Highly-sensitive electrochemiluminescence biosensor for detection of inosine monophosphate in meat based on graphdiyne/AuNPs/luminol nanocomposites
Jing Liu, Yizhong Shen, Gungxin Wng, Yodong Xing, Yemin Guo, Xi Sun,*, Yun Liu*
a School of Agricultural Engineering and Food Science, Shandong University of Technology, Zibo 255049, China b School of Food and Biological Engineering, Hefei University of Technology, Hefei 230009, China
c School of Agriculture and Biology, Shanghai Jiao Tong University, Shanghai 200240, China
Keywords:Inosine monophosphate (IMP)Electrochemiluminescence Graphdiyne (GDY)/AuNPs/luminol Bienzyme
A B S T R A C T Inosine monophosphate (IMP), as a critical umami substance, is one of the most important indicators for evaluating the quality of meat products. Here, a sensitive electrochemiluminescence (ECL) biosensor based on graphdiyne (GDY)/AuNPs/luminol nanocomposites was constructed to detect IMP. The GDY/AuNPs/luminol nanocomposites were synthesized by using simple one-pot method. GDY utilized its 2D framework to disperse and fix gold nanoparticles, which inhibited the agglomeration of gold nanoparticles and greatly improved its stability and catalytic properties. Importantly, GDY/AuNPs/luminol nanocomposites showed excellent catalytic ability and superior ECL activity towards luminol-H2O2 systems due to the synergistic effect of GDY and AuNPs. Under optimal conditions, the prepared biosensor exhibited a wide linear range from 0.01 g/L to 20 g/L, a satisfactory limit detection of 0.001 3 g/L, as well as an excellent specificity. Moreover, we carried out the precise analysis of IMP in actual meat samples with acceptable results compared to the liquid chromatography. We believe that this work could offer an eff icient ECL platform for accurate and reliable report of IMP levels, which is signif icant for maintaining food quality and safety.
1. Introduction
Inosine monophosphate (IMP) is usually regarded as one of the most powerful tools to judge the quality of meat products [1,2]. It is an excellent flavor enhancer with low threshold and high umami efficiency, mainly derived from the degradation of ATP after the animals have been killed. IMP is not only valuable to the meat f lavor formation and development, but is also closely related to the aging and freshness of the meat, as it could be further degraded to hypoxanthine during the aging processing, leading to the bitterness of the meat [3-5]. In order to provide consumers with meat products of appreciated f lavor and fresh quality, it is very important to precisely monitor IMP level.
Up to now, several assays have been employed to detect IMP,including high performance liquid chromatography (HPLC) [6],nuclear magnetic resonance (NMR) spectroscopy [7], and electrochemistry [8-12]. However, these assays still have some limitations such as complicated pretreatment process, low sensitivity,high cost, and time consuming. Electrochemiluminescence (ECL)as a promising analytical technology integrates the advantages of electrochemistry and chemiluminescence [13,14]. It has attracted great attention due to its superior features such as near-zero background noise, excellent robustness, low-cost, as well as nice temporal and spatial controllability [15-19]. Luminol as an ideal luminophore can produce a strong ECL signal with H2O2as co-reactant, and luminol-H2O2ECL system as a commonly used ECL systems have been successfully applied to the construction of ECL biosensors in various enzymatic reaction systems [20-22].
Carbon-based nanomaterials have attracted significant interest in ECL sensors because of their prominent electrical conductivity,large surface area, and enhanced loading capacity [23-26]. In particular,graphdiyne (GDY) with a two-dimensional layered in-plane porous structure composed of sp and sp2 hybrid carbon atoms, features large specific surface areas and excellent electron transport performance [27,28].The unique structure of GDY with uniformly distributed pores and highly π-conjugated systems makes it an excellent platform to facilitate strong adsorption to biomolecules and increase the conductivity of the electrode [29-31]. However, there are few studies on GDY for the construction of ECL biosensor due to lack of effective catalytic performance. To expand its application fields, nanoparticlefunctionalized carbon nanomaterials are proven to be an effective strategy due to their enhanced physical and excellent synergistic properties. Gold nanoparticles (AuNPs) have been extensively used to construct sensing matrixes because of their unique properties such as the large surface-to-volume ratio, high conductivity, and excellent catalytic ability [32]. Compared with AuNPs/luminol and carbon nanomaterials/luminol, AuNPs-carbon nanomaterials in ECL sensors exhibit superior catalytic efficiency and ECL activity towards luminol-H2O2system. This is because carbon nanomaterials can increase the interface area of the modified electrode to capture more AuNPs and luminol through Au-π interaction and π-π stacking, and AuNPs can facilitate the electron transfer at the electrode interface and catalyze the ECL reactions of luminol [33-36]. Inspired by this,the combination of GDY and AuNPs has great potential in fabrication of ECL biosensors with excellent performance. GDY utilizes its unique structure to provide specific chelating agent sites for the immobilization of nano-gold particles. GDY as an adsorption layer can greatly improve the stability and catalytic properties of nanogold particles by inhibiting the agglomeration of nano-gold particles.In addition, luminol used as a reducing agent for nanogold formation improves the ECL efficiency of the nanocomposites. However, few studies have focused on the ECL biosensors based on GDY/AuNPs/luminol nanocomposites for IMP detection.
Herein, a novel ECL sensor for sensitive detection of IMP was developed based on GDY/AuNPs/luminol nanocomposites. HAuCl4was reduced to AuNPs on GDY using the inherent reduction ability of luminol to form GDY/AuNPs/luminol nanocomposites. It can provide an excellent carrier for the immobilization of 5’-nucleotidase and xanthine oxidase due to its superior biocompatibility and large surface areas. We chose IMP as target analyte model, and IMP was first catalyzed by 5’-nucleotidase to generate inosine and H2O2, and then inosine was catalyzed by xanthine oxidase to generate hypoxanthine and H2O2. As a result, the H2O2obtained by a two-step enzymatic hydrolysis reaction remarkably amplified ECL signal due to excellent catalytic ability and superior ECL activity of GDY/AuNPs/luminol nanocomposites towards luminol-H2O2systems. The as proposed ECL sensor exhibited low detection limit, high sensitivity, and outstanding selectivity, and provided a promising application of GDY/AuNPs/luminol nanocomposites in the development of a novel sensing platform for analysis IMP in food.
2. Materials and methods
2.1 Reagents and materials
The chemical and biological reagents used in this experiment were purchased from commercial suppliers without further processing. In detail, GDY was purchased from Nanjing Xianfeng Nanomaterials Technology Co., Ltd. (Nanjing, China). 5’-Nucleotidase, xanthine oxidase, IMP, gold chloride trihydrate (HAuCl4·3H2O) were supplied by Sigma-Aldrich Chemical Corporation (St Louis, MO, USA). Uric acid, hydrogen peroxide, acetic acid and bovine serum albumin (BSA)were purchased from Macklin Reagent Company (Shanghai, China).Phosphate buffer solution (PBS) used in this work was prepared by mixing standard stock solutions of Na2HPO4and NaH2PO4. Deionized water (D.I. water, 18.2 MΩ resistivity) used throughout experiments was acquired on a Milli-Q system (Millipore, USA).
2.2 Instrumentations
All ECL signals were detected on MPI-A electroluminescence analyzer (Xi’an, China). Electrochemical measurements are all carried out on CHI 760E (Shanghai Chenhua Instrument Co., Ltd.,China). The conventional three-electrode system consists of Ag/AgCl(saturated KCl) as the reference electrode, platinum electrode (PE) as the counter electrode and the working electrode. Raman spectroscopy was measured using NanoWizard Ultra Speed & in Via Raman(RENISHAW & JPK, Germany) with a laser excitation wavelength of 532 nm. A transmission electron microscope (TEM) image was obtained on JEM-2100 TEM (Electronics Co., Ltd., Japan). A scanning electron microscope (SEM) image was obtained by using a field emission SEM JSM-7800F (JEOL, Japan).
2.3 Synthesis of GDY/AuNPs/luminol nanocomposites
The GDY was used as the substrate material, and then the gold chloride trihydrate was reduced to AuNPs on the surface of GDY by using the reducing power of luminol. Firstly, AuNPs were deposited on GDY in a solution with a mass ratio of GDY to tetrachloroauric acid of 1:2.5. The solution of GDY and tetrachloroauric acid was heated to 90 °C, and then a certain amount of luminol was quickly added to the above solution under vigorous stirring. At this time,the color of the resulting solution gradually changed from light yellow to light purple, which means the successful assembly of the GDY/AuNPs/luminol composite. Finally, the resulting solution was centrifuged and washed three times with ultrapure water. The obtained material was placed in brown bottles and stored at 4 °C for later use.
2.4 Fabrication of ECL biosensor
The ECL biosensor was prepared by layer-by-layer assembly.Before modifying the electrode, the PE was polished with 1.0, 0.3 and 0.05 mm alumina powder, followed by cleaning and ultrasonic treatment in ethanol and ultrapure water to remove the residual alumina powder. After that, they were blown dry with purified nitrogen. The multilayers films of BSA/5’-nucleotidas-xanthine oxidase/GDY-AuNPs-luminol/PE were prepared at ambient temperature according to the following steps. First, 8 µL GDY/AuNPs/luminol was dropped on the surface of the PE and dried in air. Then, 6 μL of nucleotide oxidase-xanthine oxidase solution(5’-nucleotide:xanthine oxidase = 1:1) was fixed on the surface of GDY/AuNPs/luminol/PE and dried. Finally, 6 µL of BSA (0.5%)solution was dropped on above electrode to block non-specific sites and dried in air. All the obtained biosensors were thoroughly washed with PBS and stored at 4 °C. The manufacturing process and mechanism of the ECL biosensor are shown inFig. 1.
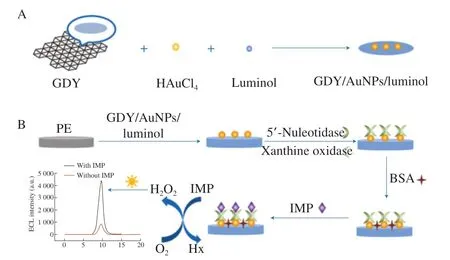
Fig. 1 Schematic illustration of (A) the synthesis processes of GDY/AuNPs/luminol nanocomposites and (B) the fabrication processes and proposed mechanism of the designed ECL biosensor for IMP monitoring.
2.5 Electrochemical and electrogenerated chemiluminescence behaviors
Cyclic voltammetry (CV) was carried out in 5.0 mmol/L[Fe(CN)6]3-/4-solution containing 0.1 mmol/L KCl. The scan rate was 100 mV/s and the potential range was -0.2-0.6 V. To monitor the changes of electrochemical signal, ECL detection was performed in a three-electrode system including a PE electrode as a working electrode, a PE as an auxiliary electrode, and Ag/AgCl as a reference electrode. Usually, the three-electrode system was immersed in 0.3 mL of 0.1 mmol/L PBS (pH 7.4), and then 10 µL of IMP with different concentrations was dropped into the sensor. The ECL intensity was monitored at a scan rate of 100 mV/s with the scan range of 0.2-1 V, and the photomultiplier tube voltage was 800 V.
2.6 Preparation of real meat samples
Commercial pork, beef, chicken and lamb were randomly selected as representative meat samples to prove the practicality of GDY/AuNPs/luminol ECL biosensors. Fresh pork, beef, lamb tenderloin and chicken breast meat slaughtered within 48 h were selected from the supermarket in Zibo, China, which had a good glossy color,uniform red color, no off-flavor and good elasticity. A total of 40 meat samples (n= 10, for each meat product) were transported to the laboratory and stored at 4 °C for further experiments. In order to report the IMP content in these actual meat samples, 4 g of each meat sample were thoroughly mixed with 20 mL of 5% perchloric acid using a homogenizer (JRA-35S, China) at 12 000 r/min, and then diluted with ultrapure water to 25 mL. After that, the resulting homogenate was centrifuged at 15 000 ×gfor 10 min at 4 °C, and then the supernatant was collected and adjusted to pH 5.5 with 2 mol/L KOH. Finally, the supernatant was centrifuged again under the same conditions as above, and collected for HPLC analysis.
3. Results and discussion
As mentioned before, a novel GDY/AuNPs/luminol nanocomposites were synthesized using simple one-pot method, and then 5’-nucleotidase and xanthine oxidase were well immobilized onto sensor substrates. On this basis, double enzyme catalyzed IMP to produce a large amount of hydrogen peroxide, which efficiently amplified the ECL signal of luminol-H2O2system. Therefore, highsensitive detection of IMP levels monitored by ECL biosensor was realized. The experimental procedure for fabrication and mechanism of ECL biosensor was presented in Fig. 1.
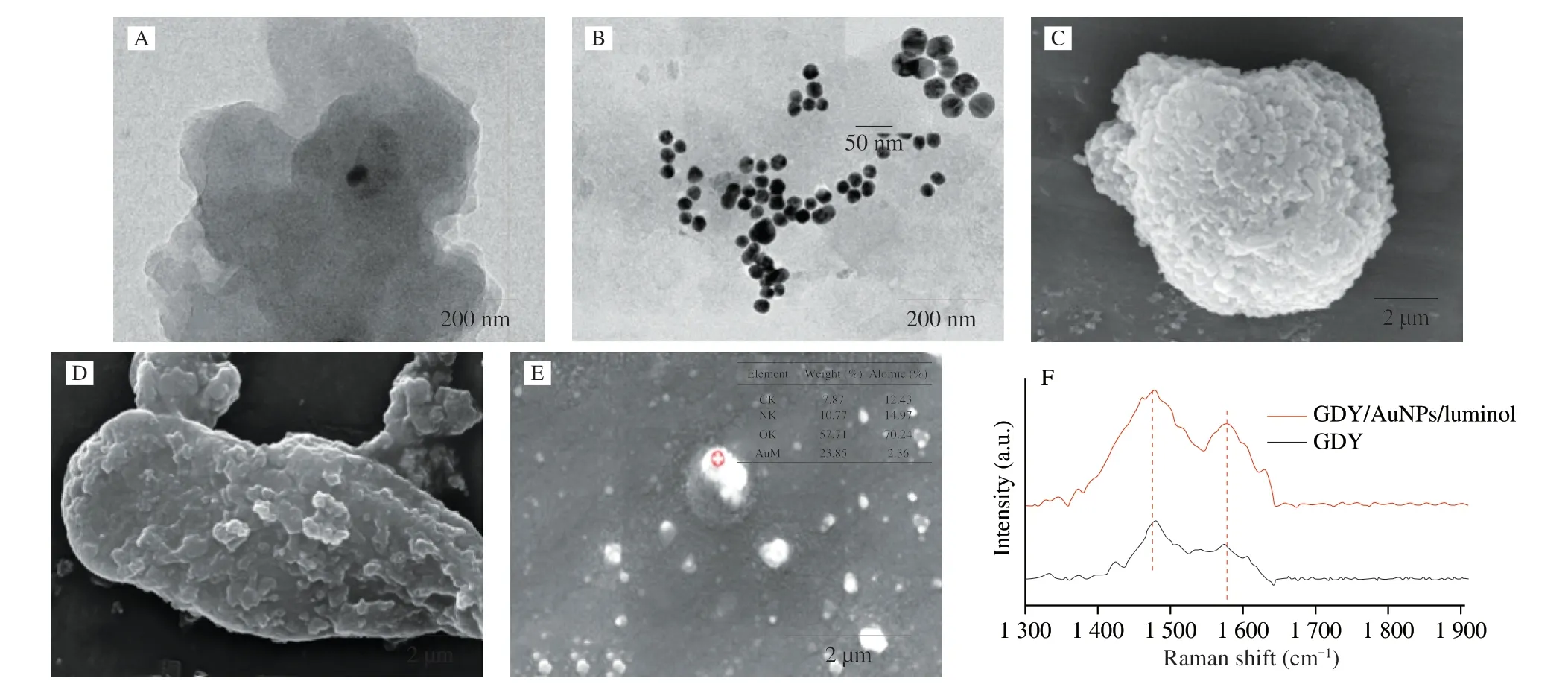
Fig. 2 TEM images of (A) GDY and (B) GDY/AuNPs/luminol. SEM images of (C) GDY and (D) GDY/AuNPs/luminol. (E) SEM-EDS image and (F) Raman spectroscopy of GDY/AuNPs/luminol.
3.1 Assembly and characterization of GDY/AuNPs/luminol nanocomposites
The morphology and structure of GDY/AuNPs/luminol composites were characterized by TEM and SEM. As shown in Fig. 2A, GDY showed a uniform and continuous layered microstructure with stacked layers, providing a larger area for AuNPs loading. After luminol reduced HAuCl4on the surface of GDY,AuNPs with a uniform size of 10 nm were well monodispersed on the surface and between the layers of GDY (Fig. 2B). Then,the morphology of the original GDY and GDY/AuNPs/luminol composites were further studied by SEM. The SEM in Fig. 2C shows the porous structure of the GDY powder. This porous structure is very important for the effective diffusion of AuNP on the surface of GDY.As shown in Fig. 2D, the surface of GDY/AuNPs/luminol became rough compared to the original GDY (Fig. 2C). In addition, the unique porous structure of GDY/AuNPs/luminol can provide many binding sites for strong adsorption of enzymes, thereby enriching the available substrate concentration to react with enzyme electrodes.The element composition of GDY/AuNPs/luminol was analyzed using energy dispersive spectrometer (EDS) technology. Fig. 2E confirmed the presence of C, N, O and Au elements, indicating that AuNPs are formed. The results showed that the GDY/AuNPs/luminol nanocomposites were successfully synthesized.
To evaluate the structural changes, the Raman spectroscopy was carried out. As shown in Fig. 2F, the original GDY had two major peaks which located at 1 472 and 1 576 cm-1, respectively.The peak at 1 472 cm-1corresponded to the stretching of aromatic bonds, similar to graphene [37-39]. The peak at 1 576 cm-1should be attributed to the first-order scattering of the E2gmode and it was observed in the in-phase tensile vibration of the sp2carbon domain in the aromatic ring [40], which was the characteristic peak of the GDY [41,42]. Compared with the original GDY, the Raman signals of GDY/AuNPs/luminol were all enhanced. The ratio of Raman peak intensity between GDY/AuNPs/luminol and GDY was 1.47:1. This is because AuNPs can enhance the Raman signals of other substances through the surface plasmon resonance effect of AuNPs [43,44].Therefore, these results were fully in line with the synthesis of GDY/AuNPs/luminol.
3.2 Electrochemical and ECL behaviors of the modified electrodes
CV and electrochemical impendence spectroscopy (EIS) have been employed to monitor the interface properties of different modified electrodes. Fig. 3A showed the CV curves of the modified PE. A pair of distinct of redox peaks was obtained from the bare PE (curve a)due to the oxidation and reduction of [Fe(CN)6]3-/4-, indicating the PE was well polished. When the GDY/AuNPs/luminol was modified onto the PE, the peak currents of the electrode increased remarkably (curve d),and this might be the result of excellent electron transfer of AuNPs and luminol [45]. After the modification of 5’-nucleotidase-xanthine oxidase onto above electrode, enzymes hindered the charge transfer between liquid and the electrode, resulting in an obvious drop (curve c). Finally, after modification of BSA, the amperometric signal further decreased (curve b). These results are due to the electronically inert feature of enzymes and BSA that hindered the electron transfer and mass transfer of Fe(CN)64-/3-ions on the electrode surface, and it can also prove that the enzymes and BSA were well loaded on the GDY/AuNPs/luminol.
The Nyquist chart consists of a semicircular part in the higher frequency range and a straight part in the lower frequency range. The diameter of the semicircle is equal to the electron transfer resistance(Ret) at the electrode interface. As shown in Fig. 3B, the bare glassy carbon electrode (GCE) showed a large semicircular area and the Ret was 3 469 Ω (curve a). After modifying GDY/AuNPs/luminol on GCE, the Ret value decreased to 3 045 Ω (curve d), which is the result of the good conductivity of GDY and AuNPs. Subsequent GDY/AuNPs/luminol/GCE was stepwise modified with 5’-nucleotidasexanthine oxidase and BSA, and the semicircle gradually increased.The Ret value of 5’-nucleotidase-xanthine oxidase/GDY/AuNPs/luminol/GCE and BSA/5’-nucleotidase-xanthine oxidase/GDY/AuNPs/luminol/GCE were estimated as 3 085 and 3 413 Ω,respectively. These results were consistent with the outcomes in CVs,proving the successful assembly of biosensor.
Feasibility of the developed ECL biosensor for IMP detection was studied by ECL measurement. As illustrated in Fig. 3C, a very small and even unobservable ECL signal was observed on bare PE (curve a).After the modification of GDY, the ECL intensity enhanced due to excellent conductivity of GDY. When the GDY/AuNPs/luminol was modified onto the electrode, a stronger ECL signal was detected on the electrode.It may be explained that GDY and AuNPs could enhance the ECL of luminol-H2O2efficiently due to their excellent electrical conductivity and high electrocatalytic activity. After immobilizing the 5’-nucleotidase-xanthine oxidase and BSA on GDY/AuNPs/luminol/GCE surface, the ECL signal declined (curve d) owing to the weak electroconductivity of BSA and hindrance of nonconductive multiple enzymes which had severely obstructed electron transfer.Upon addition of IMP (10 g/L), a strong ECL signal was detected on the electrode (curve e). This phenomenon could be explained by the double enzymes-catalyzed generation of H2O2using IMP as substrate and the catalytic ability of GDY/AuNPs/luminol composites towards H2O2. These results indicated that GDY/AuNPs/luminol played an important role in amplifying the ECL signal of luminol. GDY could increase the interface area of the modified electrode to capture more AuNPs and luminol through Au-π interaction and π-π stacking; and AuNPs could facilitate the electron transfer at the electrode interface and catalyze the ECL process of luminol in both electrode and chemical reaction. In addition, GDY/AuNPs/luminol could provide an ideal substrate for the mobilization of enzymes. Thus, the developed ECL biosensor was quite effective for IMP detection.

Fig. 3 CVs (A) and EIS (B) curves of bare PE (a), BSA/5’-nucleotidase-xanthine oxidase/GDY/AuNPs/luminol/PE (b), 5’-nucleotidase-xanthine oxidase/GDY/AuNPs/luminol/PE (c), and GDY/AuNPs/luminol/PE (d) in 5 mmol/L [Fe(CN)6]3-/4- containing 0.1 mol/L KCl solution (pH 7.0). The scan rate is 100 mV/s. Inset is the R(C(RW)) equivalent circuit model, including the solution resistance (Rs), double layer capacitance (Cdl), warburg impedance (Zw) and electron transfer resistance (Ret). ECL profiles (C) of bare PE (a), GDY/PE (b) in 0.1 mol/L PBS containing 0.20 mmol/L luminol and 10 mmol/L H2O2, BSA/5’-nucleotidasexanthine oxidase/GDY/AuNPs/luminol/PE (c), GDY/AuNPs/luminol/PE (d) in 0.1 mol/L PBS containing 10 mmol/L H2O2, and BSA/5’-nucleotidase-xanthine oxidase/GDY/AuNPs/luminol/PE (e) in 0.1 mol/L PBS (pH 7.4) with 10 g/L IMP.
3.3 Experimental condition optimization
To maximize the performance of the biosensor, experimental conditions such as the concentration of luminol, the temperature and pH value of PBS, etc. were studied. Fig. 4A showed the effect of luminol concentration from 0.1 mmol/L to 0.35 mmol/L on the ECL intensity of the developed biosensor. It was observed that the ECL intensity increased with the increase of luminol concentration, and then stabilized after 0.25 mmol/L, indicating that the luminol solution had reduced HAuCl4to AuNPs. Therefore, 0.25 mmol/L luminol was used as the optimal concentration for developing biosensors. The pH value is an important factor that affects the ECL performance and enzyme activity of the biosensor. It can be seen from Fig. 4B that the ECL intensity reached its maximum value at pH 7.5, and then decreased significantly at higher pH value. Therefore, a pH 7.5 solution was selected as the optimal concentration for subsequent experiments. The influence of temperature is another parameter in the measurement. As indicated in Fig. 4C, the ECL intensity gradually increased with increasing the temperature up to 35 °C, and decreased significantly at higher temperature. Accordingly, 35 °C was chosen as the optimal condition for the ECL biosensor. Fig. 4D depicted the ECL intensity reached a maximum at 50% of 5’-nucleotidase in the mixture of two enzymes, and decreased with the increase in the proportion of 5’-nucleotidase. It is because all IMP have reacted with enzymes, and the remaining enzymes in the reaction can decrease the ECL intensity.In addition, we optimized the concentration of the two enzymes,and the optimal concentration of 5’-nucleotidase and xanthine oxidase were 0.3 and 0.25 mg/mL, respectively (Figs. S1, S2).Hence, the optimal proportion and concentration of enzymes were employed as for the developed biosensor.
3.4 Performance of the ECL biosensor
Under the optimal conditions, the ECL response of the developed ECL biosensor to different concentrations of IMP was tested. As shown in Fig. 5A, the ECL intensity had a good linear relationship with IMP concentration with the range from 0.01 g/L to 20 g/L(Fig. 5B). The linear regression equation wasI= 388CIMP+ 739 with a correlation coefficient of 0.994. Moreover, Fig. 5B illustrated the detection limit was 0.001 3 g/L (S/N = 3). Compared with other biosensors (Table 1), the developed ECL biosensor exhibited a lower detection limit and a wider detection range. The excellent detection performance of the improved ECL method is attributed to the high catalytic activity of GDY/AuNPs/luminol. This indicated that the designed ECL biosensor can be used for highly sensitive IMP detection.
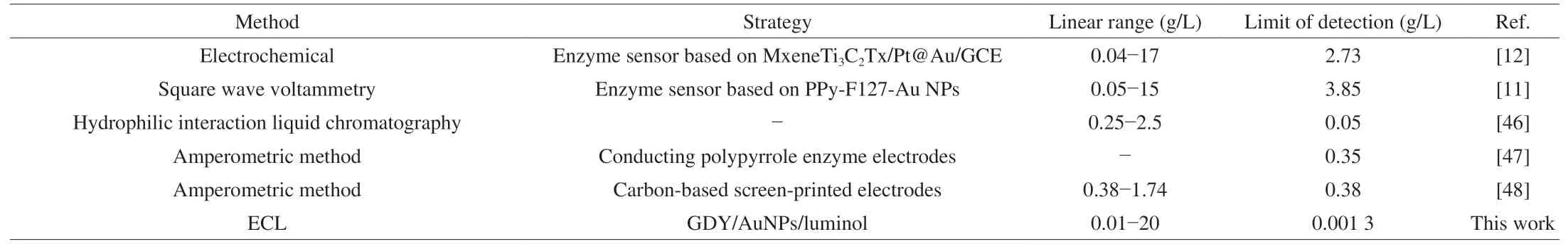
Table 1Comparison of the constructed ECL enzyme biosensor with other reported methods for IMP detection.
To investigate the specificity and anti-interference ability of the developed ECL method, some species that coexist with IMP in muscles including inosine diphosphate, inosine triphosphate,cysteine, and methionine were used as interfering compounds. Fig. 5C displayed that the interfering compound did not cause a significant ECL response signal compared with the background signal, while IMP caused a strong ECL signal. In addition, the ECL intensity obtained from the mixture of IMP with other interfering compounds was approximated to that of the IMP alone. Therefore, the proposed ECL biosensor had great specificity for IMP. Furthermore, after storing the used ECL biosensor at 4 °C for 7 days, up to 93.2% of the initial ECL response signal can be retained, indicating that the developed ECL biosensor has ideal long-term storage stability (Fig. 5D). Moreover,the reproducibility of the ECL biosensor was assessed by analyzing the ECL response of 6 independently fabricated electrodes at 10 g/L IMP. As shown in Fig. 5E, the relative standard deviation (RSD) was 3.48%, indicating that the proposed ECL biosensor maintained good repeatability.

Fig. 4 The effects of the concentration of luminol (A), the different pH (B), temperature of PBS (C) and proportion of 5’-nucleotidase (D) on ECL intensity in 0.1 mol/L PBS (pH 7.4) with 10 mg/mL IMP.
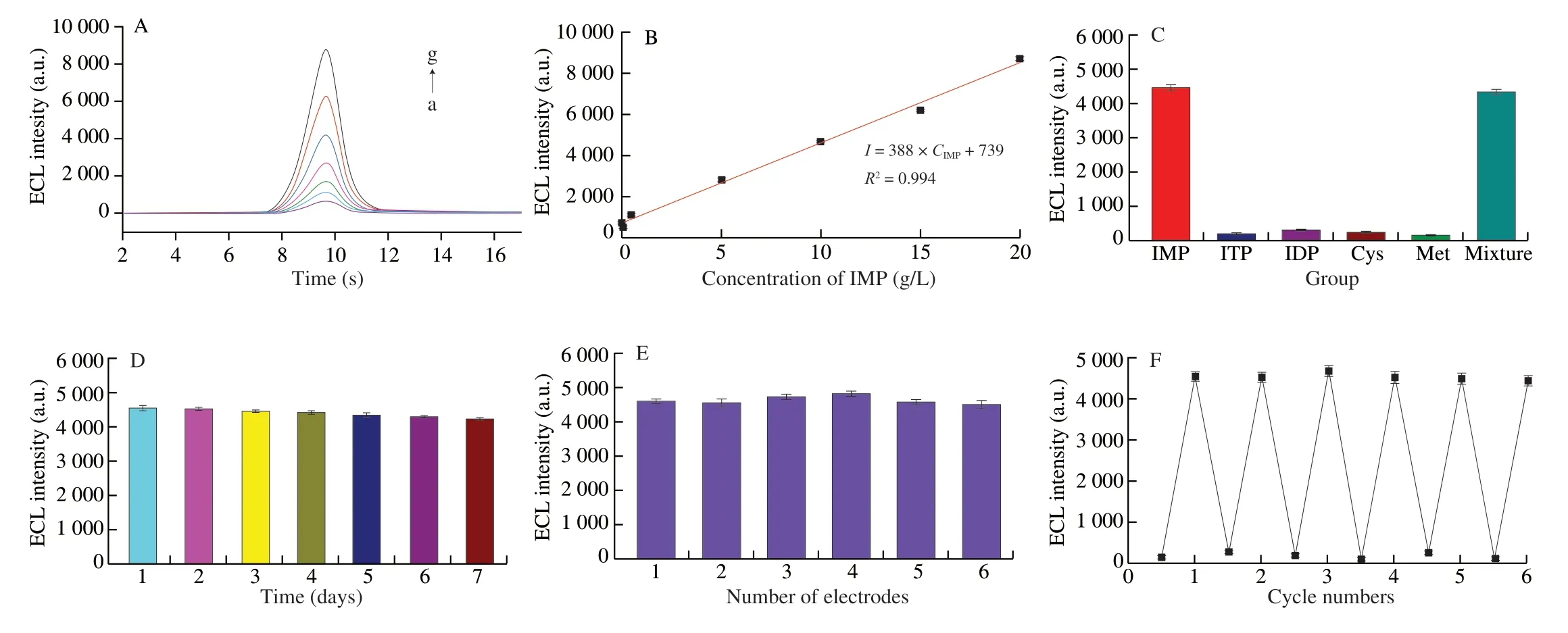
Fig. 5 (A) ECL intensity of the biosensor to different concentrations of IMP. (a) 0.01, (b) 0.05, (c) 0.5, (d) 5, (e) 10, (f) 15, (g) 20 g/L. (B) Calibration curve of the ECL biosensor for IMP detection. (C) Stability and (D) selectivity of the proposed ECL biosensor. (E) Repeatability and (F) reproducibility of the ECL biosensor.
In order to evaluate the reproducibility of the sensor, the ECL biosensor with the IMP captured was incubated in 1.0 mol/L NaOH for 5 min to denature the enzymes and detach the IMP, then thoroughly washed with PBS buffer and the ECL response of sensors were detected. After that, the sensors were regenerated by incubating the enzymes and the ECL signal of sensors were detected again in the presence of 10 g/L IMP. After 6 consecutive and repeated regeneration processes, our sensors still maintained its original recognition ability during the detection process. The RSD of multiple measurement results was less than 5.5%, and its background signal was negligible, indicating that the ECL biosensors have excellent reproducibility.
3.5 Real sample analysis
To evaluate the practical application performance of this ECL biosensor, the designed ECL biosensor was applied to detect the IMP in different meat samples, including chicken, pork, beef, and lamb.The quantification of IMP in the actual samples was obtained by using the external calibration plot. This is because the contents of IMP in meat products were all within the detection range of the biosensor.The results obtained by biosensor were compared with the reference value of the HPLC. As shown in Table 2, the RSD was less than 2.41%,indicating that our ECL biosensor was consistent with the HPLC method. The analysis results ofF-test andt-test showed the Sigs of the four samples were all greater than 0.05 within the 95% confidence interval, indicating that there is no significant difference between the HPLC method and the sensor detection method in the actual sample detection. These results suggested that our ECL biosensor could provide a promising alternative to detect IMP in actual meat samples.

Table 2Analysis results of IMP in actual samples by two methods.
4. Conclusion
In conclusion, a sensitive ECL sensor was successfully developed for the detection of IMP based on the GDY/AuNPs/luminol composites that have excellent catalytic effect on luminol-H2O2ECL system. The proposed ECL biosensor showed excellent detection performance for IMP, with a low detection limit of 0.001 3 g/L,high sensitivity and good stability. More importantly, this ECL biosensor has been successfully applied to the determination of IMP in actual meat samples, which is significant for the development of an ultrasensitive biosensing platform for meat quality evaluation.
Conflict of interest
The authors declare no conflicts of interest.
Acknowledgments
This work was supported by The National Natural Science Foundation of China (31972198, 31622042) and The National Key R&D Program of China (2016YFD0400803, 2016YFD0401501).
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://doi.org/10.1016/j.fshw.2022.10.040.
- 食品科学与人类健康(英文)的其它文章
- Emerging natural hemp seed proteins and their functions for nutraceutical applications
- A narrative review on inhibitory effects of edible mushrooms against malaria and tuberculosis-the world’s deadliest diseases
- Modulatory effects of Lactiplantibacillus plantarum on chronic metabolic diseases
- The role of f lavonoids in mitigating food originated heterocyclic aromatic amines that concerns human wellness
- The hypoglycemic potential of phenolics from functional foods and their mechanisms
- Insights on the molecular mechanism of neuroprotection exerted by edible bird’s nest and its bioactive constituents

