Physicochemical, structural characterization, and antioxidant activities of chondroitin sulfate from Oreochromis niloticus bones
Jun Yang, Mingyue Shen, Ting Wu, Xianxiang Chen, Huiliang Wen, Jianhua Xie*
State Key Laboratory of Food Science and Technology, Nanchang University, Nanchang 330047, China
Keywords:Oreochromis niloticus by-products Chondroitin sulfate Structural characterization Antioxidant
A B S T R A C T In this study, chondroitin sulfate was extracted from Oreochromis niloticus bones (OCS) and isolated to three fractions (OCS-1, OCS-2, and OCS-3). The physicochemical properties and structure characterization including monosaccharide, disaccharide compositions, molecular weight (Mw) of OCS were determined by HPAEC, HPLC-SAX, HPGPC, FT-IR spectra, and 1D/2D NMR. Moreover, their thermal properties,crystalline structure, and microstructure were also analyzed. Results showed that their Mw were between 10 kDa and 50 kDa. CS-6 was the predominant disaccharide unit in four OCS, and the CS-4/CS-6 ratios were close to CS from shark cartilage. Besides, the results of antioxidant activity showed that different fractions of OCS had a distinct DPPH radical, hydroxyl radical, and ABTS+ radical scavenging activity.OCS-1 has the highest scavenging activities in DPPH and hydroxyl radical compared with other fractions,which showed a higher medicinal value. Those findings may lay some theoretical basis for the potential application development of OCS.
1. Introduction
Oreochromis niloticus(Tilapia), a major aquatic product cultivated in China. Due to its delicious taste and rich nutrition, it is deeply loved by people. China has become the largest market of tilapia products. However, there are large amounts of the problem of tilapia by-products such as fish skin and fish bones, which has caused both large amounts of environmental pollution and many wastes. Therefore, the use of scientif ic methods to create sustainable development and increase additional income from these by-products have aroused widespread interest. Studies have shown that fish by-products contain some active substances beneficial to human health, such as chondroitin sulfate (CS). Therefore, extracting CS from tilapia by-products may be an effective method to solve such problems [1,2].
CS, a product from terrestrial animal cartilage and marine organism, is a natural sulfated polysaccharide. It is composed of repeating disaccharide units of →4)-β-D-GlcA-(1→3)-β-DGalNAc (1→, which can be divided into CS-O (chondroitin),CS-A (chondroitin-4-sulfate), CS-C (chondroitin-6-sulfate), CS-D(chondroitin-2, 6-sulfate) and CS-E (chondroitin-4, 6-sulfate)according to the position of the sulfate group [3]. The structure of CS was affected by its source. For example, CS was extracted from the cartilage of chicken, and pig was mainly composed of CS-A and CS-C. Whereas, CS was extracted from marine organisms such as shark cartilage has a higher amount of disulf ide disaccharide unit of CS-C and CS-D [4]. Besides, a new type of CS extracted from sea cucumbers has been identif ied as fucosylated CS [5]. Therefore, the source extracted of CS plays a key role in its essential structure.
CS has been reported to have a lot of bioactivities such as antitumor, antioxidant, anti-inflammatory, and anticoagulation activities [4]. Moreover, as a major component of the extracellular matrix, CS was related to the conduction of signal molecules,cell growth, and differentiation [6]. Hence, CS has a lot of pharmaceutical applications especially for the treatment of osteoarthritis. Ren et al. [7] found a new CS extracted fromScophthalmus maximushas a potential therapeutic effect for osteoarthritis by protecting chondrocytes, inhibiting the release of pro-inflammatory cytokines such as interleukin-1, tumor necrosis factor-α, and prostaglandin E2. However, the biological activity of CS is greatly influenced by its structure, which varies mainly in disaccharide composition and molecular weight (Mw). CS-A was confirmed that have higher antioxidant activity than CS-C [8].The anti-inflammatory activity of CS was greatly influenced by its disaccharide composition andMw[9]. Hence, figuring out the structure information of CS fromO. niloticuswill be beneficial to understand its bioactivity and explore its potential value.
In this study, CS was extracted fromO. niloticusbones and separated into three components using graded alcohol precipitation.The characterization of CS includingMw, monosaccharide composition, disaccharide composition,, microstructure, crystalline structure, and thermal stability were analyzed. Meanwhile, the antioxidant activitiesin vitroof each component were evaluated and compared. This study may provide a feasible method to solve the pollution and waste problems caused by the processing of fish products, and provide some theoretical basis for the further application of CS fromO. niloticus.
2. Material and methods
2.1 Material
O. niloticusbones were kindly supplied from Zhaoqing(Guangdong, China). CS-A sodium salt (purity > 85%) was purchased from Aladdin (Shanghai, China). Chondroitinase ABC (fromProteus vulgaris, 0.3-3 units/mg) was purchased from Sigma (Shanghai,China). Other agents used in this study were analytical grade.
2.2 Extraction and isolation of CS form O. niloticus bones
The extraction process of CS formO. niloticusbones was referring to previous method with some modification [6]. After crushing, soak the bones in 95% ethanol for 2 h to remove alcoholsoluble substances such as pigments. Discarding the supernatant solution, the fish bone were oven-dried to remove the ethanol. Add water five times the mass of the raw materials and 1% sodium hydroxide, and water bath at 50 °C for 4 h. The supernatant solution were rotary evaporated, after centrifugation, 3% trypsin was added,and the protein was removed at 37 °C for 2 h. After centrifugation, the supernatant was added with Sevage reagent and shaken continuously to remove the remaining protein. The supernatant solution were collected by centrifugation and precipitated with 95% ethanol overnight. The precipitate was re-dissolved in water and dialyzed for three days to remove small molecules, and then lyophilized.
Isolation of CS formO. niloticusbones was carried out according to the previous method with some modification [10]. In brief, the crude extract was dissolved in ultrapure water, then precipitated overnight in 25% ethanol (total concentration), After centrifugal,precipitates were discarded. The supernatants were rotary evaporated to remove ethanol, and then precipitated overnight in 50% ethanol.The precipitates were collected and were redissolved in ultrapure water. The sulution was rotary evaporated to remove the ethanol, then dialyzed, and lyophilized. The supernatant solution were repeated the above procedures with 66% and 80% ethanol. The crude extracts and final three fractions obtained were named OCS, OCS-1 (25%-50%),OCS-2 (50%-66%), and CS-3 (66%-80%), respectively.
2.3 The determination of physicochemical properties
The uronic acid content of OCS, OCS-1, OCS-2, and OCS-3 were determined by the Carbazole-sulfuric acid method [11] using glucuronic acid as a standard. The protein contents were determined by the Coomassie brilliant blue G-250 method using BSA as a standard [12], and the total phenol contents were determined by the Folin method using gallic acid as a standard [13,14]. The CS content of OCS was calculated by glucuronic acid according to previous study [15].
2.4 Structure analysis of OCS
2.4.1 Determination of Mw
TheMwof OCS, OCS-1, OCS-2, and OCS-3 were determined by high-performance gel permeation chromatography (HPGPC)with Agilent 1260 HPLC system (Agilent, USA) equipped with an UltrahydrogelTM-1000 linear column (300 mm × 7.8 mm, Waters,USA) and using a series of differentMwof glucosans as a standard.
2.4.2 Monosaccharide composition
Monosaccharide composition of OCS, OCS-1, OCS-2, and OCS-3 were determined by high-performance anion-exchange chromatography (HPAEC) with pulsed amperometric detection.Samples are hydrolyzed with 12 mol/L sulfuric acid, and then it is placed in an oil bath at 105 °C for 2 h and passed through a 0.22 µm membrane.
2.4.3 Disaccharide composition
Disaccharide composition of OCS, OCS-1, OCS-2, and OCS-3 were determined according to the previous method with some modifications [16]. In brief, OCS was depolymerised by chondroitinase ABC, and analyzed by HPLC (Agilent, USA) with diode array detector at 245 nm equipped with a strong anion-exchange(SAX) column (Waters, USA), and using CS-A as a standard.
2.4.4 Fourier transformed infrared spectroscopy (FT-IR)analysis of OCS
FT-IR analysis of OCS, OCS-1, OCS-2, and OCS-3 was determined using potassium bromide tablet method by an infrared spectrometer (Nicolet 5700, USA). The Scanning wavelength is 4 000-400 cm-1, scanning 32 times, and the resolution ratio is 4 cm-1.
2.4.5 Nuclear magnetic resonance (NMR)
The1H NMR spectrum of OCS-1 was performed refer to previous reports [17,18]. In brief, OCS-1 was lyophilized three times with D2O, and then 30 mg OCS-1was dissolved in 0.5 mL D2O.The sample was acquired by a Bruker AV 600 Mhz spectrometer(Bruker, Switzerland) at 295.2 K and performing at 600.58 MHz. 2D NMR spectra including COSY, HSQC, and TOCSY were obtained according to previous study with some modifications [19]. Parameter:5 mm PATXI 1H probe, 600.58 MHz, 295.2 K. MestReNova Software (version: 5.3.1-4 696) was used for further spectral analysis.
2.4.6 Crystalline structure of OCS
Crystal structures of OCS, OCS-1, OCS-2, and OCS-3 were determined by an X-ray diffractometer (D8 Advance, Bruker Inc.,Germany). The diffraction angle (2θ) was ranges from 4° to 55°.
2.5 Thermogravimetric analysis (TGA)
Thermogravimetric curves of OCS, OCS-1, OCS-2, and OCS-3 were determined by a thermogravimetric analyzer (TGA 4000, PE Instruments Inc., USA). The test temperature was 30-720 °C.
2.6 Microstructure of OCS
The microstructure of OCS, OCS-1, OCS-2, and OCS-3 was observed using a scanning electron microscope (SEM, JSM6701F,Japan). After coated with gold, the samples were observed at 250×and 2 000× times.
2.7 Antioxidant of OCS
2.7.1 DPPH radical scavenging activity
The DPPH radical scavenging activities of OCS, OCS-1, OCS-2,and OCS-3 were measured according to previous methods [20,21].In brief, 0.5 mL sample and 1.5 mL of ultrapure water were added to 2 mL of DPPH (0.2 mmol/L) solution. The mixture was violently shaken and reacted 30 min at 37 °C in the dark. The absorbance was determined at 517 nm by a Thermo Scientific Varioskan Flash(Thermo, USA). 95% ethanol instead of DPPH was used for the blank control. DPPH radical scavenging activity was calculated as the following equation:

WhereA0is the absorbance of DPPH;A1is the absorbance of the mixture without DPPH;A2is the absorbance of mixture of samples and DPPH, respectively.
2.7.2 Hydroxyl radical scavenging activity
Hydroxyl radical scavenging activities of OCS, OCS-1, OCS-2,and OCS-3 were determined according to previous study [22]. In brief, 0.5 mL sample, FeSO4solution (5 mmol/L), and H2O2solution(30%) were mixed for cultivating 10 min at 37 °C. Then 0.5 mL salicylic acid-ethanol solution (3 mmol/L) was added to mixture solution for cultivating 30 min at 37 °C. The absorbance was determined by a Thermo Scientific Varioskan Flash (Thermo, USA)at 510 nm. Hydroxyl radical scavenging activity was calculated as the following equation:

WhereA0is the absorbance of mixture solution without sample;A1is the absorbance of mixture solution;A2is the absorbance of mixture solution without H2O2, respectively.
2.7.3 ABTS+ radical scavenging activity
ABTS+radical scavenging activities of OCS, OCS-1, OCS-2, and OCS-3 were performed referring to previous method [12,23]. ABTS+working solutions were prepared and stored 12 h in the dark. Then 10 µL different concentration (200, 400, 600, 800, 1 000 µg/mL) of OCS reacted with 200 µL ABTS+working solutions for 10 min in the dark. The absorbance of mixture solution at 734 nm was determined.The ABTS+radical scavenging activity was calculated as the following equation:
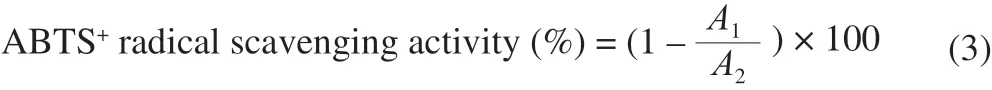
WhereA1was the absorbance of ABTS+working solutions with OCS sample;A2was the absorbance of ABTS+working solutions without OCS sample.
2.8 Statistical analysis
Dates were performed in triplicate and analyzed by Origin software (version 8.0, Stat-EaseInc., Minneapolis, MN, USA). The results were expressed as mean values ± standard deviations.
3. Results and discussions
3.1 Physicochemical properties of OCS
The OCS was extracted fromO. niloticusbones and OCS-1,OCS-2, and OCS-3 were obtained using graded alcohol precipitation.The physicochemical properties of OCS, OCS-1, OCS-2, and OCS-3 are shown in Table 1. The crude extracts have an extremely low content of uronic acid, indicating the CS content in OCS was low.However, the content of uronic acid of the isolated fraction (OCS-1 and OCS-2) greatly enhanced compared with OCS (P< 0.05),indicating that graded alcohol precipitation was a simple and effective method to enrich the content of CS from crude extracts ofO. niloticusbones. Graded alcohol precipitation is a common, inexpensive, and effective way for the separation and purification of polysaccharide based on differential solubility of differentMw. Usually theMwof polysaccharide is negatively correlated with the concentration of ethanol. Therefore, graded alcohol precipitation can enrich the components in a specific range ofMw[24]. OCS-1 have highest CS content, indicating 25%-50% alcohol have the best enrichment effect.Others fractions also were detected a small amount of CS. Besides,OCS, OCS-1, OCS-2, and OCS-3 also have a small number of proteins and phenolic substances, indicating they are both mixture that contain other bioactive substances even after a series of separation and purification processes. Duan et al. also extracted CS from Tilapia byproducts by a combination of ultrasonic and microwave, and the extracted CS was of high purity [1].

Table 1The physicochemical properties of OCS, OCS-1, OCS-2, and OCS-3.
3.2 Structure of OCS
The essential structure of CS was tightly related to its bioactivities. Hence, figuring out the essential structure including monosaccharide composition, disaccharide composition, andMwof OCS was important for its further application. The information on the essential structure of OCS, OCS-1, OCS-2, and OCS-3 was listed in Table 2. TheMwof CS is correlated with its bioactivity,so it is necessary to determine the molecular weight of OCS. The HPGPC images of OCS, OCS-1, OCS-2, and OCS-3 before and after depolymerised by chondroitinase ABC were displayed in Fig. S1. TheMwof OCS-1 (44.6 kDa) and OCS-2 (43.7 kDa) were close, whereas OCS-3 has lowerMw. Cho et al. [25] reported that lowMwof CS have better preventive effects on arthritis compared with high lowMwof CS. Besides, the HPGPC image of OCS was heterogeneous. In contrast, OCS-1, OCS-2, and OCS-3 exhibited more uniform peaks than OCS, indicating that graded alcohol precipitation could enhance the purity of OCS.
In order to further identified the disaccharide composition of OCS, chondroitinase ABC was used to depolymerised OCS and its isolated fractions. The hydrolysis solution of OSC was analyzed by SAX-HPLC and the disaccharide composition was shown in Fig. 1A.CS-A was subjected to the same treatment as a stardard. As shown in Fig. S1, there is a significant increase of retention time of OCS,and its three fractions, which confirmed that the enzymolysis was successful. Compared with CS-A standard, OCS, OCS-1, OCS-2,and OCS-3 all showed that it is composed of CS-0, CS-4, and CS-6. Moreover, CS-A standard displayed a higher content of CS-4 than CS-6. Whereas, OCS, OCS-1, OCS-2, and OCS-3 all showed a higher content of CS-6 than CS-4. But the ratio of CS-4/CS-6 of OCS, OCS-1,OCS-2, and OCS-3 has slight distinction (Table 2). These results were similar to the previous study [1]. This CS-4/CS-6 ratio was close to CS extracted from shark cartilages, which is below 0.7 [26].
Table 2The essential structure of OCS, OCS-1, OCS-2, and OCS-3.

Fig. 1 The structural properties and thermal stability of OCS, OCS-1,OCS-2, and OCS-3. (A) SAX-HPLC profiles and disaccharide composition analysis, CS-A as a standard, (B) The FT-IR spectra from 4 000-400 cm-1,(C) The XRD patterns from 4° to 55°, (D) The TGA and derived thermogravimetric curves (DTG) curves from 30 to 720 °C.
3.3 FT-IR analysis
FT-IR spectra informations of OCS, OCS-1, OCS-2, and OCS-3 were shown in Fig. 1B, which showed a typical FT-IR spectrum of CS. The large characteristic peaks around 3 400 cm-1were attributed to the stretching vibration of O-H and N-H [27]. The majorcharacteristic peaks at 1 648, 1 560, and 1 253 cm-1were attributed to the stretching vibration of C=O, C-N, and S=O, respectively. This proved that the existent of the -COOH group, -NHCOCH3group,and -SO3H group [28]. Besides, the characteristic peak observed at 850 cm-1and 819 cm-1was related to CS-A and CS-C [4], which is consistent with the results of disaccharide composition.
3.4 XRD analysis
XRD is a useful tool to determine the crystal structure of a substance, which can be used to qualitatively analyze the amorphous or crystal structure of polysaccharide. The XRD patterns of OCS, OCS-1, OCS-2, and OCS-3 were displayed in Fig. 1C.All of them displayed a main flat peak at about 21, indicating an amorphous structure. These results are similar to those of previous study [29]. However, the diffraction pattern peak intensity of OCS and OCS-3 was lower than OCS-1 and OCS-2, which it may be related to the content of CS.
3.5 Thermal properties analysis
TGA is an effective method to evaluate the thermal property of polymer with the increase of temperature. It could observe that both the weight of OCS, OCS-1, OCS-2, and OCS-3 gradually lost as the temperature rose from 30 °C to 720 °C (Fig. 1D). The first weightloss stage at a maximum degradation rate of 78 °C was related to the evaporation of water molecules. The second major weight loss stage is around 174-463 °C in the derived thermogravimetric curves (DTG)curve, which corresponds to the degradation of functional groups such as the -COOH group and the -SO3H group. Moreover, this stage may involve the breakage of glycosidic bonds and pyranose rings of CS [2].However, the isolated fraction including OCS-1, OCS-2, and OCS-3 have higher weight loss at the same temperature compared with the OCS, indicating lower thermal stability. OCS showed multiple stages of weight loss at the second weight-loss stage, which may be attributed to the heterogeneity of OCS. There is a large amount of impurity in OCS and influence its thermal property. Oliveira et al.also found the thermogravimetric curve of CS extracted from tilapia exhibited a multiple-stage weight loss trend [2].
3.6 NMR analysis
The further structural characteristics of OCS-1 were analyzed by 1D and 2D NMR spectra (1H, COSY, HSQC, and TOCSY). At 1D1H NMR spectrum (Fig. 2A), there are three spectral regions, first is the anomeric H signals (δ4.5-5.0), the second is the acetamido methyl H signals (δ1.87-1.9), and the others H signals are focused onδ3.16-4.47. All anomeric proton signals are below 5.0, confirming theβ-configuration of OCS-1. The obvious acetamido methyl protons signals proved the GalNAc structure of OCS-1. The proton signal atδ4.61 andδ4.5 was considered as GalNAc 6S H1 and GlcA 6S H1 [30],which confirmed the presence of CS-6 unit in OCS-1. These results consist with the results of disaccride composition and FT-IR.
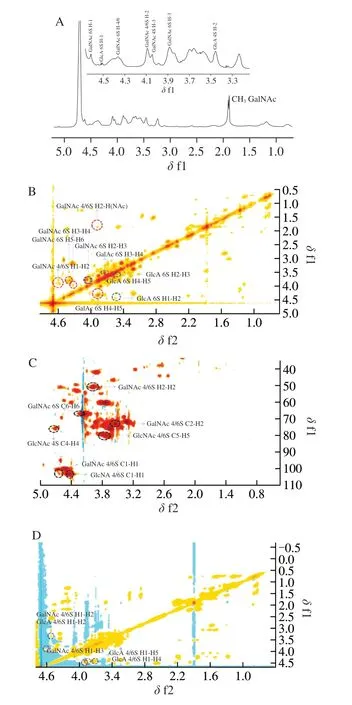
Fig. 2 The NMR spectra of OCS-1 was acquired by a Bruker AV 600 Mhz spectrometer at 295.2 K and performing at 600.58 MHz. (A) 1H spectrum,(B) 2D COSY spectrum, (C) 2D HSQC spectrum, (D) 2D TOCSY spectrum.
Due to the overlap of protons signal atδ3.16-4.47, the 2D NMR spectra (COSY, HSQC, and TOCSY) were applied to assign those proton signals. COSY spectrum provides the coupling relationship between adjacent H in the same molecule of OCS-1. Therefore, it can start with anomeric hydrogen and find the signals of hydrogen on the next carbons in turn (H1-H2, H2-H3, H3-H4, H4-H5, and H5-H6). Combing the HSQC spectrum, the carbon corresponding to hydrogen can be assigned. Moreover, TOCSY can detect the coupling of anomeric protons in sugar molecules with protons passing down the carbon chain (H1-H2, H1-H3, H1-H4, H1-H5, and H1-H6),which can be used to verify the1H assignment of OCS-1. Thus, those overlap proton signal is possible to assign and the results are shown in Figs. 2B-D and Table S1 according to those procedures and previous report [19,30-32]. Besides, the1H of GlcA 2S was hard to assign,indicating the content of disulfide unit in OCS-1 was extremely low.
3.7 The microstructure observation
The surface morphology of OCS, OCS-1, OCS-2, and OCS-3 are observed by SEM and the images (250× and 2 000×) were shown in Fig. 3. The microstructures of OCS, OCS-1, OCS-2, and OCS-3 have significant distinct. OCS displayed a relatively rough and rugged surface structure compared with others, which attributed to its heterogeneity. OCS-1 showed a flake-like structure with a smooth surface. However, OCS-2 exhibited a poriferous surface with an irregular and folded shape. OCS-3 became more unfolded compared with OCS-2, whereas there are a lot of solid particles on its surface that might be some small molecular substances such as oligosaccharide, polyphenol, etc. The microstructure of polysaccharide was affected by various factors such as origin,extraction method, temperature, pH, humidity [33]. Hence, the effect factors of the microstructure of OCS need to further explore.

Fig. 3 The microstructure of (A) OCS, (B) OCS-1, (C) OCS-2, (D) OCS-3 at 250 and 2 000 times were observed by a scanning electron microscope.
3.8 Antioxidant activities
There are a lot of studies that showed CS has favorable antioxidant activities such as DPPH radical, hydroxyl radical, and ABTS+radical scavenging activity [4]. The DPPH radical and hydroxyl radical scavenging activities of OCS, OCS-1, OCS-2, and OCS-3 are displayed in Figs. 4A-B. In general, OCS and its three fractions have good antioxidant activity and the antioxidant activity of the four types of CS is different. Their antioxidant activity all increased with increasing concentrations (200-1 000 µg/mL). OCS-1 has the highest antioxidant activity of DPPH radical and hydroxyl radical scavenging activity. When the concentration of OCS-1 reaches 1 000 µg/mL, the DPPH radical and hydroxyl radical scavenging activities were 49% and 70%, respectively. Those may be attributed to OCS-1 possessed the highest content of CS. The content of CS in OCS-3 was the lowest and it also showed the lowest antioxidant activity. Moreover, the antioxidant activity of OCS was also affected by the content of the phenolic substance. Phenolic substances have been reported to have favorable antioxidant activity [34,35]. OCS has a higher content of total phenol compared with OCS-3, thus the antioxidant activity of OCS was higher than OCS-1, especially for hydroxyl radical scavenging activity. Besides, OCS, OCS-1,OCS-2, and OCS-3 exhibited similar scavenging activity on ABTS+radical (between 50% and 60%) without obvious dose-dependence behavior at concentrations of 200-1 000 µg/mL (Fig. 4C). In general,all these results showed that OCS and its isolated fractions have good antioxidant activities.

Fig. 4 The antioxidant activities of OCS, OCS-1, OCS-2, and OCS-3.(A) The capacity of DPPH radical scavenging activity, (B) the capacity of Hydroxyl radical scavenging activity, (C) the capacity of ABTS+ radical scavenging activity. Different superscript letters in the same curve indicate the significant difference (P < 0.05).
The antioxidant activity of polysaccharide was affected by a lot of elements such asMw, monosaccharide composition, and connection mode of the glycosidic bond [36]. Moreover, the antioxidant activity of CS was also influenced by the type of disaccharide unit, and the discrepancy of the antioxidant activity of OCS and its isolated fraction was related to theirMwand the ratio of CS-4/CS-6. Zhu et al. [6]showed the CS fromAndrias davidianuscartilage has favorable antioxidant activities and its antioxidant activity was similar to CS from bovine trachea. These results showed that OCS and its isolated fractions may have potential applications as nutrient supplements.
4. Conclusions
In conclusion, OCS was extracted fromO. niloticusbones, and OCS-1, OCS-2, and OCS-3 were isolated from OCS through graded alcohol precipitation. OCS-1, have the highest CS content that isolated by 25%-50% ethanol, was the mian fraction. The physicochemical property and essential structure of OCS, OCS-1, OCS-2, and OCS-3 includingMw, monosaccharide composition, disaccharide composition were determined and compared. The results showed OCS-1 has the highest CS content and theMwis around 44 kDa. The disaccharide composition of OCS and its isolated fractions were both composed of CS-O, CS-4, and CS-6 with a CS-4/CS-6 ratio lower than 0.7, which close to CS from shark cartilage. The analysis of 1D and 2D NMR of OCS-1 has also confirmed these results. Moreover,OCS, OCS-1, OCS-2, and OCS-3 have good antioxidant activities,especially for OCS-1. OCS may have some potential pharmacological applications as a substitution for shark cartilage CS.
Conflict of interest
The authors declare that they have no conflict of interest.
Acknowledgments
The financially supported by the Program of The National Youth Talent Support Program of China.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://doi.org/10.1016/j.fshw.2022.10.027.
- 食品科学与人类健康(英文)的其它文章
- Emerging natural hemp seed proteins and their functions for nutraceutical applications
- A narrative review on inhibitory effects of edible mushrooms against malaria and tuberculosis-the world’s deadliest diseases
- Modulatory effects of Lactiplantibacillus plantarum on chronic metabolic diseases
- The role of f lavonoids in mitigating food originated heterocyclic aromatic amines that concerns human wellness
- The hypoglycemic potential of phenolics from functional foods and their mechanisms
- Insights on the molecular mechanism of neuroprotection exerted by edible bird’s nest and its bioactive constituents

