Probiotics supplementation for management of type II diabetes risk factors in adults with polycystic ovarian syndrome: a meta-analysis of randomized clinical trial
Chengheng Zhang, Yingyue Sheng, Jinhi Jiang, Yuzheng Xue, Leilei Yu,Fengwei Tian, Jianxin Zhao, Hao Zhang,d,e, Jian Jin*, Qixiao Zhai,*
a State Key Laboratory of Food Science and Technology, Jiangnan University, Wuxi 214122, China b School of Food Science and Technology, Jiangnan University, Wuxi 214122, China
c Department of Gastroenterology, Aff iliated Hospital of Jiangnan University, Wuxi 214122, China
d National Engineering Research Center for Functional Food, Jiangnan University, Wuxi 214122, China
e Wuxi Translational Medicine Research Center and Jiangsu Translational Medicine Research Institute Wuxi Branch, Wuxi 214122, China f School of Pharmaceutical Sciences, Jiangnan University, Wuxi 214122, China
Keywords:Polycystic ovary syndrome Type 2 diabetes Glucose homeostasis Probiotic Meta-analysis Randomized clinical trial
A B S T R A C T This meta-analysis of randomized controlled trials aimed to evaluate the effects of probiotic supplementation on glucose homeostasis in patients with polycystic ovary syndrome (PCOS). The meta-analysis was performed in accordance with the Cochrane Handbook guidelines and relevant the preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement criteria. Of 825 identif ied reports, 11 randomized clinical trials were included in the meta-analysis. An analysis of pooled extracted data revealed that supplementation with probiotics signif icantly decreased fasting blood glucose (FBG, n = 7; standardized mean difference (SMD) = -0.40; 95% conf idence interval (CI): -2.02, -0.02; P = 0.04) and insulin levels (n = 6;SMD = -0.57; 95% CI: -0.89, -0.25; P = 0.000 4) and the homeostatic model assessment of insulin resistance(n = 7; SMD = -0.64; 95% CI: -0.96, -0.31; P = 0.000 1) while increasing the quantitative insulin sensitivity check index (QUICKI, n = 5; SMD = 0.58; 95% CI: 0.08, 1.09; P = 0.02) in patients with PCOS. The FBGreducing effect decreased as the baseline body mass index (BMI) and mean age of the participants increased.Indeed, a greater number of bacterial species and a higher bacterial dose were shown to reduce QUICKI effectively. The systematic review indicated that probiotic supplementation may help to control glucose homeostasis in adults with polycystic ovarian syndrome.
1. Introduction
Polycystic ovary syndrome (PCOS) is a common endocrine metabolic disorder affecting women of reproductive age, with a worldwide prevalence of 4%-21% [1]. PCOS is often accompanied by ovulatory dysfunction and insulin resistance [2,3]. Approximately 44%-70% of patients with PCOS exhibit symptoms of insulin resistance [4]. These patients, who tend to have higher fasting blood glucose (FBG) and insulin levels and exhibit insulin resistance, are at risk of developing type 2 diabetes [2,4]. Various mechanisms have been proposed to explain the inf luence of probiotic on glucose homeostasis and type 2 diabetes, such as metabolic endotoxemia [5],modifications in the secretion of the incretins [6], and short-chain fatty acids (SCFAs) production [7]. A number of clinical randomized trials suggested that probiotics consumption could promote the glucose homeostasis [8-10]. Improved metabolic health is associated with improving the richness and diversity of gut microbiota [8,9].Probiotics are live microorganisms that when administered in adequate amounts confer a health benefit on the host [10]. Over the last decade, several studies have evaluated the effectiveness of probiotics in promoting glycemic control in patients with diabetes.The results have suggested that the effects of these supplements are mediated by the regulation of aberrant glucose homeostasis via actions targeting the intestinal microbiota, leading to the production of metabolites such as bile acid and SCFAs [11-15].
Several meta-analyses have reported that probiotics may also improve glucose homeostasis in patients with PCOS [16-19].However, the included studies exhibited a high level of heterogeneity,which was not clearly explained [16,17]. In addition, these metaanalyses did not evaluate the associations of dose responses, body mass index (BMI) values, ages, number of species and type of species with glucose homeostasis [18,19].
With this meta-analysis, we aimed to conduct a broad assessment of the clinical outcomes of probiotic supplementation in women with PCOS. We further aimed to determine whether the number of probiotic species, probiotic dose supplementation, BMI and age of participants would be associated with the most effective control of glycemic homeostasis in patients with PCOS.
2. Methods
2.1 Data sources and searches
This meta-analysis was conducted and reported in accordance with the guidelines of the Cochrane Handbook and the relevant criteria from the referred reporting items for systematic reviews and meta-analyses (PRISMA) statement [20,21]. This study was registered in PROSPERO (CRD42020169045). The Cochrane Central Register of Controlled Trials (http://onlinelibrary.wiley.com/cochranelibrary/search; 1900 to 1 Mar. 2021), Medline (http://www.ncbi.nlm.nih.gov/pubmed; 1945 to 1 Mar. 2021), Web of Science (http://isiknowledge.com; 1900 to 1 Mar. 2021), and Google Scholar (https://scholar.google.com/; 1900 to 1 Mar. 2021) were subjected to a systematic search of the published literature. The following search terms were applied: (polycystic ovarian syndrome OR polycystic ovary syndrome OR PCOS) AND (random OR randomized OR randomized controlled trial OR controlled clinical trial OR randomized studies) OR(probiotics OR synbiotics ORBifidobacteriumORLactobacillusORBacteriaOR fermented milk). The wild-card term “*” was also used to increase the sensitivity of the search strategy. The search was not language restricted. The search was not language restricted. The final search was conducted on Mar. 1, 2021.
2.2 Study selection
Relevant articles were included if they fulfilled the following inclusion criteria. 1) A randomized controlled trial with a parallel or cross-over design. 2) Adult (≥ 18 years old) population with PCOS.3) Sufficient reporting of anthropometric indices in both the intervention and placebo groups. 4) Reported mean ± standard deviation (SD)or standard error (SE) for at least one parameter (FBG, homeostatic model assessment for insulin resistance (HOMA-IR), quantitative insulin sensitivity check index (QUICKI), insulin) in both the control and treatment groups at baseline and at the end of trial. 5) Use of
probiotics in any formulations or in dairy products and any species/strains/treatment/dose of live probiotics or symbiotics. The following exclusion criteria were also applied: 1) non-randomized clinical trials;
2) uncontrolled studies (lack of a placebo control); 3) reported duplicate data; or 4) reviews, letters or case reports. Table 1 presents the participants, intervention/exposure, comparisons, outcomes and study design (PICOS) criteria used to define the research question.

Table 1Inclusion and exclusion criteria following the participants, intervention, comparison, outcomes, and study design (PICOS) approach.
2.3 Data extraction and quality assessment
Two authors independently extracted the following data from the 15 studies deemed eligible for inclusion: study design, first author’s name, publication year, mean age and BMI of the participants,number of participants, sample size, duration of intervention, general details of the intervention (type, form, dose of probiotics), details of both the experimental intervention (type of oral supplementation,dosage, number of species) and the control and outcomes (FBG,insulin, QUICKI, HOMA-IR).
The Cochrane risk-of-bias tool was used to assess the risk of bias of each individual study via a domain-based evaluation [22]. The following criteria were assessed by the two authors: random sequence generation (selection bias), allocation concealment (selection bias),blinding of participants and personnel (performance bias), blinding of the outcome assessment (detection bias), incomplete outcome data(due to the amount, nature or handling of incomplete outcome data),selective reporting (reporting bias) and other bias. Disagreements were resolved by a third adjudicator (J.C.J.). As per the recommendations of the Cochrane Handbook, each item was determined to have a “low”,“high”, or “unclear” risk of bias. Trials with a low or high risk of bias in key domains were categorized as having a low or high risk of bias,respectively. Otherwise, trials were categorized as having an unclear risk of bias.
2.4 Data synthesis and analysis
The meta-analysis was conducted using RevMan version 5.3 (Cochrane Collaboration, Oxford, UK) and Comprehensive Meta-Analysis 2.0 (Biostat, Englewood, NJ, USA). For all PCOS symptoms, mean difference (MDs) in the outcome data were used in the meta-analysis if they were reported by at least three studies. Foroutcomes that could be measured using different units, the reported effects were presented as standardized mean difference (SMD). The meta-analysis also implemented a random-effect model based on the detected heterogeneity among studies [22]. The effect sizes are presented as mean differences with 95% confidence intervals, andP< 0.05 were considered statistically significant. Heterogeneity was explored quantitatively using Cochran’s Q-test [23] and I-square statistics (I2) [24]. Here,I2values of < 25%, 25%-75%and > 75% were considered to indicate low, moderate and high heterogeneity, respectively. When theI2≥ 25%, the possible reasons for heterogeneity were investigated using the following methods:1) subgroup analysis; 2) sensitivity analysis; and 3) random effect meta-regression, to identify which trials caused heterogeneity and how the trials contributed to the overall analysis [25]. Begg’s rank correlation test and Egger’s regression intercept test were applied to each included trial to evaluate publication bias [26-28]. Again,P< 0.05 was considered to indicate significance.
3. Results
3.1 Studies characteristics
The PRISMA statement flowchart in Fig. 1 depicts the process of study selection and reasons for study exclusion. A total of 825 relevant articles were identified through an electronic database search.After eliminating duplicate studies (the same study), the titles and abstracts of the remaining 532 studies were screened. Subsequently,501 studies that did not fulfill the inclusion criteria were eliminated.The remaining 31 studies were subjected to a full-text screen.Finally, 11 qualitative studies met the eligibility criteria and were included in the review. These included 10 randomized, double-blind,placebo-controlled trials [29-38] and 3 randomized, non-blinded,placebo-controlled trials [8]. However, 1 of the 11 included trials comprised 2 intervention groups, and these groups were treated as separate studies, resulting in the inclusion of 12 studies in the metaanalysis [8]. Probiotics were administered in various forms, including beverages [8] and encapsulated freeze-dried powders [29-38].The study interventions involved 3 [31,32,34,37], 4 [29,30,35,36],5 [8], 6 [33] or 7 [38] bacterial species (Table 2). The daily probiotic supplement dose was reported in units of CFU/day in the
meta-analysis.
3.2 Quality assessment
Fig. 2 depicts the results of the risk-of-bias assessment. Overall, 3 studies [29,34,37] were deemed to have a low risk of bias, 4 [30-32,35]had an unclear risk of bias and 4 [8,33,36,38] had a high risk of selection bias [38] or attrition bias [8,33,36]. In the included studies,the participant dropout rates during follow-up ranged from 6.5% to 8.1%. Generally, an attrition rate of < 20% is considered acceptable for randomized clinical trials, especially when the studies have similar numbers of dropouts. Although few studies reported the blinding of outcome assessments, this was less prone to affect the primary efficacy outcome.
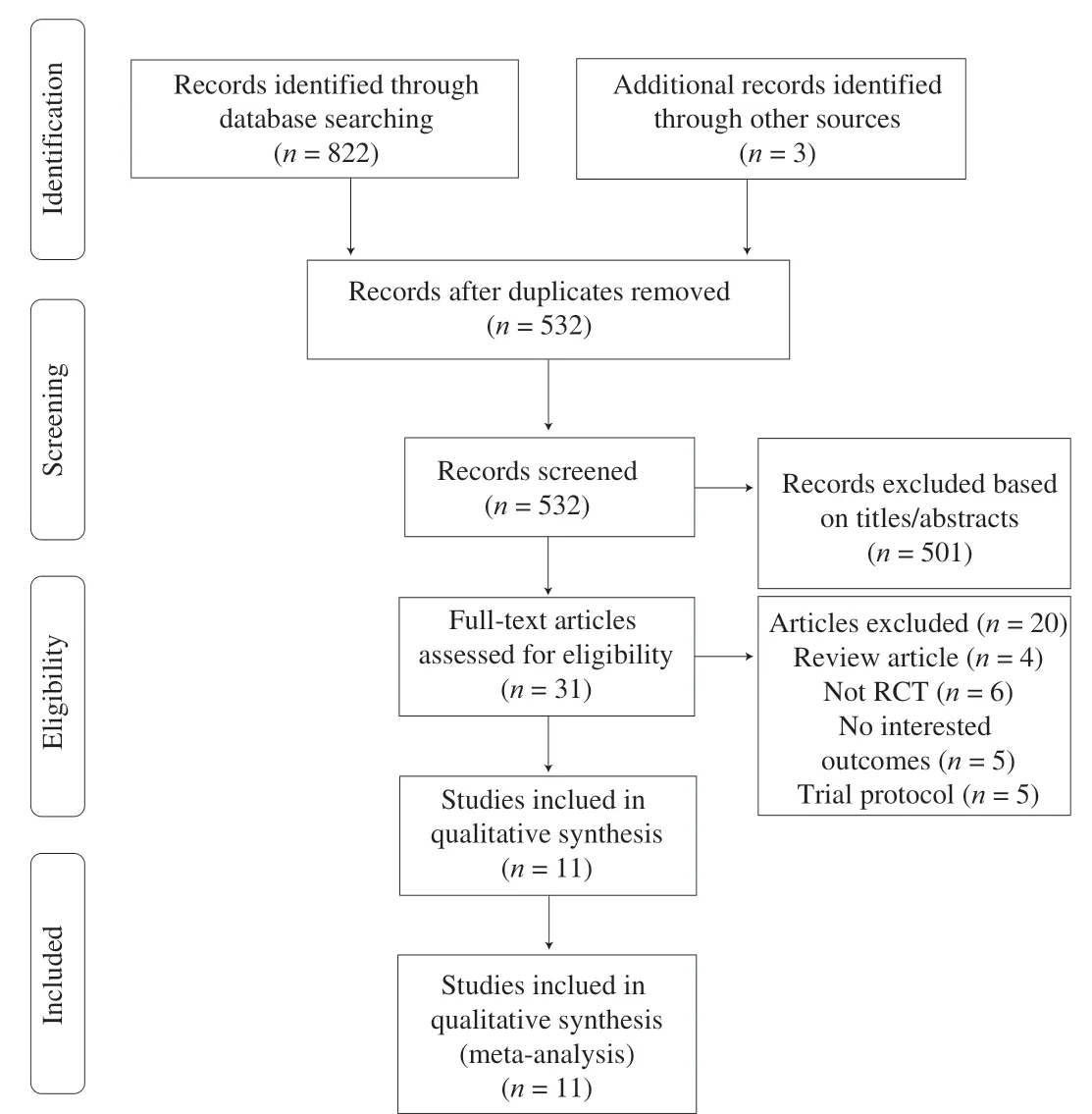
Fig. 1 Flow diagram of the systematic review and meta-analysis.
3.3 Meta-analysis, meta-regression and sensitivity analyses(FBG, Insulin, HOMA-IR, QUICKI)
3.3.1 FBG
Six studies reported the FBG. However, 1 study consisted of 2 intervention groups (both included probiotic), which compared with one placebo group, these were treated as separate studies to reducing heterogeneity among studies in the meta-analysis according to the recommendations of the Cochrane Handbook. Accordingly, 7 studies were included in our meta-analysis. Overall, probiotic supplementation led to significant reductions in the FBG levels of patients with PCOS when compared with placebo (n= 7; SMD = -0.40; 95%confidence interval (CI): -2.02, -0.02;P= 0.04) (Fig. 3A).However, the results of the included studies were heterogeneous(n= 7;I2= 74%;P= 0.000 8). A subgroup analysis did not reveal any significant findings, although a meta regression analysis indicated some effects of mean age and the baseline BMI of the participants.The FBG-reducing effect decreased as the baseline BMI (slope:0.10; 95% CI: 0.04, 0.17;P= 0.003) (Fig. 3B) and mean age of the participants (slope: 0.18; 95% CI: 0.06, 0.31;P= 0.003) (Fig. 3C).The results of the meta regression analysis did not indicate significant associations between the FBG effects of probiotic supplementation and the probiotic dose (slope: 0.15; 95% CI: -0.23, 0.52;P= 0.44),and number of species (slope: -0.05; 95% CI: -0.19, 0.08;P= 0.45)(Fig. S1).
3.3.2 Insulin
Seven studies reported the participants’ insulin levels. A pooled data analysis revealed a significant difference in the changes in insulin levels in the intervention groups when compared with those in control groups (n= 7; SMD = -0.65; 95% CI: -0.96, -0.34;P< 0.000 1)(Fig. 4A). However, significant heterogeneity was observed between the studies (n= 7;I2= 61%;P= 0.02). A sensitivity analysis attributed this heterogeneity to a study by Shoaei et al. [38], and the removal of this study reduced the heterogeneity by up to 55% without altering the insulin-reducing effects (n= 6; SMD = -0.57; 95% CI: -0.89, -0.25;P= 0.000 4) (Fig. 4B). However, a high level of heterogeneity remained among the groups. The insulin-reducing effect became less significant as the mean BMI of the participants (slope: 0.11; 95% CI:0.04, 0.18;P= 0.001) (Fig. 4C). However, no significant associations between the age of the participants, number of probiotic species and probiotic dose with insulin reduction after probiotic administration were observed (Fig. S2).

Fig. 3 Forest plot for data pooling of FBG from eligible studies and meta-regression graph. (A) Overall data pool of FBG. (B) Meta-regression graph for participants’ BMI. (C) Meta-regression graph for participants’ age.

?

?
3.3.3 HOMA-IR
Six studies evaluated the homeostatic model assessment of insulin resistance (HOMA-IR) index. However, one study comprised two intervention groups, which were compared with two placebo groups,and these were treated as separate studies [8]. Accordingly, the metaanalysis included 7 separate studies. The results demonstrated that probiotic use had a significant reducing effect on the HOMA-IR(n= 7; SMD = -0.64; 95% CI: -0.96, -0.31;P= 0.000 1) (Fig. 5A).However, the heterogeneity analysis yielded unacceptable results(n= 7;I2= 64%;P= 0.01), indicating the need for more evaluation.A sensitivity analysis revealed that this heterogeneity was attributable to a study by Karimi et al. [33], and the removal of this study reduced heterogeneity by up to 0 without affecting the HOMAIR-reducing effect (n= 6; SMD = -0.79; 95% CI: -1.01, -0.57;
P< 0.000 01) (Fig. 5B).
3.3.4 QUICKI
Five studies reported the QUICKI. An overall meta-analysis revealed that probiotic supplementation led to a significant increase in QUICKI compared with a placebo (n= 5; SMD = 0.58; 95% CI:0.08, 1.09;P= 0.02) (Fig. 6A). However, the results of these studies yielded significant heterogeneity (I2= 81%;P= 0.000 3). A meta regression analysis based on the probiotic dose and the number of bacterial species in the supplements reduced this heterogeneity.Indeed, a greater number of bacterial species (slope: -0.49; 95% CI:-0.63, -0.35;P= 0.000) (Fig. 6B) and a higher bacterial dose(slope: -0.86; 95% CI: -1.28, -0.43;P= 0.000 07) (Table 2 and Fig. 6C) were shown to increase QUICKI effectively. The mean age and baseline BMI of participant were not shown to be significantly associated with an increasing in QUICKI after probiotic administration (Fig. S3).

Fig. 4 Forest plot for data pooling of insulin from eligible studies and meta-regression graph. (A) Overall data pool of insulin. (B) Data pool of insulin after sensitivity analysis. (C) Meta-regression graph for participants’ BMI.
3.4 Publication bias
A plot of the standard error for each glycemic homeostasis indicators against the corresponding SMD exhibited a typical funnel shape. The effect sizes were distributed symmetrically around the pooled effect sizes for FBG, insulin, HOMA-IR and QUICKI (Fig. 7).No significant publication bias was identified for the meta-analyses of FBG (Begg’s test,P= 0.27; Egger’s test,P= 0.21), insulin (Begg’s test,P= 0.38; Egger’s test,P= 0.11), QUICKI (Begg’s test,P= 0.11;Egger’s test,P= 0.57). Although visual inspection of funnel plots suggested asymmetry for HOMA-IR and Trim and Fill analyses suggest small study effects, Begg’s tests (P= 0.07) and Egger’s tests(P= 0.06) were not significant and adjusted pooled effect estimate after Trim and Fill did not change direction or significance (Table 3).
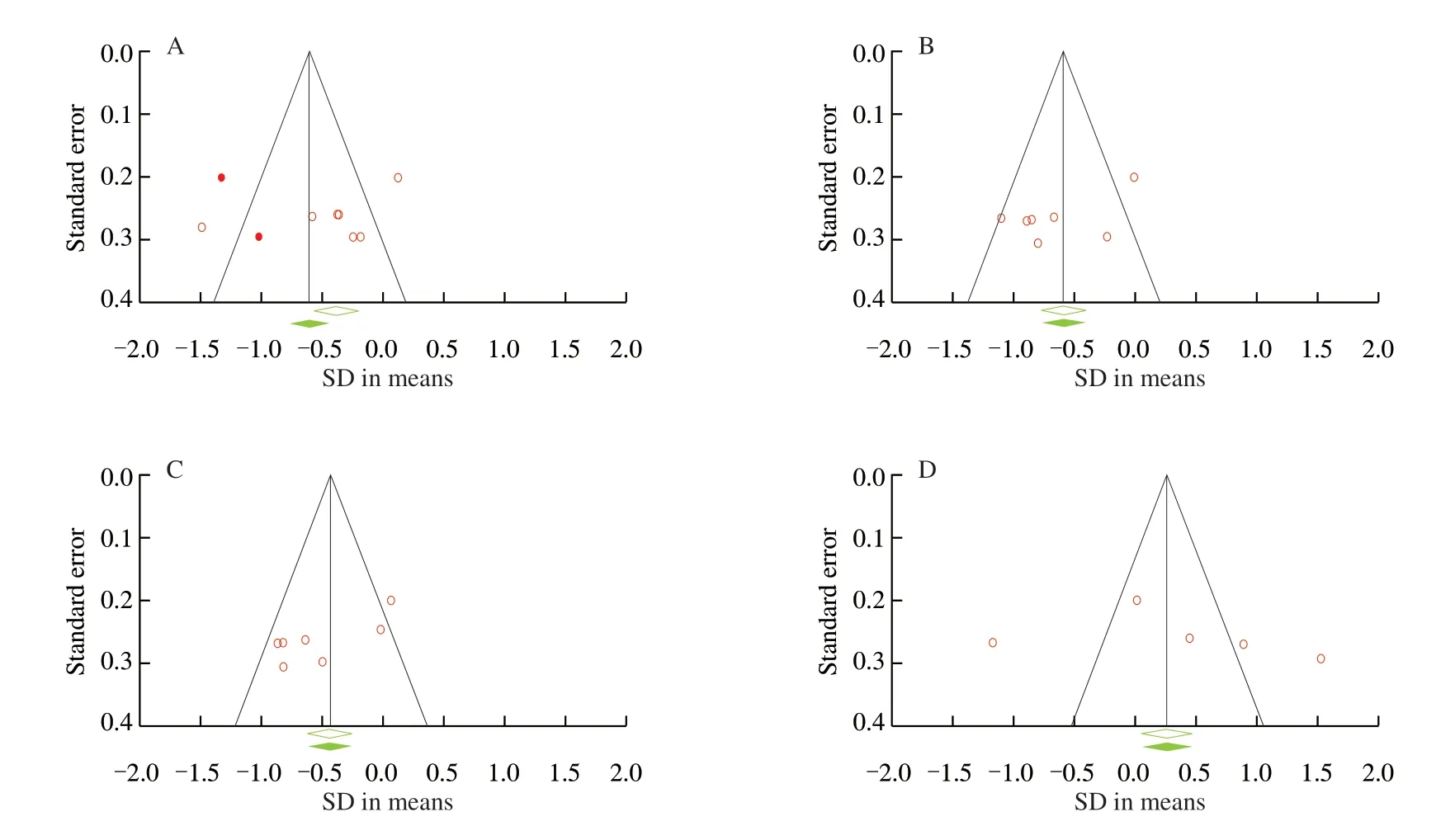
Fig. 7 Funnel plots detailing publication bias in the studies reporting the effects of probiotic supplementation on glucose homeostasis. (A) HOMA-IR. (B) FBG level. (C) Insulin level. (D) QUCKI.
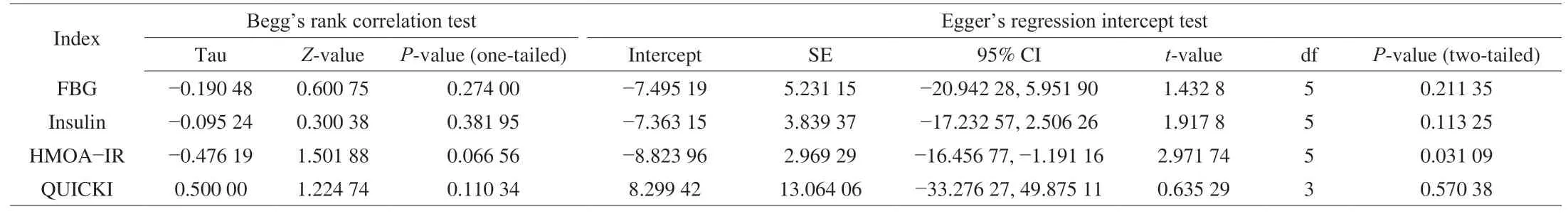
Table 3Publication bias assessment by Begg’s rank correlation test and Egger’s regression intercept test.

Fig. 5 Forest plot for data pooling of HMOA-IR from eligible studies. (A) Overall data pool of HMOA-IR. (B) Data pool of HMOA-IR after sensitivity analysis.

Fig. 6 Forest plot for data pooling of QUCKI from eligible studies and meta-regression graph. (A) Overall data pool of QUCKI. (B) Meta-regression graph forprobiotic species. (C) Meta-regression graph for probiotic dose.
3.5 Adverse events and compliance
Only 5 studies reported adverse events, and no side effects were reported after probiotic supplementation [29,30,32,34,37]. Four
studies reported compliance rate in included studies and compliance
rates were above 90% in all 4 studies [30,32,34,37].
4. Discussion
This meta-analysis suggests that probiotic supplementation may help to control glucose homeostasis in patients with PCOS.Notably, probiotics supplementation could significantly reduce HOMA-IR, insulin and FBG levels and increase QUICKI. Our results were consistent with those of other studies in which probiotic supplementation significantly affected glucose homeostasis in patients with PCOS. Tabrizi et al. [17] reported that probiotic consumption significantly reduced the levels of fasting glucose, fasting plasma insulin and HOMA-IR. However, those meta-analyses did not include a meta-regression of the relationships between participants characteristics (e.g., age, BMI), intervention characteristics (e.g., dose,species, strain) and glycemic homeostasis in PCOS patients. This meta-analysis indicated the potentially beneficial effect of probiotics on glucose homeostasis in patients with PCOS and identified a variety of factors on which this effect depended. Overall, probiotic supplementation led to significant reductions in the FBG levels (SMD of -0.40,P= 0.04), HOMA-IR (SMD of -0.64,P= 0.000 1), insulin levels (SMD of -0.65,P< 0.000 1) and significant increasing in QUCKI (SMD of 0.58,P= 0.02) of PCOS patients when compared with placebo.
A meta-regression based on the participants’ mean age revealed a more significant improvement in glucose homeostasis following probiotic supplementation in younger individuals than in older individuals. These results are consistent with a recent review suggesting an association of the effect of probiotic supplementation on type 2 diabetes with the age of the participants [39].
The effectiveness of probiotic on the insulin and FBG-reducing decreased as the patients’ baseline mean BMI increased. Previous study has previously been demonstrated that insulin and FBG in obese participants (BMI > 30 kg/m2) were less controlled compare with non-obese ones [40]. Arner et al. [40] performed a randomized controlled trials (RCTs) and reported that gut microbiota transplantation from lean donors significantly improve insulin sensitivity in obese patients with metabolic syndrome. These observations, along with ours, suggest that obesity may cause more difficulty in controlling insulin and FBG, and weight loss could increase the effect of probiotics on reducing FBG [41-43].
Previous studies have indicated a direct relationship between probiotic dose and therapeutic effect [44]. The studies included
in our meta-analysis administered a range of probiotic doses
(2 × 109-6 × 1010CFU/day). The meta-regression analysis indicated a more significant improvement in glucose homeostasis indicators with high-dose than low-dose probiotics. Gao et al. [45] reported that 1011CFU yielding superior outcomes and fewer gastrointestinal events compared to 5 × 1010CFU in adult patients. The efficacy of probiotics depends on the survival of probiotic strains through the gastrointestinal tract [46]. As some probiotic strains have a low survival rate, high probiotic doses may ensure the survival of sufficient numbers of live strains during gastrointestinal transit.
A meta-regression based on the number of species revealed a more significant improvement in glucose homeostasis in patients who received a high number of probiotic strains than in those who received a low number. The superior effects of multi-strain probiotics may be attributable to synergistic interactions between individual probiotic strains with various therapeutic activities [13,47]. Different strains of probiotics have different probiotic functions, and the combination of multiple strains can play a synergistic effect. One meta-analysis reported that used multiple probiotic strains showed a statistically significant reduction of necrotizing enterocolitis (NEC) and mortality and enhanced very low-birth-weight (VLBW) infant weight gain,whereas trials with a single strain did not [48]. The probiotics that can effectively regulate blood glucose homeostasis mainly come fromLactobacillusandBifidobacteriain the studies included in the review.However, more well conducted in good design, large sample size and long follow-up time of studies are needed to identify the most effective species or strains and determine which types of bacteria should be included in multi-species or multi-strain supplements.
Probiotic supplementation led to significant reductions in FBG,insulin levels, HOMA-IR and a significant increasing in QUICKI in PCOS patients compared with a placebo, which may through restoring the gut microbial balance [49-51]. Dysbiosis, alterations of the collection of microbes in the gut which mostly observed in PCOS patients, has previously been demonstrated to be restorable by probiotics supplementation [49,50]. Yang et al. [51] reported thatBacteroides acidifaciensadministration resulted in amelioration of insulin resistance and reduced glucose levels in serum in the mice,which may through subsequently alter the commensal bacteria community. Guo et al. [50] reported that microbiota interventions through fecal microbiota transplantation from healthy rats were beneficial for the treatment of PCOS in rats. Therefore, probiotics that influence the richness and diversity of gut microbiota can help to manage glucose homeostasis of PCOS patients.
5. Limitations
Our meta-analysis also had some limitations. There was significant heterogeneity in indices of FBG, insulin and QUICKI,indicating variation between the studies in the evaluation of the effect of probiotics on these outcomes. This could be explained by the different probiotic interventions (e.g., species, dose, number and duration) and participant’s characteristics (e.g., age, BMI). In addition, small sample sizes and differences in the methods used to measure outcomes might contribute to heterogeneity. Further, not all of the studies included analyzed fecal samples collected before and after probiotic supplementation to determine the exact changes in gut microbiota caused by the intervention. The individual characteristics of the baseline microbiome may influence effects of probiotics on health a disease [52], however, all of included references did not report the fecal microbiota, which may be a risk of increased heterogeneity. In addition, most included studies involved relatively modest sample sizes. Therefore, conclusions to be drawn will be limited at least by methodologic problems [53]. In addition, all subjects without hyperinsulinism, with or without hyperglycemia,could have undervalued the association due to a dilution of the effect.This limitation was partially resolved with meta-regression analysis.Finally, there was evidence of publication bias. Although the visual inspection of the funnel plots showed asymmetric for HOMA-IR and Trim and Fill analyses showed small-study effects, we selected not to downgrade for the publication bias, as Begg’s and Egger’s tests were not significant and the adjusted effect estimate after Trim and Fill analyses did not alter direction or significance.
6. Conclusion
In summary, probiotic supplementation can help to reduce the risk of type 2 diabetes mellitus (T2DM) in a patient with PCOS. The FBG-reducing effect decreased as the baseline BMI and mean age of the participants increased. Indeed, a greater number of bacterial species and a higher bacterial dose were shown to reduce QUICKI effectively. However, the FBG-and insulin resistance-reducing effects of probiotics are not sufficiently strong to consider them as alterative nonpharmacologic therapies. This review suggests that probiotic supplements could be beneficial for the management of glucose homeostasis in patients with PCOS. Future clinical studies with superior study designs, large sample sizes and long follow-up periods are needed for the development of clinical practice guidelines.Besides, future studies must also consider the patient characteristics(i.e., age, sex, BMI, microbiome composition, baseline FBG, and baseline HbA1c) and intervention characteristics (i.e., probiotic dose,number of probiotic strains) when evaluate the effect of probiotic supplements on reducing risk of T2DM in a patient with PCOS.
Conflicts of interest
The authors declare no conflict of interest.
Acknowledgments
This work was supported by the National Natural Science Foundation of China Program (31871773 and 32001665); the Natural Science Foundation of Jiangsu Province (BK20200084); National First-Class Discipline Program of Food Science and Technology(JUFSTR20180102); Collaborative Innovation Center of Food Safety and Quality Control in Jiangsu Province; the Wuxi Health and Family Planning Commission (ZDRC039); High-level Health Talents in Jiangsu Province (LGY2018016).
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://doi.org/10.1016/j.fshw.2022.10.023.
- 食品科学与人类健康(英文)的其它文章
- Emerging natural hemp seed proteins and their functions for nutraceutical applications
- A narrative review on inhibitory effects of edible mushrooms against malaria and tuberculosis-the world’s deadliest diseases
- Modulatory effects of Lactiplantibacillus plantarum on chronic metabolic diseases
- The role of f lavonoids in mitigating food originated heterocyclic aromatic amines that concerns human wellness
- The hypoglycemic potential of phenolics from functional foods and their mechanisms
- Insights on the molecular mechanism of neuroprotection exerted by edible bird’s nest and its bioactive constituents

