Anti-lipid-oxidation effects and edible safety evaluation of the oil extracted by a supercritical CO2 process from coix seed fermented by Monascus purpureus
Haiying Zeng, Anran Zhu, Shengling He, Mingjun Wu, Muhammad Mazhar,Anyan Wen, Na Liu, Likang Qin,*, Song Miao*
a Key laboratory of Plant Resource Conservation and Germplasm Innovation in Mountainous Region (Ministry of Education), Collaborative Innovation Center for Mountain Ecology & Agro-Bioengineering (CICMEAB), College of Life Sciences/Institute of Agro-bioengineering, Guizhou University, Guiyang 550025, China b School of Liquor and Food Engineering, Guizhou University, Guiyang 550025, China
c Teagasc Food Research Centre, Moorepark, Fermoy, Co.Cork P61 C996, Ireland
Keywords:Coix seed oil Composition Edible safety Mutagenicity Genotoxicity
A B S T R A C T The physicochemical properties and composition of coix seed oil produced by Monascus purpureus fermentation and supercritical CO2 extraction were determined. Anti-lipid-oxidation and edible safety were evaluated using a cholesterol-fish oil model, acute oral toxicity assay, and genetic toxicity assay in vitro and in vivo, respectively. The results show that the extraction oil from fermented coix seed (FCS-O) had good physicochemical quality and abundant active components with physiological function. In particular,γ-tocotrienol, γ-oryzanol, coixenolide and oleic acid concentrations reached 72.83 µg/g, 745.96 µg/g,9.65 mg/g and 316.58 mg/100 g DW, respectively. The FCS-O exhibited higher antioxidant capability in inhibiting lipid oxidation and peroxidation. Compared to the blank control, the concentrations of 7-ketocholestreol and peroxide only were 8.42 µg/mL and 16.16 mmol/kg at 168 h of oxidation (P < 0.01). In addition, the FCS-O has been conf irmed to be a very safe edible oil, with no acute toxicity (LD50 > 10 g/kg bw,considered actually non-toxic) and no induced mutagenicity, cytotoxicity or genotoxicity. These results serve as a good safety reference for future application of the oil from fermented coix seed. The development and utilization of this kind of oil will be benef icial as a food, food ingredient, nutritional supplement, or natural food antioxidant to promote good health function.
1. Introduction
Coix (Coix lachryma-jobiL.) is an annual plant that belongs to the grasses family, and it is a close relative of maize [1]. As a special cereal crop used for both medicine and food, it is widely cultivated in Asian countries, including China, Japan, Thailand, Burma, Laos,and India [2]. The ripe seed kernel of coix is coix seed, also known as adlay, Job’s tear, or Chinese pearl barley. Recently, many reports have recognized coix seed for its excellent nutrition because it is rich in various bioactive compounds and has numerous important health and medical functions, such as anticancer, anti-inflammatory, antiallergic, anti-oxidant, serum calcium and blood glucose reducing effects [3-5]. Coix seed has generally been considered a high-end nourishing cereal, and it is currently accepted as a whole food product in Western countries.
Oil is an important component of coix seed, with many beneficial functions to human health, including anti-tumor, antiinflammatory, analgesic, antiviral, immune boosting and weight loss effects [6,7]. Coix seed oil, in particular, is rich in coixenolide,which is a unique and important functional ingredient for reducing inflammation, purulence and pain, as well as providing anti-tumor effects [8]. Although coix seed oil is well-known for its broad antitumor spectrum, its low bioavailability and oxidation stability limit its application [9]. A lot of lipophilic active compounds in coix seed are bound with the fiber of coix seed. It is difficult to free the bound compounds for absorption by the human body. Thus, they have lower bioavailability or bio-absorption [10]. Fermentation is a better biotransformation pathway to improve the bioavailability,antioxidation and biological actives of raw food materials with high efficiency [11-14]. Our previous study found that fermentation by
Bacillus subtilisorMonascus purpureuscan significantly enhance the antioxidant and anticancer activities as well as health benefits of coix seed [15,16]. The fermented seed could be used as a food ingredient or supplement to offer lipophilic coix seed antioxidants with high bioavailability to increase their efficacy for health promoting function. A new product, coix seed tea fermented byM. purpureus,has been described in China Patent No. CN 201810372538.3.However, the coix seed oil from microorganism fermentation has not been well studied. As coix seed is rich in oil, the study of the extracted oil from fermented coix seed, including its physicochemical properties, bioactive compounds and anti-lipid-oxidation properties,will be necessary for developing new products and promoting the development of the coix seed industry.
Typically, coix seed oil is obtained using mechanical or chemical processes. Mechanical processes are often associated with low yields,while chemical extraction methods often involve the use of harmful organic solvents [17]. In this study, supercritical CO2extraction, a safe, clean and environmentally friendly process, was performed to extract the oil of fermented coix seed and to maximize the retention of the active ingredients in the extract with no solvent residue. The physicochemical properties and compositions of the extracted oil were characterized, and the anti-lipid-oxidation effects were evaluated. The results of this study could be helpful in understanding the quality and application of the oil in fermented cereals. The oil of fermented cereal could be a healthy processed food or ingredient with highly bioavailable functional compounds and enhanced health-promoting function.
Currently, food security is a global concern and a great scientific challenge. Therefore, prior to bringing a new microorganismfermented oil onto the market for human consumption, the safety of the ingredient must first be demonstrated [18]. In this study, the edible safety of coix seed oil produced byM. purpureusfermentation and supercritical CO2extraction was also assessed in a 14-day acute oral toxicity study in mice, anin vitrobacterial reverse mutation assay, anin vivobone marrow cell micronucleus assay and anin vivochromosome aberration assay. The goal was to evaluate the acute toxicity, mutagenicity, cytotoxicity and genotoxicity of fermented seed oil and determine its safety for use in dietary supplements.
2. Materials and methods
2.1 Chemicals and animals
Citrinin, sterols, tocopherols, tocotrienols,γ-oryzanol,fatty acids and cholesterol, 7-ketocholesterol, menhaden fish oil, 2,2’-azobis(2-methylpropionamidine) dihydrochloride(AAPH), methyl methanesulfonate, 2-acetylaminofluorene,1,8-dihydroxyanthraquinone, and cyclophosphamide were purchased from Sigma Aldrich (St. Louis, MO, USA). HPLC-grade hexane and acetic acid were purchased from Tianjin Kermel (Tianjin, China).SPF (Specific Pathogen Free)-grade Kun Ming mice were purchased from the Experimental Animal Center of Guizhou Medical University(animal production license NO. SCXK [Qian] 2012-0001; use license NO. SYXK [Qian] 2014-001; quality certificate NO. 1201085). All mice weighing (20 ± 2) g were housed in a well-ventilated room and maintained under standard laboratory conditions at (22 ± 2) °C,(55 ± 10)% relative humidity and 12 h artificial photoperiod. All animal care and experimental protocols complied with the Animal Management Rules of local authorities and care and use of the Laboratory Animals of the Experimental Animal Center of Guizhou University.
2.2 Preparation and extraction of coix seed oil
Coix seed was provided by Guizhou Xinlong Green Development Co., Ltd. (Guiyang, China).M. purpureusCGMCC 3.4629 was purchased from the China General Microbiological Culture Collection Center (CGMCC, Beijing, China). The activatedM. purpureus(≥ 106total spores/mL) were inoculated into sterilized coix seed(10 mL/100 g) to ferment at (30 ± 1) °C for 10 days. Then, fermented coix seed was low-temperature dried (≤ 8%) and milled using a McGill No. 2 mill (McGill, Brookshire, TX, USA). The milled seed was collected and stored at -20 °C before extracting the oil. The oil of fermented coix seed was extracted using a supercritical CO2extraction apparatus (Model-HT500-5L, Guizhou Aerospace Wujiang,Ltd., Guizhou, China) at 40 °C and 26 MPa. The extraction time was 120 min, and the CO2flow rate was 3.5 L/h. For each extraction, 1 000 g of fermented coix seed powder (0.3-0.4 mm) were placed into the extractor, and the pressure was carefully maintained at a constant 26 MPa in the extractor when the set temperature was reached. The extraction oil from fermented coix seed (FCS-O) was weighed (dry weight basis) and stored at -20 °C before determination. The oil of raw coix seed (CS-O) was prepared by the same methods. The extraction yield of the oil was calculated using the following formula:

Where,M0is the initial mass of sample put into the extractor;Mtis the mass of extracted oil.
2.3 Determination of physicochemical properties and compositions in FCS-O
Physicochemical properties of extraction oil were analyzed and determined according to the state standard for edible plant oil and method described by Szabo et al. [18]. The measurement methods of each index are shown in the Table 1. Citrinin was determined via an Agilent 1260 chromatographic system with a fluorescence detector (Agilent Technologies Inc., Santa Clara, CA, USA). HPLC analysis conditions were as described by Huang et al. [19]. According to methods described by Shen et al., sterols and fatty acids were determined by GC-MS and GC-FID, respectively [20]. Tocopherols,tocotrienols and oryzanol were determined using a normal-phase HPLC system with a fluorescence detector. The HPLC analysis conditions were as described by Jang et al. [21] and Xu et al. [22]. The concentration of each component was calculated based on standard curves. Coixenolide was determined by employing the method of Yang et al. [23]. The total-sterols, total-tocols, total unsaturated and total saturated fatty acids were calculated, respectively.

Table 1Physicochemical properties of FCS-O.
2.4 Anti-lipid-oxidation effects of FCS-O
Preparation of cholesterol-fish oil emulsion. Cholesterol-fish oil emulsion (500 mL) consisted of 500 mg cholesterol, 5 g menhaden fish oil, 50 mg AAPH, and 5 mL Tween-20. The emulsion was adequately mixed and diluted with a pH 7.0 PBS solution to volume. Next,1 mL FCS-O and 20 mL cholesterol-fish oil emulsion (1:20,V/V)were combined in a 50 mL centrifuge tube. The solution was fully mixed by ultrasound for 10 min. Then, the tube was placed in a 37 °C water bath and shaken at 200 r/min for 7 days. The emulsion without extraction oil was set as the blank control. Every day,1 mL mixture emulsion was analyzed to monitor the concentration change of 7-ketocholesterol until the 7thday of oxidant reaction.The 7-ketocholesterol was determined using a normal phase HPLC system with a series of fluorescence and UV detectors. The HPLC analysis conditions were as described by Tian et al. [24]. In addition,the change in peroxide concentration in mixture emulsion was also monitored (Methods refer to 2.3., GB 5009.227-2016, China).
2.5 Safety evaluation of FCS-O
Acute oral toxicity of FCS-O was evaluated according to the limited method of National Standard for oral acute toxicity assay (GB 15193.3-2014, China). Twenty-four mice (50% males and females)were randomly divided into three experimental groups and one control group. The acute oral toxicity of FCS-O was determined according to the acceptable daily intake (ADI) estimation.
The experimental doses of the extracted oil were 10, 1, and 0.1 g/kg bw. Following administration, mice were closely observed every day until they were sacrificed at day 14 for signs of general conditions, clinical symptoms and the incidence of mortality. Body weights of mice were measured at days 0, 7 and 14. During the testing period, animal drinking water was unlimited, and food was rationed and refilled daily.
The mutagenicity of FCS-O was determined using the plate incorporation method of National Standard for bacterial reverse mutation assay (GB 15193.4-2014, China).Salmonella typhimuriumstrains TA97a, TA98, TA100, TA102 and TA1535 were purchased from Moltox Molecular Toxicology Inc. (Molecular Toxicology,Boone, NC, USA). Their genetic backgrounds were confirmed and controlled according to National Standard for Ames (GB 15193.4-2014, China). Then, these strains were activated, reaching approximately 109cells/mL. The experiment was carried out with two concentration groups. For the first group, the doses were 50, 158,500, 1 580, and 5 000 µg/plate. For the second group, the doses were 8, 40, 200, 1 000, and 5 000 µg/plate. The Ames test was performed both with and without the S9 activation system (a post-mitochondrial fraction, from the livers of outbred male albino rats treated with Aroclor 1254), which was freshly prepared before each test. In the absence of S9 mix, the positive control forS. typhimuriumTA97a and TA98 was Dexon. For TA100 and TA102, the positive control was methyl methanesulfonate, and for TA1535, it was sodium azide. In the presence of S9 mix, the positive control forS. typhimuriumTA97a,TA98 and TA100 was 2-acetylaminofluorene. For TA102, the positive control was 1,8-dihydroxyanthraquinone, and for TA1535, it was cyclophosphamide. Whether or not S9 mix was present, the negative,solvent and spontaneous recovery mutation controls for all strains were dimethyl sulfoxide, corn oil and sterile water, respectively. All of the plates were prepared and tested in triplicate. The number of revertant colonies was counted for all strains. A comparison with the spontaneous recovery mutation and negative (solvent) controls was performed. If the number of the revertant colonies increased by two times or more in the test plates, and they were biologically relevant and/or dose-dependent, the assay would be judged positive for mutagenicity; in the opposite case, it was negative.
The effect of FCS-O on bone marrow cell micronucleus was performed according to National Standard for bone marrow cell micronucleus (GB15193.5-2003, China). Based on a restricted randomization procedure, 50 mice were divided into 5 groups, with 5 males and 5 female mice in each group. Experimental mice were treated twice with 2.5, 5.0 and 10.0 g/kg bw of FCS-O by an oral gavage at 24 h intervals. Positive control mice were administered 0.05 g/kg bw cyclophosphamide (CPA), and negative control mice were treated with corn oil. Six hours after the second oral administration, the mice were sacrificed by cervical dislocation.The sternal bone marrow of each mouse was removed and diluted by fetal bovine serum. The marrow was smeared on a clean and dry glass slide, fixed with methanol, and stained with 2% Giemsa for microscopic observation. Two thousand bone marrow polychromatic erythrocytes (PCE) from each mouse were examined with an oil immersion microscope, and the number of PCE with one or more micronucleus were counted. At the same time, normochromatic erythrocyte (NCE) cells were also observed and recorded.
The effect of FCS-O on chromosomal abnormality in mice spermatocytes was examined by National Standard for spermatogonial cells or spermatocyte chromosomal aberration assay (GB 15193.8-2014, China). Briefly, all of the doses were the same as that of the bone marrow cell micronucleus assay. A total of 25 male mice weighing (30 ± 5) g were randomly divided into 5 groups and gavaged for 5 consecutive days. Positive control mice were intraperitoneally injected once with 0.01 mL cyclophosphamide (0.05 g/kg bw). On the 13thday after the first oral administration, all mice were injected simultaneously with colchicine once (6.0 mg/kg bw). 5 h later, mice were sacrificed by cervical dislocation. The testis of each mouse was taken out to separate the seminiferous tubule, which was transferred to a centrifuge tube. Through hypotonic treatment, fixation, softening and fixation, the harvested cells were centrifuged and stored at 4 °C overnight. Finally, the cells were observed under a microscope. At least 500 metaphase cells should be detected to record structural chromosome aberrations (including fragment, translocation, minute chromosome, autosomal univalent, x-y univalent, and gap). The rate of chromosomal abnormality of mouse spermatocyte was expressed as a percentage, taking into account only fragment, minute chromosome and translocation.
2.6 Statistical analysis
The above determinations were carried out in triplicate or more times and expressed as the means ± standard deviations (SD). The SPSS 22.0 software (IBM Company, New York, USA) was used for statistical analysis. The significant differences between the two groups were determined by ANOVA. Difference between two groups was determined at a significant differenceP< 0.05 or at an extremely significant differenceP< 0.01.
3. Results and discussion
3.1 Physicochemical properties and compositions of the oil from fermented coix seed
Figs. 1a and 1b show the changes of coix seed before and after fermentation. The original color of raw coix seed was a yellowish white color, and it changed to reddish brown in the middle of fermentation. The pigments are the secondary metabolites of polyketides, biosynthesized by malonyl-CoA catalysis from tetraketide, pentaketide to hexaketide [25,26]. They accumulated in the solid-state aerobic fermentation ofM. purpureus. After 10 days of fermentation, the culture coix seed was inactivated by drying.Then, the oil of fermented coix seed was extracted by an optimized supercritical CO2extraction method, and the yield of the extracted oil was 12.80% (raw coix seed, 5.10%). The fermentation significantly released bound forms of lipophilic compounds in coix seed. These compounds may be attributed to enzymatic hydrolysis reactions of the microorganism during fermentation [27]. Fermentation could produce a significant amount of acids and decrease the pH of the fermented medium. Acid hydrolysis also participated in the release of bound or blocked forms of lipophilic compounds [20,28]. Additionally, the coix seed oil extracted by supercritical CO2extraction has been found to be of better quality than solvent-extracted oil. It has better color,odor, and flavor. Moreover, it is much more clear, golden, and bright(Fig. 1c, Table 1).

Fig. 1 The preparation of coix seed oil produced by M. purpureus fermentation process. (a) Raw coix seed. (b) The coix seed fermented by M. purpureus in the middle of fermentation. (c) The oil of raw coix seed (left)and the oil of fermented coix seed (middle) produced by supercritical CO2 extraction, the oil of fermented coix seed (right) produced by hexane extraction.
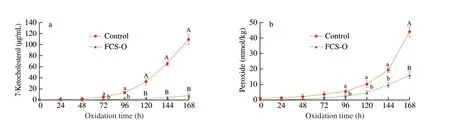
Fig. 2. Anti-lipid-oxidation effects of FCS-O. The production of 7-ketocholesterol (a) and the change in peroxide concentration (b) with or without FCS-O in the cholesterol-fish oil emulsion at different oxidation times. The concentrations with different letters at the same oxidation reaction time were significantly different(lowercase letters, P < 0.05; capital letters, P < 0.01).
The physicochemical properties and compositions of the oil from fermented coix seed (FCS-O) were determined using the state standard for edible plant oil, HPLC, GC-MS and internal methods.The physicochemical properties of the oil conformed to the standard of edible vegetable oil (Table 2). For example, the acid value, iodine value, unsaponifiable matter, peroxide value and other indicators were all far better than those of National Standard of edible oil (GB 2716-2018, China), and they were no significant difference (P> 0.05) with the oil from raw coix seed (CS-O). Furthermore, it also contained rich sterols (68.75 µg/g), tocols (125.62 µg/g),γ-oryzanol (745.96 µg/g)and coixenolide (9.65 mg/g) (Table 2), and the contents were much higher than the levels in the raw oil or most cereals [29-31].A number of studies have reported that these compositions are all active components with special physiological functions. For example,sterols have been confirmed as crucial roles in maintaining extensive cholesterol homeostasis. These sterols are characteristic functional components inMonascus-fermented rice,Cordyceps sinensisandGanoderma lucidumfor dual-purpose of drug and dietary supplements [32].γ-Tocotrienol (72.83 µg/g in the oil) has recently been reported to have greater health promoting function than other tocols [33,34].The level ofγ-oryzanol in the oil of fermented coix seed reached 745.96 µg/g. It is one type of plant sterols, and it contains ferulic acid in its structure [10]. Thus,γ-oryzanol is an antioxidant phytosterol because ferulic acid is a strong antioxidant. It has a special antioxidant activity in preventing cholesterol oxidation, which could generate toxic cholesterol oxidation products in food and the body, resulting in the development of cardiovascular diseases [35]. Coixenolide is a diol lipid uniquely present in coix seed. It is a long carbon chain with two long chain fatty acid esters. Although it may not have antioxidant function based on its chemical structure, previous studies reported that it has anticancer activity [36]. Also, oleic acid was the predominant fatty acid in the oil (316.58 mg/100 g), followed by linoleic acids (193.38 mg/100 g). Though the contents of totalunsaturated fatty acids was reduced by fermentation, but its content was till as high as 551.22 mg/100 g. The reason for the decrease of fatty acid was related to the consumption of fatty acids due to the growth ofM. purpureus.And it also reflected the relationship between the decomposition of esterase and the synthesis of lipase from the starter culture [37]. Overall, the fermentation byM. purpureusis a new approach to effectively increase the yield of extracted cereal oil and the contents of important bioactive compounds. The high levels of these active compositions could significantly enhance the health benefits of FCS-O. Fortunately, no citrinin was detected in the oil.The production of citrinin was well controlled by strain selection and fermentation regulation [38,39].

Table 2Compositions of FCS-O.
3.2 Anti-lipid-oxidation effects of the oil from fermented coix seed
The cholesterol oxidation products, especially in the serum,have been confirmed to cause various cardiovascular diseases, such as inflammation of the macrophage and endothelial cells, leading to the formation of the vessel plaque [24,40]. The higher cholesterol level could lead to a higher possibility of cholesterol oxidation,which increases the risk of chronic diseases. 7-Ketocholesterol is a stable oxidation product of cholesterol oxidation via 7-hydroperoxycholesterol and 7-hydroxycholesterol [41]. In this study, 7-ketocholesterol, the key component of cholesterol oxidation products, were used as the target analyte to evaluate the anti-lipidoxidation effects of FCS-O. It proved a satisfactory method to predict the capability of FCS-O in inhibiting cholesterol oxidation in an emulsion as well as predict the health efficacy of the oil and its future application values. Fig. 2a shows the production of 7-ketocholesterol in cholesterol-fish oil emulsion with or without FCS-O at different oxidation times. Compared with the control group, the treatment group could significantly inhibit cholesterol oxidation induced by AAPH. After 72 h of oxidation, the difference between the two groups was significant. 7-Ketocholesterol in the control group reached 109.40 µg/mL, while that in the treatment group was only 8.42 µg/mL at 168 h of oxidation. Thus, the inhibition cholesterol oxidation capability in mixture emulsion was significantly improved by adding FCS-O. The reasons were closely related to the abundance of antilipid-oxidation active components in the oil (Table 2). In previous studies, tocols extracted from rice bran have been reported to have higher anti-lipid-oxidation activity [41,42].γ-Oryzanol has a special antioxidant activity in preventing cholesterol oxidation, which could generate toxic cholesterol oxidation products in foods and the body, resulting in the development of cardiovascular diseases [35].Recently,γ-oryzanol, as a new lipophilic antioxidant, has also been studied to stabilize corn oil and fish oil in yogurt [43,44]. Meanwhile,the change in peroxide concentration in the emulsion was also monitored. Peroxide concentration is an index of rancidity. The change in peroxide value of oil indicates the resistance capability of the oil to peroxidation during storage [45]. Fig. 2b shows that although the peroxide concentration of the treatment group increased slowly, it was also significantly lower than that of the control group,especially after 96 h of oxidation. The peroxide concentration in the control group increased to 43.93 mmol/kg from 1.32 mmol/kg.The concentration in the treatment group with FCS-O was only 16.16 mmol/kg. Again, these results reveal that FCS-O has stronger antioxidant ability to inhibit lipid oxidation and delay rancidity. At the same time, unsaturated fatty acids of FCS-O (Table 2) could also further enrich the fatty acid composition and enhance the quality of the emulsion. Overall, the results indicated that FCS-O had higher anti-lipid-oxidation activity than the control group. FCS-O could be a good natural food preservative or food ingredient to reduce lipid oxidation and delay rancidity in food. In view of its potential application value, edible safety of FCS-O was further evaluated.
3.3 Safety evaluation of oil extracted from fermented coix seed
The acute toxicity of FCS-O was evaluated by oral perfusion in KM mice. The results are shown in Table 3. During the testing period,the mice had normal activity, smooth hair, no poisoning or death, and no abnormal pathological anatomy (LD50> 10 g/kg bw). According to dose classification of LD50in the National Standard for oral acute toxicity assay (GB 15193.3-2014, China), FCS-O belonged to the practically non-toxic category.
The bacterial reverse mutation assay has been used worldwide as an initial screen to determine the mutagenic potential of new foods,chemicals and drugs [18,46]. In this assay, TA97a, TA98, TA100,TA102 and TA1535, amino acid-requiring strains ofS. typhimurium(0.1 mL/plate), were used to detect point mutations, which involvesubstitution, addition or deletion of one or a few DNA base pairs.Table 4 shows that regardless of whether the S9 metabolic activation system was added, the amount of reverse mutation colonies of TA97a,TA98, TA100, TA102 and TA1535 in each dose group of FCS-O was within the normal range, and there were no significant differences from the negative, solvent and spontaneous recovery mutation controls(P> 0.05). However, the amount of reverse mutation colonies of TA97a,TA98, TA100, TA102 and TA1535 in the positive controls with S9 or without S9 activation system was more than twice that of the spontaneous recovery mutation control, and the differences were significant (P< 0.01). The experimental results show that FCS-O had not induced mutagenicity on any strains TA97a, TA98, TA100, TA102 or TA1535, regardless of whether the S9 mix was present, and the results were also consistent at different concentration groups.
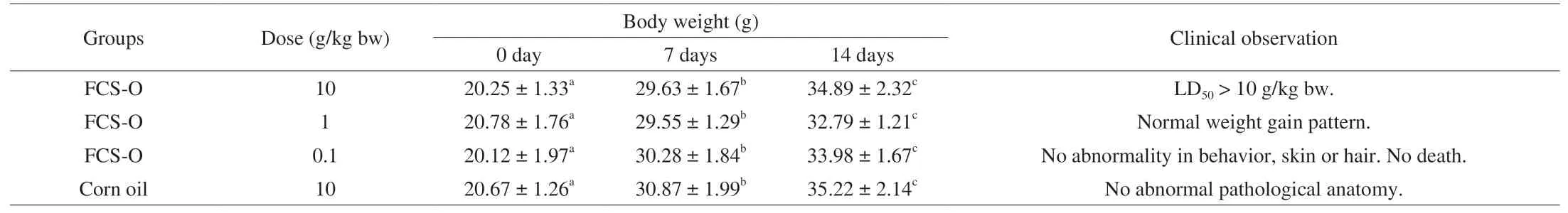
Table 3The acute oral toxicity evaluation of FCS-O.
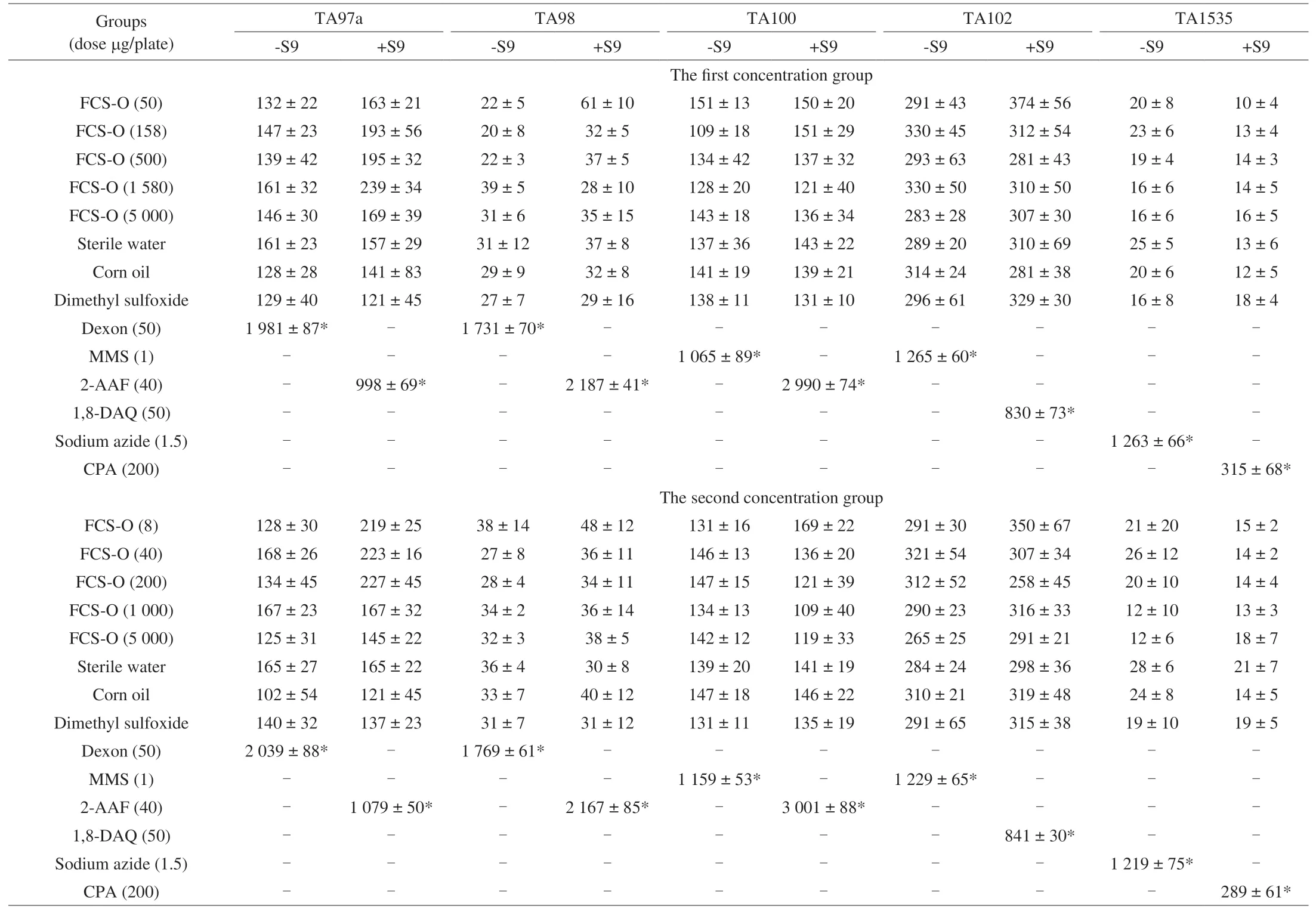
Table 4Mutagenicity of FCS-O in the bacterial reverse mutation assay (number of revertants).
Table 5 shows the effects of FCS-O on bone marrow micronucleus. No differences in sensitivity to FCS-O were observed between the male and female mice. No significant differences in the percentage of micronucleated erythrocyte cell counts were observed between the FCS-O treatment groups and the negative control group(P> 0.05). Additionally, there were no significant differences among the three dose levels of FCS-O (P> 0.05). In the positive control group, the micronucleated erythrocyte cell counts were higher than in the FCS-O groups and the negative control group (P< 0.01). The PCE and NCE ratios of all groups were not significantly different, compared to the control groups. Thus, it indicated that FCS-O at the three doses exhibited no cytotoxicity or obvious inhibition of PCE formation.
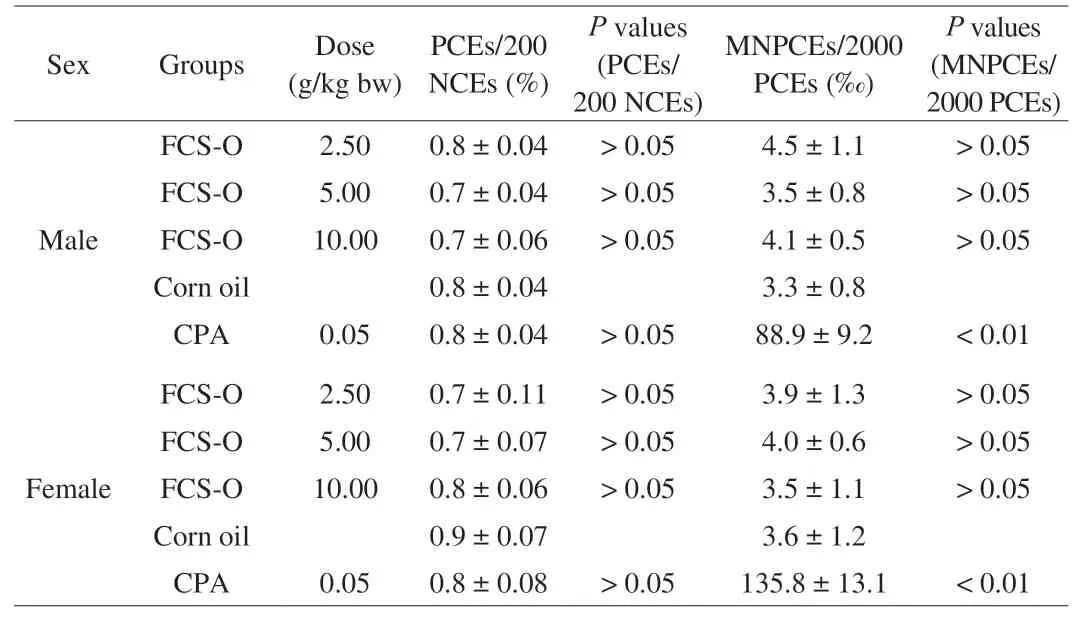
Table 5Effects of FCS-O on bone marrow micronucleus in KM mice.
Chromosomal aberrations are caused by irregularities or abnormalities in the number, distribution, structure or arrangement of chromosomes, and they are associated with genetic diseases or species differences [47]. Chromosomal aberration assay was performed to assess the mammalian cell mutagenicity and chromosomal clastogenicity of FCS-O. The chromosomal abnormality rate of spermatocytes in each dose group of FCS-O was observed and calculated. The results show no significant differences (P> 0.05)between treatments and negative control group (Table 6). However,there was a significant difference (P< 0.05) between the negative and positive control groups. The average values of abnormality rate in the negative and positive control groups were 1.0% and 15.0%,respectively. In the FCS-O groups, the percentage of the total number of chromosomal aberrations ranged from 0.4% to 1.0%. Hence, it could be determined that FCS-O has no mutagenic and clastogenic potential to chromosomes of spermatocytes in mice. That is, FCS-O has no genotoxicity.
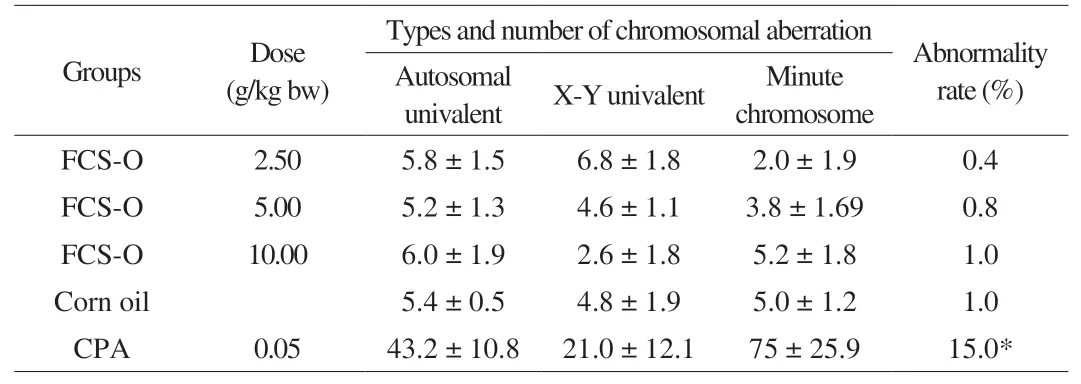
Table 6Effects of FCS-O on chromosomal abnormality of spermatocyte in KM mice.
4. Conclusions
In summary, this study examined the physicochemical properties and compositions of coix seed oil produced byM. purpureusfermentation and supercritical CO2extraction. Moreover, we evaluated its anti-lipid-oxidation, acute oral toxicity, mutagenicity and genotoxicity. The extracted oil from fermented coix seed possessed good physicochemical quality and abundant active components with physiological function, such as phytosterols, tocols (vitamin E),γ-oryzanol, coixenolide, oleic acid and linoleic acid. Particularly, the concentrations ofγ-tocotrienol,γ-oryzanol or coixenolide in fermented coix seed were much higher than those in raw or most cereals. Rich in active compounds, the extracted oil could significantly enhance the stabilization of susceptible lipids and the antioxidant activity of an emulsion. Additionally, the extracted oil inhibited cholesterol oxidation and the formation of 7-ketocholestreol, a kind of harmful oxidation product. The extracted oil from fermented coix seed is also a very safe edible oil that exhibits no acute toxicity, induced mutagenicity, cytotoxicity or genotoxicity. Therefore, the extracted oil from fermented coix seed could be used as a food, food ingredient,nutritional supplement, or natural food antioxidant to provide healthpromoting functions.
Declaration of competing interest
The authors declare that they have no competing financial interests.
Acknowledgments
The research was supported by Natural Science Foundation of China (32260583) and the Agriculture Committee of Guizhou Province [(2017) 106 & (2018) 81] and the Talent Introduction Program of Guizhou University [(2021) 76].
- 食品科学与人类健康(英文)的其它文章
- Emerging natural hemp seed proteins and their functions for nutraceutical applications
- A narrative review on inhibitory effects of edible mushrooms against malaria and tuberculosis-the world’s deadliest diseases
- Modulatory effects of Lactiplantibacillus plantarum on chronic metabolic diseases
- The role of f lavonoids in mitigating food originated heterocyclic aromatic amines that concerns human wellness
- The hypoglycemic potential of phenolics from functional foods and their mechanisms
- Insights on the molecular mechanism of neuroprotection exerted by edible bird’s nest and its bioactive constituents

