔100 μL置于96孔板中,将具有肝损伤的APAP用含10%胎牛血清的1640培养基稀释为12 mmol/L。设置模型组(含12 mmol/L APAP培养基)、正常组(含10%胎牛血清的1640培养基)、化合物组(含12 mmol/L APAP培养基+50、100、200 μg/L化合物)、阳性对照组 (含12 mmol/L APAP培养基+200 μg/L联苯双酯),分别取100 μL置于96孔板中,每组设6个复孔,在37 ℃下培养24 h,采用MTT法考察各化合物对HL-7702细胞相对存活率的影响[28],发现化合物5、10、12、20具有显著活性,在高剂量下更明显(P<0.05),见表1。
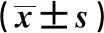
表1 各化合物对HL-7702肝细胞株相对存活率的影响
5 结论
本实验从扯根菜中分离得21个化合物,除化合物3、9外均为首次从该植物中分离得到,其中化合物5、10、12、20可提高HL-7702的相对存活率。上述结果丰富了扯根菜化学成分,可为具有保肝活性的相关新药开发提供参考。

