Inhibitory effect of coriander (Coriandrum sativum L.) extract marinades on the formation of polycyclic aromatic hydrocarbons in roasted duck wings
Yjie Yu, Yiqun Cheng,b, Chong Wng, Suhong Hung, Yng Lei, Ming Hung,*, Xibin Zhng
a Key Laboratory of Meat Processing and Quality Control, MOE, Key Laboratory of Meat Processing, MOA, Jiangsu Collaborative Innovation Center of Meat Production and Processing, Quality and Safety Control, and College of Food Science and Technology, Nanjing Agricultural University, Nanjing 210095, China
b College of Environmental Science & Engineering, Institute of Functional Food, Anhui Normal University, Wuhu 241000, China
c Shandong New Hope Liuhe Group Co. Ltd/Quality Control for Feed and Products of Livestock and Poultry Key Laboratory of Sichuan Province, Chengdu 610023, China
Keywords:Polycyclic aromatic hydrocarbons Coriander extract Roast duck Inhibition Radical Phenolic compounds
A B S T R A C T Coriander (Coriandrum sativum L.) is recognized for its antioxidant property, as a kind of natural phenolicrich ingredient. Polycyclic aromatic hydrocarbons (PAHs) present a class of heat-driven hazards in foods,especially the processed meat. In this study, the effect of coriander root and leaf extract on the formation and inhibition of PAH8 in roasted duck wings was f irstly investigated. Coriander root extract (CRE) and coriander leaf extract (CLE) with f ive concentration groups (200, 400, 600, 800, 1 000 mg/L) were prepared respectively to marinate the duck wings. CRE marinade exhibited greater inhibitory effect on PAH8 formation in roasted duck wings that ranged from 65.0%-87.4%. The electron spin resonance study indicated a significantly positive correlation between PAH8 and free radical level, suggesting the participation of radicals in PAHs formation. Also, it was speculated that the inhibitory effect on PAH8 was related to the phenolic compounds identif ied in coriander marinades. CRE made greater inhibitory effect on the formation of PAH8 and could be considered as a kind of natural source to mitigate PAHs in heat-processed meat products.
1. Introduction
Polycyclic aromatic hydrocarbons (PAHs) have been evaluated in many foodstuffs due to their ubiquity and carcinogenic potential [1]. Except for occupational exposure or smokers, food is the prior source of PAHs ingestion for human [2]. A recent study proposed a correlation between uptake of PAH-contaminated food and gastric cancer risk based on 153 cancer cases and 306 matched controls [3]. The formation mechanism of PAHs is still unclear while two factors are be of concern. Pyrolysis and pyrosynthesis of organic matters, particularly the lipids could form benzene ring structures via Diels-Adel reaction. During which, reactive radical intermediates e.g. cyclohexene or hydroperoxides is of significance to the PAHs formation [2,4]. The incomplete combustion of fuel itself could be the other motivation [5]. Wood or charcoal utilized as heat source also releases PAHs during the unstable initial combustion stage [6,7].PAH8 (a sum of benzo(a)anthracene (BaA), chrysene (Ch), benzo(b)fluoranthene (BbF), benzo(k)fluoranthene (BkF), benzo(a)pyrene(BaP), dibenz(a,h)anthracene (DhA), benzo(g,h,i)perylene (BgP),indeno(1,2,3-c,d)pyrene (IP) and PAH4 (a sum of BaA, Ch, BbF,BaP) are two main indicators for the occurrence and toxicity of PAHs in foods proposed by the European Food Safety Authority Panel on Contaminants [8].
Meat and meat products are susceptible to generate and aggregate PAHs during thermal processing, particularly the smoking, grilling,roasting and drying etc. [9]. Roast duck, famously known as Peking duck, is one of the Chinese traditional barbecue delicacies and popular for its characteristic golden-brown colour, rich aroma and thin crisp taste. In pursuit of these, ducks are usually hung vertically over the heat (hardwood or charcoal) for roasting in an open or closed stove, during which the temperature and duration maintained over 270 °C and 40-45 min [10]. Such severe conditions may result in the occurrence of PAHs. In fact, Lin et al. [10] reported 129 µg/kg of 19 PAHs (including PAH8) in Gua-lu type (roasted in an open stove) Peking duck, among which BaP was quantified as high as 8.7 µg/kg exceeding the European prescribed maximum level of 5 µg/kg in grilled and barbecued meat products [11]. According to a previous survey on commercial barbecue meat products in Hong Kong, total 15 PAHs of charcoal-roasted duck was 105.6 µg/kg [12].So, a long-term consumption of such the barbecue meal potentially bring risk to people’s health. The minimisation of PAHs intake and meanwhile, satisfying consumers’ appetite is a challenge for traditional meat products industry.
There are two major approaches namely barrier methods and removal methods on PAHs mitigation of processed meat products [13].The first focuses for the strategies before heat processing including marination, process-improvement, juice filter/collection etc. and the other concerns the end-products after heat-processing. Marination is an efficient manner to mitigate the hazards from meat products. Relevant reports mainly include herbs/spices [14], beverages [15], fruits/vegetables [16], lactic acid bacteria [17] and sugar coproducts [18].Coriander (Coriandrum sativumL.), as a kind of popularly used condiment has a long history of cuisine and the whole plant is edible [19].Most research focuses on the coriander leaf or seed for their medicinal and therapeutic values, e.g. anticancer [20,21]. While limited information was available about the coriander root for its further utilization. So far, only one literature reported the antioxidant effect about coriander root extract. According to the authors, coriander root extract possessed higher total phenolic content and antioxidant activity than those of its leaf extract, which was proposed to be mediated by a range of phenolic compounds, e.g. ascorbic acid [22].Coriander root may be considered as a natural phenolic-rich source for the hazard mitigation in processed meat products. Therefore, the present study aimed to investigate the effect and the key compositions of coriander root and leaf extract marinades on the PAH8 formation and inhibition in roasted duck wings.
2. Materials and methods
2.1 Materials and reagents
A sum of 60 duck wings (around 42 ± 3 g each) were brought from SUGUO superstore (Nanjing, China). A mix of eight standard PAHs (PAH8) was preset in acetonitrile: methanol (90:10) (Supelco,Bellefonte, PA, USA) solution. Ethanol, acetonitrile,n-hexane and dichloromethane were of HPLC grade from Merck (Darmstadt,Germany). 1,1-Diphenyl-2-picrylhydrazyl (DPPH·), gallic acid,ascorbic acid and Folin-Ciocalteu’s reagent were purchased from Sigma-Aldrich (St. Louis, MO, USA). Other chemicals of analytical purity were acquired from Sinopharm (Shanghai, China). Sep-Pak Vac silica cartridge (6 cc/1 g, Waters, Wexford, Ireland) was used for PAHs purification. Ultrapure water was prepared from a Milli-Q System (Millipore, Bedford, MA, USA).
2.2 Marinating and roasting
2.2.1 Preparation of marinades
Coriander plants (Coriandrum sativumL.) were collected from a local plantation in Nanjing City. The roots and leaves were separated, rinsed, freeze-dried and crushed into powder over 40 mesh. 5 g powder of root or leaf was dispersed in water (1:50, g/mL).Ultrasound assisted extraction was performed subsequently at 45 °C for 1 h, followed by centrifuging (10 000 ×g) for 10 min (Avanti J-26S XP, Beckman coulter, CA, USA). The steps above were repeated twice. The supernatant combined was filtered under vacuum and lyophilized. The resulting coriander leaf and root extract powder were collected coding as coriander leaf extract (CLE) and coriander root extract (CRE) separately. Five groups of concentration of two extracts (200, 400, 600, 800, 1 000 mg/L) were prepared respectively as marinated groups. Five duck wings were taken for each marinated group, control group (marinated in water) and raw group. Marinating conditions that followed the descriptions of Wang et al. [23] were 1:1 (m/V) at 4 °C for 4 h. No ingredient other than CLE and CRE was added to marinades.
2.2.2 Roasting conditions
About 1 kg bamboo charcoal (KAOKE, Guangdong, China) was horizontally placed at the bottom of an open-fire stove (59 × 39.5 ×50 cm, Guangdong, China) and kindled. Until the flame subsided, duck wings were roasted at a height of 15 cm from heat surface, turning over randomly during roasting. Charcoal was replaced among each group of duck wings. It took 8 min to get wings well-done. After cooling, the roasted wings were unboned, minced and lyophilized, then stored under-20 °C. No addictive was added for the whole roasting.
2.3 Determination of total phenolic content of coriander marinades
The total phenolic content (TPC) of marinades was determined based on descriptions of Singleton et al. [24] before and after marinating. Namely, 200 µL of marinade aliquot was mixed with 2.5 mL of Folin-Ciocalteu’s reagent (10:1 diluted in water), shaking well. Afterwards, 2 mL of sodium carbonate (7.5 mg/mL) was added. Absorbances at 760 nm were recorded by a multifunctional microplate reader (M2e, Molecular Devices, Shanghai, China) after 2 h reaction at room temperature. Water was taken as the blank.Standard curve was drawn by a series of gallic acid solutions. The results were calculated as gallic acid equivalents (GAE) per liter of marinade (mg GAE/L).
2.4 Determination of DPPH· scavenging activity of coriander marinades
DPPH· scavenging activity of marinades was evaluated according toWang et al. [23]. Two equal aliquots of 200 µL of marinade were mixed with DPPH· solution (0.2 mmol/L, dissolved in 95% ethanol)and ethanol respectively as marinade sample and sample blank.DPPH· solution and 95% ethanol were taken as control and reagent blank. All the solutions were protected from light for 2 h until the reaction achieved the balance. Absorbance values were collected at 517 nm. Results were expressed as percentage scavenging of DPPH·comparing to the control.
2.5 Determination of ferric reducing power of coriander marinades
Ferric reducing power (RP) was determined as mentioned by Zhao et al. [25]. Briefly, 100 µL of samples was mixed with 250 µL of phosphate buffer (0.2 mol/L, pH 6.6) and 250 µL of potassium ferricyanide (1 mg/mL), the mixture was incubated at 50 °C for 20 min. Afterwards, 250 µL of trichloroacetic acid (10 mg/mL) was added. The solution was centrifuged (10 000 ×g) for 10 min. And then 250 µL of supernatant was collected into a 2-mL tube with 50 µL ferric chloride (0.1 mg/mL) added subsequently. Absorbances at 700 nm were recorded. The results were calculated following a standard curve plotted by ascorbic acid solutions and expressed as ascorbic acid equivalents (AAE) per liter of marinade (mg AAE/L).
2.6 Analysis of PAHs
2.6.1 Extraction and purification
The analysis of PAHs was conducted according to Wang et al. [23].Namely, 2.5 g lyophilized meat powder was weighted into a 50 mL tube containing 20 mL of acetonitrile and 10 mL ofn-hexane saturated with acetonitrile. Ultrasound assisted extraction was performed for 30 min at 40 °C. The resulting mixtures were centrifuged(10 000 ×g) to present clear liquid-liquid interface. The acetonitrile sublayer was collected. The extraction was repeated once. The pooled solution was subjected to rotary evaporation at 35 °C(IKA, HB 10, Jiangsu, China) to remove solvent. The resulting residue was redissolved in 5 mL ofn-hexane and loaded into silica cartridge sequentially washed and preconditioned by 6 mL each of dichloromethane andn-hexane. The analytes were eluted drop by drop with 5 mL of dichloromethane/n-hexane (30:70,V/V) and then concentrated to dryness under steady nitrogen flow. 200 µL of acetonitrile:methanol (90:10) was then added and filtered through 0.22 µm membrane for instrumental analysis.
2.6.2 Chromatographic conditions
But it didn t go away. All through the flatlands of Arkansas, Oklahoma , north Texas and New Mexico it lay like a coiled snake inside of me. When we approached the high plateau of northern Arizona it began to stir. As the grades grew steeper and the curves sharper, my sense of control faltered11, It s all in your head, I kept repeating desperately12. There is no danger. It s all in your head. 。、、,。,。,,。“,”。“。。”
A Waters Acquity UPLC H-class system (Waters, Milford,MA, USA) equipped with a diode array and a fluorescence detector(UPLC-DAD/FLR) was assigned for PAHs analyzation. Separations were conducted on a SupelcosilTMLC-PAH column (10 cm ×3.0 mm, 3 µm particle size, Supelco, Bellefonte, PA, USA) thermostated at 30 °C with a flow rate of 0.64 mL/min. The binary mobile phase of water (A) and acetonitrile (B) was performed following the linear gradient: 0-2 min, 50% B; 2-8 min, 50%-100% B; 8-11.2 min,100% B; 11.2-15 min, 100%-50% B. The injection volume was 4 µL. Detection wavelength of DAD was set at 254 nm. Excitation/emission wavelengths was set as: 270/390 nm for BaA and Ch,260/430 nm for BbF, 290/410 nm for BkF, BaP, DhA and BgP,290/470 nm for IP. Empower 3 software (Waters, Milford, MA, USA)was designed for instrument control, data acquisition and processing.Analytes were identified by comparing retention times with those of mixed standards. External calibration curve method was adopted for quantification of PAHs. For method validation, the detection limit(LOD) and quantification limit (LOQ) were evaluated on a signal to noise (S/N) of 3:1 and 10:1. Recovery experiments were also conducted by spiking 50 ng/g standards in six replicates. The data was listed in Table S1.
2.7 Electron spin resonance spectroscopy analysis
An electron spin resonance (ESR) spectrometer (EMX-10/12,Bruker, Germany) was assigned to detect free radical level of roasted duck wing power. To keep the most sensitive detection range, samples were stuffed below 2.5 cm from the bottom of the narrow quartz tubes. Instrumental parameters were set below: microwave frequency of 10.55 GHz, microwave power of 21.68 mW, center field of 3 480.00 G, sweep width of 150.00 G, modulation frequency of 100.00 kHz, modulation amplitude of 3.00 G, time constant of 40.96 ms, conversion time of 163.84 ms. The whole experiment was completed at ambient temperature.
2.8 Identification of phenolic compounds of coriander marinades
An UPLC system matched with quadrupole time-of-flight mass spectrometry (UPLC/Q-TOF-MS, Xevo G2-S, Waters, Manchester,UK) was applied to analyze the phenolic composition of two kinds of marinades [23]. 2 µL of marinade was injected into a Acquity UPLC BEH C18column (2.1 × 100 mm, 1.7 µm particle size,Waters, Manchester, UK) at a flow rate of 0.35 mL/min. 0.1%formic acid in water (A) and acetonitrile (B) were used as the binary mobile phase with the gradient program arranged as: 95% A for 0.5 min; 95%-60% A, 0.5-20 min; 60%-5% A, 20-22min; 5% A,22-23.5 min; 5%-95% A, 23.5-25 min. Electrospray ionization source (ESI) was enabled both in positive and negative ion mode with a selected mass range ofm/z50-1 200. The ionization parameters followed as: capillary voltage of 2.5 kV, ion source temperature of 120 °C, curtain gas temperature of 400 °C. Flow rate of cone gas and curtain gas were 50 and 800 L/h, respectively. The lock mass option(m/zof 556.227, 554.262) was allowed to recalibration. Software Masslynx 4.1 (Waters, Manchester, UK) was ran for data acquisition and processing.
2.9 Statistical analysis
Five replicates of each group were taken for the PAHs analysis.Data was analysed by Statistical Analysis System 8.1 (SAS Institute Inc. NC27513. USA) and expressed as mean ± standard deviation.ANOVA with Duncan’s multiple range test was used for the difference among means on a significant level ofP< 0.05.
3. Results and discussion
3.1 Total phenolic content of coriander marinades
Phenolic constituents of plants are a large class of secondary metabolites accounting for the antioxidant activity [26]. As seen in Fig. 1, before marinating, TPCs of CLE groups significantly increased in parallel with concentrations, which ranged from 11.4 (CLE200)to 29.2 mg GAE/L (CLE1000). The same trend was observed in the CRE groups viz. 5.4 (CRE200)-9.7 (CRE1000) mg GAE/L, which were only half as much as CLE counterparts. The results seemed to be inconsistent with Tang et al. [22], whereby coriander root extract possessed higher TPC than that of leaf extract. Such difference may result from the extracting method and solvent, cultivate environment and processing storage condition of the plant [19]. After 4 h of marinating, a decrease of TPCs occurred ranging 5.6-21.4 mg GAE/L as well as 3.2-7.4 mg GAE/L in CLE and CRE groups, which coincided with Wang et al. [23]. The authors inferred that some substances in marinade transferred to the surface of duck wings during marinating. The decrease of TPC (%) was in a range of 26.3%-50.9%for CLE groups, as well as 23.5%-41.9% for CRE groups.
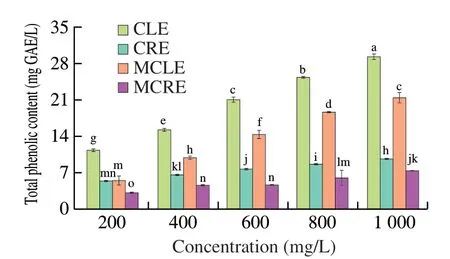
Fig. 1 Total phenolic content of five concentration groups of coriander leaf and root extract marinades respectively before soaking of duck wings(CLE/CRE) and after marinating (MCLE/MCRE). Bars with different letters represent significant differences (P < 0.05).
3.2 DPPH scavenging activity of coriander marinades
The results were expressed as percentage DPPH scavenging comparing to the control. In Fig. 2, all the samples showed DPPH scavenging activity. Before marinating, CLE and CRE marinades exhibited significantly increasing DPPH· scavenging activity with the concentration increased, 22.3%-56.3% for CLE groups and 8.1%-68.0% for CRE groups. Generally higher scavenging efficiency result from the higher TPC of marinades. Nevertheless, CRE1000 showed the strongest scavenging activity while lower TPC was determined as showed in Fig. 1. The different categories and contents of phenolic compositions in two kinds of marinade may explain the discrepancy. Similar behaviour was also described by Sulaiman et al. [27]. According to which, the degree of polymerization and interaction between phenols’ structures and the radical DPPH during purple-fading reaction also mattered.After marinating, a decrease of DPPH scavenging activity clearly appeared in all samples, which was within a range of 17.5%-44.1%as well as 24.2%-38.9% for CLE and CRE groups. Of CRE200 and CRE400 groups, no statistical difference of DPPH scavenging activity was calculated while TPCs reduced significantly before and after marinating. The reason may be the less TPCs initially in these two groups (5.4 and 6.5 mg GAE/L, respectively). Moreover,a significantly positive regression correlation was found between TPC and DPPH· scavenging of CLE and CRE groups suggesting a mediating role of coriander extracts on antiradical property(CLE,y= 1.78x+ 1.71,R2= 0.952 4,P< 0.001; CRE,y=14.62x- 77.58,R2= 0.937 5,P< 0.001).

Fig. 2 DPPH scavenging activity of 5 concentration groups of coriander leaf and root extract marinades respectively before soaking of duck wings(CLE/CRE) and after marinating (MCLE/MCRE). Bars with different letters represent significant differences (P < 0.05).
3.3 Ferric reducing power of coriander marinades
As indicated in Fig. 3, the results of RP of two marinades shared very similar trends with their TPC outcomes of concentrationdependence, which ranged 19.3-41.3 mg AAE/L for CLE groups and 7.3-11.6 mg AAE/L for CRE groups. Likewise, the RP decreased significantly with the range of 76.2%-36.2% and 52.5%-31.3% for CLE and CRE groups after marinating. The regression correlation coefficients were also calculated as 0.958 5 (CLE,y= 1.19x+7.41,P< 0.001) and 0.988 0 (CRE,y= 1.02x+ 1.80,P< 0.001)between TPC and RP of two marinades. In combination of DPPH·assay results, it can be concluded that two marinades exhibited good antioxidant activities mediated by their phenolic constituents. Similar behaviour was also reported by Sulaiman et al. [28].

Fig. 3 Ferric reducing power of 5 concentration groups of coriander leaf and root extract marinades respectively before soaking of duck wings (CLE/CRE)and after marinating (MCLE/MCRE). Bars with different letters represent significant differences (P < 0.05).
3.4 Effect of coriander marinades on PAHs formation
The PAH8 profile of roasted duck wings marinated in water(control) and five groups of CLE or CRE marinade were showed in Table 1. A sum of 22.84 ng/g of PAH8 was quantitated of control group with greater contents of DhA (12.44 ng/g), BgP (5.16 ng/g),BaA (1.71 ng/g), BbF (1.14 ng/g). The other 4 congeners Ch, BkF,BaP and IP were all below 1 ng/g. In addition, 1.85 ng/g of PAH8 in raw meat were also determined with no PAHs detected previously in marinades, which may result from PAH-contaminated packaging material or animal feed [29]. Lin et al. [10] reported 51 ng/g of PAH8 in skin samples of Peking duck with 8.7 ng/g and < 1 ng/g of BaP and DhA included, the two carcinogens classified respectively as evidently carcinogenic to humans (Group1) or probably carcinogenic (Group 2A)by the International Agency of Research on Cancer [10,30]. While 0.61 and 12.44 ng/g of these two PAHs were quantified in this study.Three main reasons may explain the discrepancy. The specialized material called “stuffed duck” for Peking duck processing contained abundant subcutaneous fat that favoured PAHs production and accumulation as the lipophilic matrix and precursor during roasting[10]. Besides, the roasting style of Peking duck viz. hanging over/in the open-flame of hardwoods which promoted the production of PAHs[31]. Finally, 1.70 ng/g of DhA was detected in raw duck in this study,so it was speculated such “DhA susceptibility” of raw meat may be a reason for the high amount of DhA during roasting subsequently. Chen et al. [32]also investigated PAH8 in duck drumsticks after sugar-smoking and none of PAH8 was detectable in all samples. These different PAHs profiles of duck samples could be interpreted to the different thermalprocessing methods.
In comparison to the control, both marinades reduced PAH8 of roasted duck wings, especially CRE marinade. Of CRE groups,CRE600 made the best performance in lowering PAH8 by 87.4%,followed by CRE800 (79.7%), CRE400 (79.0%), CRE1000 (74.2%),CRE200 (65.0%). Among the CLE groups, the highest decrease by 51.8% of PAH8 was observed in CLE800, followed by CLE600(46.1%), CLE400 (36.5%), CLE200 (16.6%). In terms of individual PAH, Ch and BgP were undetectable in 400-800 groups of CLE and CRE marinades, respectively. Except for that, BkF decreased most by 94.7% of CLE600 group, followed by 93.4% of BaP in CRE800, 89.1%, 83.6%, 81.6%, and 77.8% of IP, DhA, BbF and BaA in CRE600. Moreover, a negative correlation was also found between the concentration groups of 200-600 mg/L of CRE marinade and PAH8 (Pearson correlation = -0.941,P< 0.001), as well as the concentration groups of 200-800 mg/L of CLE marinade and PAH8(Pearson correlation = -0.991,P< 0.001). Unexpectedly, the highest concentration group (1 000 mg/L) of CRE or CLE marinade did not exhibit the strongest inhibitory effect on PAH8 formation. Manners likewise were mentioned by Gong et al. [14] whereby 120 mg/kg of rosemary extract and tea polyphenol significantly increased PAHs of Youtiao (Chinese fried bread) comparing to the 60 mg/kg of these two antioxidants. Our previous study also reported 27.3%reduction of heterocyclic amines in deep-fried chicken treated by 2.0 mg/L sugarcane-molasses extracts, which was less efficient than 29.9% reduction of 1.5 mg/L dose treatment [18]. However,Min et al. [33] observed a significant dose-dependence between PAH8 and (-)-epigallocatechin gallate (EGCG) with a range of 50-300 µg/kg. Taken together, the highest concentration marinating did not necessarily make the best inhibitory effect on PAHs formation.In other word, the inhibitory effect of coriander marinades on PAHs followed a concentration-dependent relationship within a confined range of concentrations.
3.5 Free radical levels of roasted duck wings
ESR spectroscopy was assigned to detect the total free radical level of roasted duck wing samples. As seen in Fig. 4, a classic asymmetric signal was observed at 3 480 G of magnetic field.Generally, the stronger signal intensity (peak to peak height) means more radicals contained in the material. As showed in Fig. 4,the highest free radical intensity was 5 749.50 of control group.Correspondingly, the radical intensities of marinated samples were 4 530.51, 3 886.48, 1 708.75, 3 379.83, 4 256.63 for 200-1 000 mg/L groups of CRE marinade, and 5 423.50, 5 115.59, 4 774.80, 4 329.43,5 716.55 for 200-1 000 mg/L groups of CLE marinade. Obviously,CRE600 exhibited the lowest radical intensity among all the marinated groups, which agreed with the PAH8 profile showed in Table 1. Also,a positive correlation was found between the free radical level and PAH8 in two marinades suggesting the participation of radicals in PAHs formation (CRE: Pearson correlation = 0.883,P= 0.019; CLE:Pearson correlation = 0.966,P= 0.001). Taken the results of TPC and antioxidation together, the antiradical activity of phenolics in coriander marinades may explain the decay of radical intensity.Generally, phenolic compounds could break the chain-transfer reaction of free radicals by the chelation of catalytic metal ions or providing hydrogen/electro to unstable intermediate radicals e.g. lipid hydroperoxides, which played a key role in PAHs generation [4,34].Relevant research was also reported by Min et al. and Cheng et al. [18,33].Whereas, in Fig. 4B, CLE1000 showed the highest radical intensity among all marinated groups. It seemed that marinating of the highest concentration tended to promote the production of radicals.Jongberg et al. [35] found Bologna sausages mixed with green tea extract dramatically enhanced the radical intensities than those ofcontrol (phenolics-free) ones. The authors attributed this to the accumulation of phenoxyl radicals produced during initial selfoxidation of catechins. Other researchers pointed out hydroxyl radicals could produce through oxidation of phenolic compounds coupled with reduction of oxygen. The resulting hydroxyl radicals could accelerate lipid peroxidation inducing the occurrence of mutagens e.g.PAHs [36]. Therefore, it is significant to comprehensively understand and evaluate the effect of phenolic compounds given the different categories and contents, food systems, cooking styles etc.
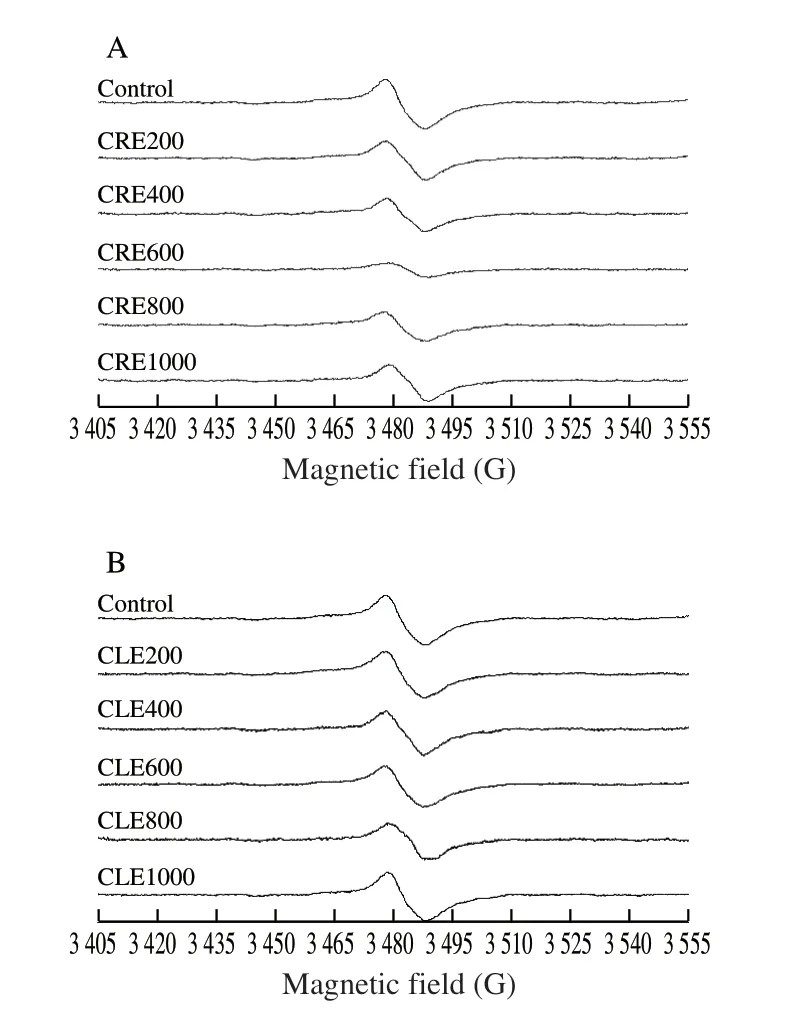
Fig. 4 ESR spectra of lyophilized roasted duck wings powder (n = 3). ESR spectra obtained from roasted duck wings samples marinated in water (control)or (A) marinated in 5 concentration groups of CRE or (B) marinated in five concentration groups CLE.
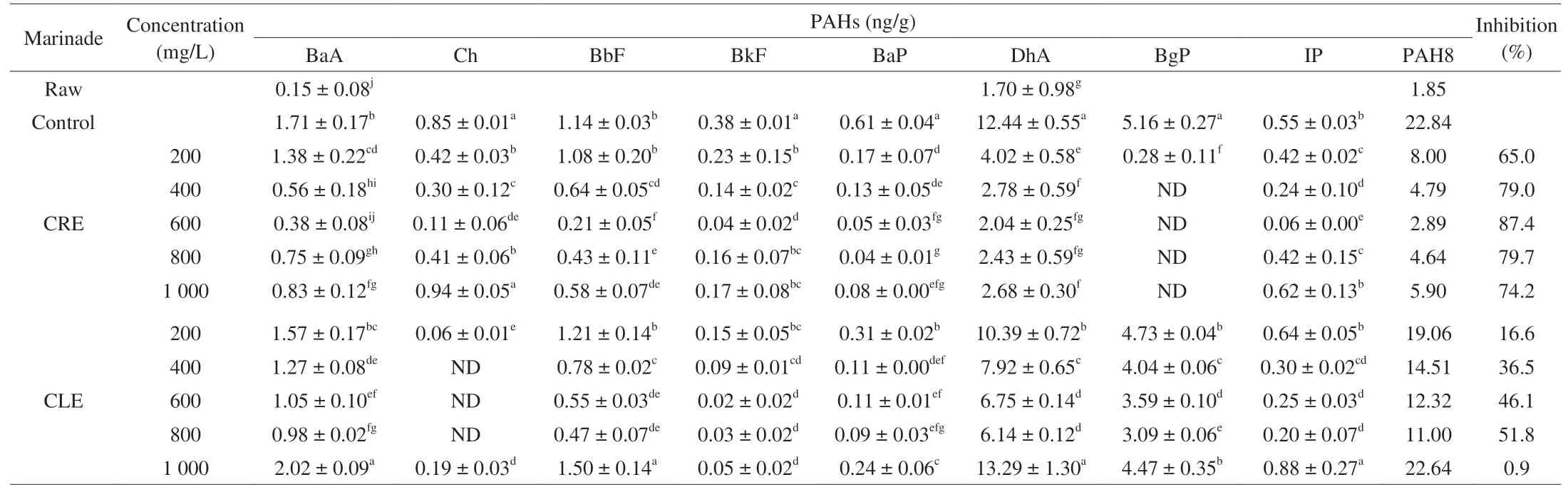
Table 1Formation and inhibition of PAHs of roasted duck wings, unroasted (raw), marinated in water (control), marinated in five concentration groups of CRE and CLE.
?
3.6 Identification of phenolic compositions in coriander marinades
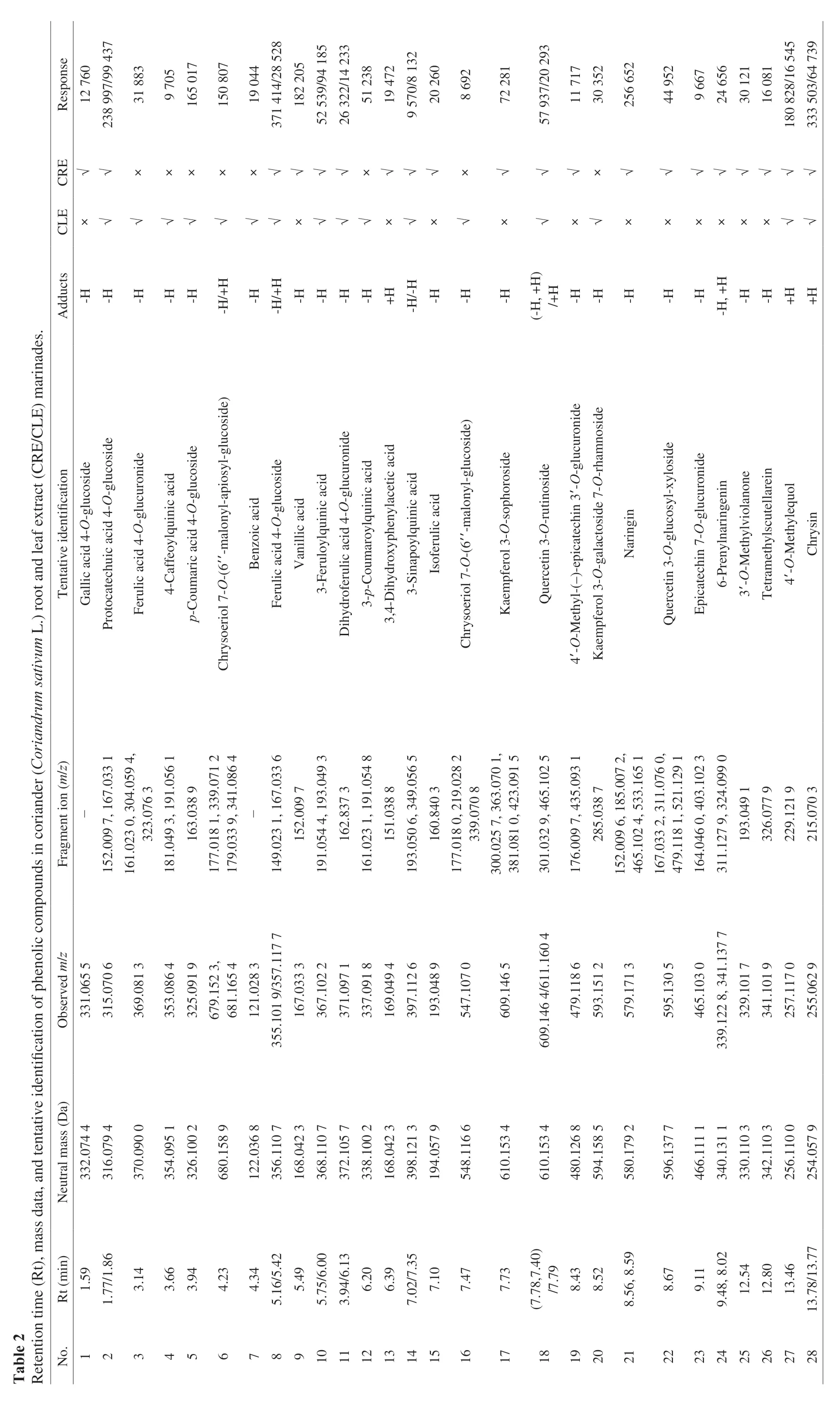
As displayed at Table 2, different compositions were determined respectively in CRE and CLE marinades by UPLC-MS/MS system.A total of twenty phenolic compounds were identified in CRE marinade, among which naringin, vanillic acid, protocatechuic acid 4-O-glucoside and 3-feruloylquinic acid were the dominating compounds according to the higher response values. While only sixteen phenolic compounds were detected in CLE marinade, among which ferulic acid 4-O-glucoside, chrysin, protocatechuic acid 4-O-glucoside,p-coumaric acid 4-O-glucoside and 4’-O-methylequol constituted the main compounds. The results complied to the published ones [22,37,38]. The different categories and contents of phenolic compounds in CRE marinade may explain the better inhibitory effect on PAH8 formation than that of CLE marinade.Min et al. [33] investigated the effect of individual antioxidantsα-tocopherol, EGCG and sesamol on PAH8 production in meat model system, which showed 37%-49% decrease of PAH8 than the control. Also, Wang et al. [39] screened eleven kinds of phenolic compound from beer that decreased more than half amounts of PAH8 in grilled chicken wings. Taken together, it could be speculated that a correlation between the existence of phenolic compounds and inhibition of PAH8.
4. Conclusion
In summary, coriander root extract made greater inhibitory effect on the formation of PAH8 in roasted duck wings than that of coriander leaf extract. A positive correlation was observed between the PAH8 amount and free radical level by ESR study. And the
phenolic compositions identified in coriander marinades may be the
key contributor to the formation and inhibition of PAH8.
Conflict of interest
The authors declare no competing interest among them.
Acknowledgements
This work was funded by the National Key R&D Program of China (2016YFD040040303).
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://doi.org/10.1016/j.fshw.2022.10.038.
- 食品科学与人类健康(英文)的其它文章
- Emerging natural hemp seed proteins and their functions for nutraceutical applications
- A narrative review on inhibitory effects of edible mushrooms against malaria and tuberculosis-the world’s deadliest diseases
- Modulatory effects of Lactiplantibacillus plantarum on chronic metabolic diseases
- The role of f lavonoids in mitigating food originated heterocyclic aromatic amines that concerns human wellness
- The hypoglycemic potential of phenolics from functional foods and their mechanisms
- Insights on the molecular mechanism of neuroprotection exerted by edible bird’s nest and its bioactive constituents

