Filtration assisted pretreatment for rapid enrichment and accurate detection of Salmonella in vegetables
Bin Li, Hnling Wng, Jinguo Xu, Wei Qu, Li Yo,*, Bngen Yo,, Cho Yn, Wei Chen,*
a Engineering Research Center of Bio-Process, Ministry of Education, School of Food and Biological Engineering,Intelligent Manufacturing Institute, Hefei University of Technology, Hefei 230009, China
b Anhui Provincial Institute of Product Quality Supervision and Inspection, Hefei 230051, China
Keywords:Vegetables Salmonella Filtration enrichment Culture-free detection Enzymatic recombinase amplif ication (ERA)Lateral f low strip (LFS)Rapid detection Food safety
A B S T R A C T Rapid detection of target foodborne pathogens plays more and more signif icant roles in food safety, which requires the eff iciency, sensitivity, and accuracy. In this research, we proposed a new st rategy of isothermalmolecular-amplif ication integrated with lateral-f low-strip for rapid detection of Salmonella without traditional enrichment-culture. Th e designed syringe-assisted-filtration can contribute to simultaneous collection and concentration of target bacterium from vegetable samples in just 3 min, resolving the drawbacks of traditional random sampling protocols. After simple and convenient ultrasonication, samples can be directly amplif ied at 39 °C in 25 min and the amplicons are qualitatively and quantitatively analyzed with the designed lateral-flow-strip in 5 min. Finally, satisf ied results have been achieved within 40 min, which greatly improve the eff iciency while the accuracy is also guaranteed. Furthermore, all detection steps can be completed under instrument-free conditions. This method will hold great promise for target pathogen detection in the resource-limited district,or for emergency on-site identif ication.
1. Introduction
Presently, healthy diet has been paid wide attention globally. Less oil, salt, sugar, and calories are the critical items of the healthy diet.Especially, salad, as one kind of health diet, has become a fashion and health symbol [1]. It is also a common sense that the fresh vegetables and fruits of the salad are the potential source of biosafety threatens [2,3].Usually, food safety accidents induced by the common bacteria including theSalmonella,Listeriamonocytogenes,Staphylococcus aureus,EscherichiacoliO157:H7, etc. have been frequently reported [4]. Numerous reports have suggested that the food poisoning caused bySalmonellaoften ranks the first, accounting for about 40% globally [5,6]. Infection ofSalmonellacan cause the strong stress response and obvious clinical symptoms including the fever,nausea, vomiting, diarrhea and abdominal cramps [7]. What makes it even worse is that it is life-threating for the in fants, the elderly and patients with low immunity [8]. Accurate and rapid detection of target potential food-borne pathogenic contaminations is of great signif icance for the food safety guarantee.
It is well known that most traditional detection methods for pathogenic bacteria are mainly based on microbiology [9] including the cultivation of the bacteria, bacteria screening, biochemical identif ication et al. [10]. Such processes are tedious, time-consuming,and professional personnel needed [11,12], and these shortcomings greatly limit their on-site applications. For this reason, a variety of rapid detection strategies for common bacteria have been extensively reported, including enzyme linked immunosorbent assay (ELISA) [13]and electrochemical sensors [14]. To a certain extent, these methods have improved the detection efficiency. But for the sensitivity and specificity related issues, there is great room for further improvement.The development of molecular biology protocols has partially improved this situation [15]. For example, the detection sensitivity is greatly improved by polymerase chain reaction (PCR) [16] due to its excellent exponential replication performance. Besides, with its unique genetic information and design of primer set, the specificity can also be guaranteed.
However, the requirement of precise heat-controller and trained personnel also limit its possibility for on-site detection. With continuous exploration, many isothermal amplification techniques have been frequently applied in pathogen detection [17-19], making signal amplification more convenient. Among these methods,recombinase polymerase amplification (RPA) is a relatively new isothermal protocol. Similar to the traditional PCR, the amplicons can be produced by two oligonucleotide primers with RPA [20].Currently, RPA is also used inin situpathogen detection for its advantage of convenient operation [21]. Meanwhile, as one kind of recombinase isothermal amplification protocol, enzymatic recombinase amplification (ERA) has also been developed, which can be performed at a constant temperature without thermal cycling as RPA [22]. Coupled with novel CRISPR-Cas12a, RPA has also been adopted in ultrasensitive, rapid, and specific Porcine circovirus 3 detection [23]. Although the above isothermal amplification techniques have simplified the detection of pathogenic bacteria to certain extent, fluorescent reading module are still expensive and professional personnel required. In recent years, lateral flow strip (LFS) has played the significant role in on-site detections because of its excellent characteristics of efficiency, simplicity and visual results judgement [24].
In this research, we adopted the isothermal amplifications and LFS to construct a new detection strategy for on-site detection of target bacteria. With the filtration assisted pretreatment for samples, the genetic template can be easily collected for amplification by ERA at the constant temperature with a common water-bath device in less than 30 min [25]. And taking advantage of the designed functional primer set, the amplified products can be qualitatively and quantitatively measured with the designed LFS in less than 5 min. With this culture-free and extraction-free sample treatment and the ERA-LFS integrated procedures, most limitations of conventional protocols for on-site detection of pathogenic bacteria have been well resolved.
2. Materials and methods
2.1 Reagents and apparatus
Chloroauric acid tetrahydrate (HAuCl4·4H2O), streptavidin (SAV)and microfiltration membrane were all purchased from the J&K Co.,Ltd. (Shanghai, China). Bovine serum albumin (BSA) was purchased from the Bio-Dee Biotech. Co., Ltd. (Beijing, China). Basic nucleic acid isothermal amplification kits were purchased from the Synda Gene Technology Co., Ltd. (Suzhou, China). Conjugate pads, sample pads, polyvinyl chloride (PVC) adhesive backing pads, absorption pads, and nitrocellulose (NC) membranes were all purchased from the Jie-Ning Biotech. Co., Ltd. (Shanghai, China). Injection syringe(50 mL) were purchased from the Sinopharm Chemical Reagent Co.,Ltd. (Shanghai, China). Filter membranes were purchased from the Sangon Biotech Co., Ltd. (Shanghai, China).
2.2 Preparation of Salmonella contaminated vegetables samples
The pathogenic bacteria used in this research were all collected from Anhui Product Quality Supervision and Inspection Institute,China (Salmonellatyphimurium: ATCC14028;L. monocytogenes:ATCC19111;S. aureus: ATCC6538;E. coliO157:H7: CICC10907;Vibrio parahaemolyticus: ATCC17802). High concentrations ofSalmonellawill be diluted in turn into the concentration of 107,106, 105, 104, 103, 102, 101, 100CFU/mL. Accurately weigh 8 equal portions of vegetable leaves and spray 1 mL ofSalmonellasolution at different concentrations confirmed by the gold standard culture method. TheSalmonellacontaminated vegetables will be kept at 4 °C to simulate the routine storage conditions for detection.
2.3 Sample pretreatment and DNA extraction
Take 10 g above preparedSalmonellacontaminated vegetables into the homogenized bag, add 100 mL ddH2O into the sealed bag and shake for 3 min to elute theSalmonellainto the solution [26].The washing solution was taken out into a 50 mL syringe and filtered through a 0.45 µm membrane [27]. The pathogenic bacteria can be detained on the microporous membrane. The retained bacterium was collected for direct ultrasonic treatment. The membrane was immersed into 200 µL Tris-EDTA buffer solution (TE buffer, 10 mmol/L Tris-HCl,1 mmol/L EDTA, pH 8.0) and the solution was treated with ultrasound for 5 min. The power of the ultrasound instrument we chose was 100 W and the frequency of 40 kHz. When 200 µL sample solution containingSalmonellawas placed in the liquid medium with ultrasonic wave, the high-frequency alternating pressure produced by ultrasound in water expands the cavity in the solution, causing an explosion which will break the cell wall. Finally, DNA was released into the supernatant. Then, the large fragments of impurities will be deposited at the bottom by centrifugation with 8 000 r/min for 3 min,and the supernatant included extracted DNA template ofSalmonellawas collected and adopted for subsequent analysis directly.
2.4 Preparation of AuNPs and the AuNP-antibody conjugates
Gold nanoparticles (AuNPs) were prepared based on our previously reported protocol with slight modifications [24]. Typically,50 mL of chloride gold acid solution with a concentration of 0.1 g/L in flask was heated to boiling with vigorous stirring. Then, 900 µL 1% sodium citrate was injected quickly after 2 min of continuous stirring and heating, the color of the mixture would turn from light yellow to black, and finally a stable color of purplish red can be obtained. Then,remove heating source and keep stirring for additional 5 min. After cooling, the solution would be stored at 4 °C in refrigerator.
Subsequently, 8 µL 1 mg/mL FITC-Ab was added in the colloidal gold solution which adjusted pH in advance with 0.1 mol/L K2CO3,and the mixture was incubated for 1 h at room temperature, 10% BSA was added and reacted for 30 min for blocking the unreacted sites.The mixture was centrifugated at 11 500 r/min for 10 min and the sediment was redissolve in 80 µL 10% BSA solution, then,AuNP-antibody conjugates were obtained. Finally, the conjugates solution was sprayed onto the conjugation pad and dried at 37 °C overnight for subsequent LFS assembly.
2.5 Design and assembly of LFS
The LFS is composed with 5 parts: the back-plastic plate, sample pad, conjugation pad, NC membrane, and absorbent pad. The late four parts were overlapped with the adjacent part for 2 mm on the back-plastic plate to ensure the migration of the solution. The test line (T line) and control line (C line) were prepared as follows with the Biodot sprayer, 20 µL 2 mg/mL SAV and 20 µL 1 mg/mL of goat-anti-mouse second antibody were sprayed onto the NC membrane at the rate of 0.5 µL/cm to form the T line and C line,respectively. Finally, the whole strip sheet was cut into the separated LFS with the width of 3 mm.
2.6 Rapid isothermal amplification of target genes of Salmonella
ERA was adopted for the rapid and isothermal amplification of the target genes ofSalmonellato produce the dual-labeled functional amplicons with the designed functional primer set. The detailed sequence information and modification description of primer set can be found in Table 1. Typically, 1 µL of forward primer (10 µmol/L)and 1 µL of reverse primer (10 µmol/L) were mixed with the ERA solution containing recombinase, DNA polymerase and single-strand DNA binding protein (SSB) and so on, to a final volume of 25 µL.Then, the amplification was conducted at 39 °C for 20 min. Finally,the amplicons were diluted with 960 µL phosphate buffer (PB,10 mmol/L) and 40 µL of the diluted amplicon sample was loaded onto the LFS. The results can be judged after 3 min by observing the optical intensity of T line on the LFS.

Table 1The sequence and functionalization information of primer set.

Table 2The comparison results with other common bacteria detection methods.
2.7 Verification of universal applicability of the strategy for common food samples
For verifying the universality of our detection method, we selected three common food samples for testing, including beverages,eggs and dairy products. The samples were treated in a similar manner to the lettuce.
3. Results and discussion
3.1 Detection principle of the proposal for rapid and isothermal detection of Salmonella contaminated vegetables
The schematic diagram of the detection strategy is demonstrated in Fig. 1, the isothermal amplification and LFS are integrated to challenge the culture-free rapid detection ofSalmonellacontaminated vegetables. And the filtration assisted collection of target bacteria and subsequent direct ultrasonic treatment can guarantee the efficiency and convenience for rapid detection ofSalmonella. Collected bacterium samples were ultrasonically treated quickly to release the genome from the bacterium as the template for direct isothermal amplification. The functional primer set labeled with biotin and fluorescein isothiocyanate (FITC), respectively, was adopted for EAR and then, great number of dual-labeled amplicons will be produced.These dual-labeled amplicons can be simultaneously recognized by both the AuNPs labeled FITC antibody on the conjugation pad and the SAV on the T line of LFS. The formed AuNPs-FITC antibodydsDNA-SAV structured will be retained on the T line of LFS and induce the observable red signal on T line of LFS. For negative results, there will be no amplicons, resulting in no optical signals on the T line. Nevertheless, the AuNP-antibody will be captured by the second antibody on C line of LFS and induce the optical signal of C line, which indicates the validity of the test. Of great note, three critical steps including the sample pre-treatment and collection,amplification and colorimetric detection can be completed in 10,25 and 3 min, respectively. And the whole process of culture-free detection of targetSalmonellacan be finished in 40 min without any complicated instruments, which can well meet the requirement of on-site screening of target bacteria.
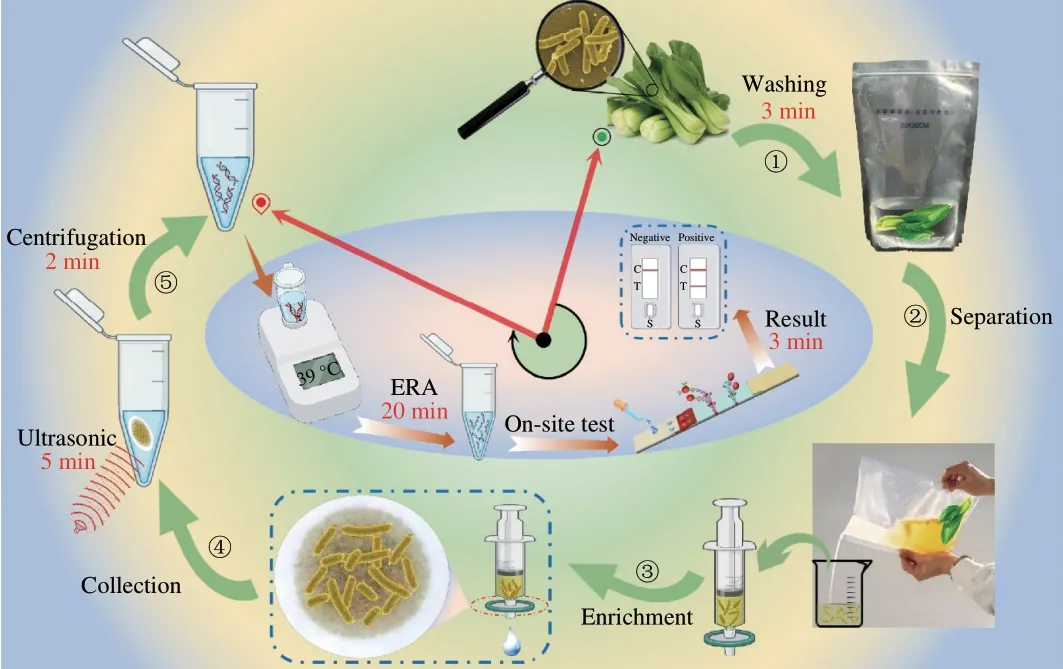
Fig. 1 Schematic diagram of the culture-free rapid detection of Salmonella contaminated vegetables.
3.2 Optimization of sample pretreatment operations
In this research, collection of target bacterium is a significant process which would affect the accuracy of the whole detection strategy. To ensure the efficient lysis and release of genome, different ultrasonic time was performed and compared. Results in Fig. 2 strongly demonstrated that with the increase of ultrasonic time, the extraction efficiency is improved greatly, and the best performance can be obtained in 5-10 min. If the ultrasonic processing time is too long, the intensity of the final visual test results inversely decreases.The reason can be ascribed to the fact that the longer sonication time can induce the break or destroy of the genome template and further influence the amplification efficiency. The corresponding quantitative analysis results were also shown in detail in Fig. 2. Finally, 5 min was selected as the optimal ultrasonic time for rapid extraction of genomic template for isothermal amplification.

Fig. 2 Sample pretreatment device and ultrasonic time optimization result diagram. (a-c) The filtration equipment for sample eluate, including syringes, filter plugs, microporous membrane, and vials. (d) Histogram of DNA concentration and ultrasonic time. (e) The electrophoresis diagram of DNA amplification products obtained at different ultrasonic time and the analysis results of LFS.
3.3 Conditions optimization of LFS
Besides the pretreatments, the preparation conditions of the LFS were also of great significance for the sensitivity. Firstly, the AuNPantibody conjugates was prepared by the electrostatic effect. Before the conjugation, the amount of the K2CO3was optimized to adjust the pH of AuNPs. Typically, 3, 5, 8, 10, 15 and 20 µL K2CO3were selected in the optimization process. Results in Fig. 3a demonstrated that 10 µL K2CO3achieved an optimal visualization result. Besides, in the conjugation AuNPs with antibody, an appropriate blocking reagent could effectively help to avoid non-specific adsorption, which usually caused false positive. For this case, 4 common proteins including casein, casein sodium, human serum albumin (HSA) and BSA were adopted for optimization. It can be found in Fig. 3b that using BSA as the blocking agent can effectively guarantee the avoidance of false positive effect and the well occurrence of high-quality T line for results judgements.

Fig. 3 Optimization results of lateral flow strip measurements. (a) The optimization results of the amount of K2CO3 added to the AuNPs for pH adjustment. The effects of 5,8, 10, 15 and 20 µL addition on the system were compared. (b) Optimization of resuspension solution including casein, casein-Na, HSA, BSA. (c) and (d) Comparison results of the amount of amplified products for loading and the type of loading buffer for LFS.
In this study, the amplification was ingeniously integrated with LFS for results analysis. Loading of the amplified products will directly determine the sensitivity and performance of detection.Different amounts of amplified products were mixed with the loading buffer for direct sampling. Too many products will increase the probability of local saturation and exhaustion of AuNPs probes,inducing the reversely decreased signal on T line. As demonstrated in Fig. 3c, 1 µL amplified products are sufficient for LFS detections with obvious difference between negative and positive group. Then,1 µL was adopted for the following LFS measurements. Finally,the loading buffer for the LFS was also considered. PB, PBST,Tris-HCl and Ac-AcNa were evaluated, respectively. And related results were shown in Fig. 3d. Except the Ac-AcNa system cannot form the integrated T line, results of other three systems were almost the same. Comparatively, the intensity of optical signals on T line was better under the condition of PB system, which was adopted for the rapid LFS detection. Of note, actually, the 10 mmol/L phosphate buffer solution containing 0.05% Tween-20 (PBST) system was almost the same. But the presence of Tween-20 in the system will inevitably increase the viscosity of the solution and the migration rate is decreased accordingly. This is also the reason for the adoption of PB for detection.
3.4 Detection sensitivity and stability of the isothermal amplification integrated LFS for Salmonella
Finally, the proposed filtration assisted detection strategy was applied for the direct and on-site detection ofSalmonellacontaminated vegetables in the culture-free model to identify the sensitivity and stability. Typically, theSalmonellacontaminated vegetables were pretreated as described in the experimental part, and then determined with the isothermal amplification-integrated LFS. Results in Fig. 4a indicated that with the increase ofSalmonellaconcentration,the signal on T line becomes stronger from 100CFU/mL to 105CFU/mL while there is no signal of blank group. When the concentration is higher than 105CFU/mL, the signal on T line comes to the saturation, which can be attributed to the extra-high efficiency of isothermal amplification. The visual detection limit can be defined as 100CFU/mL. The ImageJ treated quantitative results are also shown in Fig. 4a and strongly support the visual observed results.
According to the ImageJ results, the calibration relationship was constructed and shown in Fig. 4b. In the concentration range from 100CFU/mL to 105CFU/mL, the linear relationship is comparable better with theR2of 0.998 26, which can provide the evaluation standard for quantitative analysis ofSalmonella. The repeatability of the strategy was investigated with the repeated measurements of 10 samples at the same concentration as shown in Figs. 4c-f.Results demonstrated that the signals of the 10 repeated measurements were comparable and the RSD of the detections were in the range from 3%-6%, which can meet the requirement of practical applications.
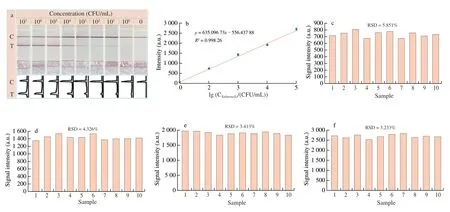
Fig. 4 Sensitivity and repeatability of Salmonella detection. (a) LFS detection results of Salmonella at different concentrations (100-107 CFU/mL) and corresponding ImageJ analyzed results. (b) The correlation between the concentration of Salmonella and the optical intensity value of T line (R2 = 0.998 26).(c-f) Stability and repeatability results of Salmonella detection at different concentrations ((c) 102, (d) 103, (e) 104 and (f) 105 CFU/mL).
In addition, a comparison with other reported methods for common bacteria detection was conducted as demonstrated in Table 2.The category of the methods, the detection time and detection limit were all listed and compared. Comparison results in Table 2 well demonstrated the excellent performance of this culture-free method forSalmonelladetection with the least requirements of hardware,indicating the powerful potential for the on-site and accurate screening of bacteria contaminations.
3.5 Detection specificity of the isothermal amplification integrated LFS for Salmonella
To identify the specificity of our strategy, the common pathogenic bacteria were adopted for evaluation. On one aspect, when other non-target bacteria were determined with the proposed strategy, there were no signal on the T line; on the other, when the targetSalmonellawas mixed with other non-target bacteria, the signals on T lines can be easily observed and distinguished. All these results in Fig. 5 demonstrate the excellent specificity and anti-interference property of this isothermal amplification integrated LFS strategy.

Fig. 5 The specificity and anti-interference investigation results. Intensity in response to E. coli O157:H7, L. monocytogenes, S. aureus, V. parahaemolyticus,and Salmonella. The concentration of each bacterium is 104 CFU/mL.
3.6 Practical application of the isothermal amplification integrated LFS strategy in other food matrix
Detection of targetSalmonellain fresh vegetables was successfully conducted with this filtration-assisted isothermal amplification-integrated LFS. In order to evaluate the wide applicability of this strategy,Salmonellain other common samples including milk, orange juice and eggs were also determined with this strategy in the cultural-free mode. Detection results of different food samples were depicted in Fig. 6. It well indicated that detection ofSalmonellacan be well achieved in different food samples. Meanwhile, the visual detection limit is of little difference among these foods, which is 102CFU/mL for milk, 101CFU/mL for orange juice and 102CFU/mL for eggs.This difference may be induced by the different matrix of the samples, which directly affect the filtration efficiency in the rapid pretreatment procedures. Anyway, all these results have well proved the powerful application potential of this filtration assisted isothermal amplification-integrated LFS for rapid detection of target pathogenic bacteria in various food samples.
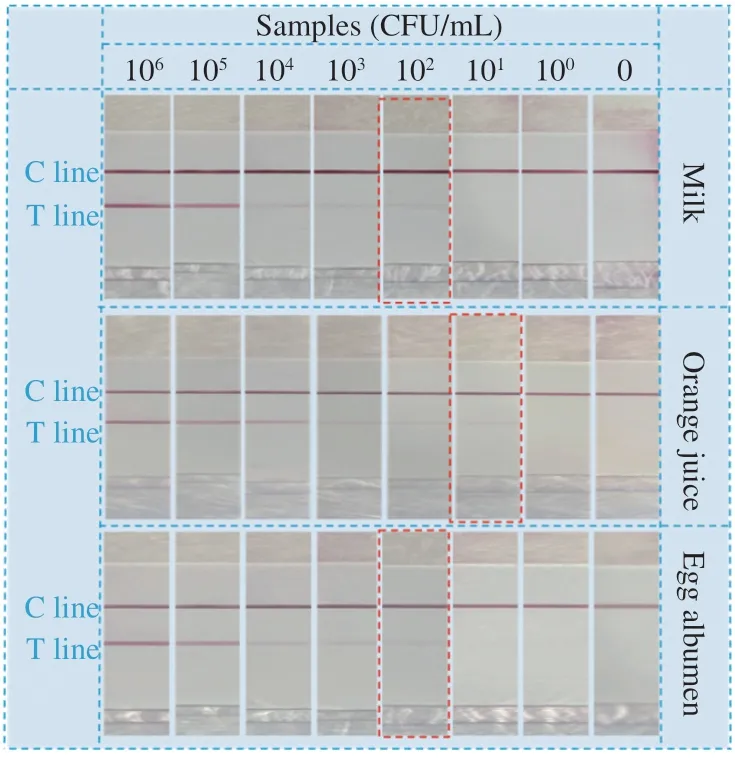
Fig. 6 Detection results of common food samples with the isothermal amplification integrated LFS in the culture-free mode (egg, orange juice and milk). The concentration range of pathogenic bacteria was 100-106 CFU/mL.Red box: The visual detection limit is of little difference among these foods.
4. Conclusions
In this study, we have developed a filtration assisted pretreatment protocol for rapid enrichment and accurate detection ofSalmonellain vegetables in the culture-free mode. The wholeSalmonelladetection process can be finished in 40 min including the steps of sample pretreatment, DNA isothermal amplification and LFS test. Under the optimized conditions, visual detection limit of 100 CFU/mL of targetSalmonellahas been achieved. Semi-quantitative results were realized in the range from 100CFU/mL to 105CFU/mL. Besides, in addition to vegetables, we also applied this strategy in other common food samples include milk, juice, and eggs for detection ofSalmonella.And satisfactory results were also obtained, indicating the universality of this designed culture-free rapid bacteria screening strategy. This new developed strategy has been evaluated with high sensitivity,good specificity, and various foods applicability for detection ofSalmonella, and it also provides a promising platform for on-site screening of other foodborne pathogens.
Declaration of competing interest
The authors declare that they have no competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was financially supported by the grants of the NSFC (32172295, 21804028), the key R&D program of Anhui(201904d07020016), the Anhui Provincial NSF (1908085QC121),the Fundamental Research Fund for central university(JZ2019HGTB0068), the China Postdoctoral Science Foundation(2019M652167), the Fund of State Key Lab of Chemo/Biosensing and Chemometrics (Hunan University), the postdoc grant of Anhui(2020B412) the Young and Middle-aged Leading Scientists,Engineers and Innovators of the XPCC (2019CB017) and China Agriculture Research System-48 (CARS-48).
- 食品科学与人类健康(英文)的其它文章
- Emerging natural hemp seed proteins and their functions for nutraceutical applications
- A narrative review on inhibitory effects of edible mushrooms against malaria and tuberculosis-the world’s deadliest diseases
- Modulatory effects of Lactiplantibacillus plantarum on chronic metabolic diseases
- The role of f lavonoids in mitigating food originated heterocyclic aromatic amines that concerns human wellness
- The hypoglycemic potential of phenolics from functional foods and their mechanisms
- Insights on the molecular mechanism of neuroprotection exerted by edible bird’s nest and its bioactive constituents

