In vivo anti-aging properties on fat diet-induced high fat Drosophila melanogaster of n-butanol extract from Paecilomyces hepiali
Akang Dan, Yushi Chen, Yongqi Tian*, Shaoyun Wang*
a College of Biological Science and Technology, Fuzhou University, Fuzhou 350108, China
b College of Chemical, Fuzhou University, Fuzhou 350108, China
Keywords:Paecilomyces hepiali Anti-aging Drosophila Dimerumic acid
A B S T R A C T The purpose of this study was to explore the potential of the development and application of Paecilomyces hepiali, a fungus with edible and medicinal value, as a foodborne antioxidant and anti-aging agent. Its n-butanol extract (PHE) from rice cultures was selected for anti-aging experiment because of significant free radical scavenging activity in vitro. In vivo, PHE could signif ciantly prolong the mean lifespan, 50% survival days, and the maximum lifespan of Drosophila on a high-fat diet. It is amazing that the mean lifespan increased from 19.1 days to 32.9 days, 50% survival days increased from 15.7 days to 34.3 days, and the maximum lifespan extended from 44.7 days to 52.7 days, when the high-fat female Drosophila model was fed with 10 µg/mL PHE. Further research showed that PHE reduced the accumulation of peroxide products and increased the activity of antioxidant 3.4 µg/mLonDPPH freeradicals scavengingactivity),4,5-dihydroxy-3-methoxypentanoicacid (compound2,enzymes.Then,throughantioxidant activitytracking, dimerumicacid (compound1,theIC50valueof new compound), and thymidine (compound 3) were isolated from PHE. It is worth mentioning that dimerumic acid, the major antioxidant compound of PHE (content up to 3%), was discovered in P. hepiali for the f rist time.It was concluded that PHE showed excellent anti-aging activity at a very low concentration on fat diet-induced high fat Drosophila melanogaster, and dimerumic acid may be its main material basis. These results indicated that PHE had the potential to be developed as antioxidant and anti-aging agent in the healthcare industry.
1. Introduction
The reactive oxygen species (ROS) production by mitochondria,which are indispensable for normal life activities during the metabolism of organisms. They include superoxide anion radicalshydroxyl radicals (·OH), and hydrogen peroxide radicals (HO2·) [1].Under normal circumstances, antioxidant enzymes (superoxide dismutase, SOD; catalase, CAT) in organisms can eliminate these free radicals and maintain them in a dynamic balance [2]. However, when the organism is exposed to external adverse stimuli, it will cause damage to mitochondria and a large accumulation of ROS, which will destroy the cells and tissues of the organism and cause aging.Based on this, Harman [3] proposed the free radical aging theory.This theory shows that excessive accumulation of free radicals is a key cause of aging. In summary, an important means to delay aging is to maintain the dynamic balance of free radicals in the organism, that is, to improve the ability to resist oxidative damage. However, due to the accelerated pace of life, modern people often consume a lot of high-fat and high-calorie food, which may form a burden on the organism and causes oxidative damage to cells and tissues [4].Therefore, it is necessary to f ind effective ways to reduce the harmful effects of a high-fat diet.
Paecilomyceshepialiis a medicinal and edible fungus belonging to the genus ofPaecilomyces Bainin the Moniliaceae family [5,6].Studies have shown thatP.hepialihas a tremendous capacity to produce structurally diverse metabolites with a variety of potent biological activities, such as antioxidants, anti-inflammatory,antibacterial, anti-tumor, immunomodulatory, and other biological activities [7]. In addition, our preliminary experimental results also showed that then-butanol extract (PHE) ofP.hepialirice cultures had excellent antioxidant activityinvitro, so that PHE had good antioxidant activityInvivowas speculated. At present, there are almost no reports on the antioxidant activity of secondary metabolites ofP.hepialiinvivo.
As an animal model commonly used in epigenetic studies,Drosophila melanogasteris very similar to mammals in the occurrence and pathogenesis of many metabolic pathways and nervous system diseases, and studies have also shown that the aging ofD. melanogasteris often accompanied by declines in motor and cognitive abilities [8,9]. Over and above that, the growth, development,and reproduction ofDrosophilaare very similar to humans, and they are homology with more than 75% of the known pathogenic genes in humans [10]. Meanwhile, according to the Functional Evaluation Procedure and Inspection Method of Health Food issued by the National Health Commission of the People’s Republic of China, if theDrosophilasurvival test, lipid peroxide, and antioxidant enzyme are positive, the tested products can be judged to have an anti-aging effect [11-13]. These advantages prove thatDrosophilais an excellent animal model to study oxidative damage leading to accelerated aging [14].
Recent studies have demonstrated that the decline in the lifespan ofDrosophilais closely related to long-term high-fat food intake [15].ForD. melanogaster, long-term intake of high-fat diets easily promotes the accumulation of peroxide products, leading to a large amount of reactive oxygen free radicals accumulationinvivo,resulting in oxidative damage to the organism and the disorder of normal metabolic function, which ultimately accelerates the aging and death ofD. melanogaster[16]. Therefore, in this study, then-butanol extract of the fermentation product ofP.hepiali(PHE)from the natural fermentation of rice was prepared. While measuring its antioxidant capacityinvitro, the aging model ofDrosophilafed a high-fat diet was utilized to study its antioxidant effectinvivo. At the same time, the antioxidant secondary metabolites ofP.hepialiwere separated and purified by the activity tracking method, and the main active compounds were characterized using MS and NMR spectrometry.
2. Materials and methods
2.1 Materials
TheP.hepialiused in this study was obtained from the China Forestry Microbial Culture Collection and Management Center (CFCC 5494). TheDrosophilaused in this study was the wild-typeD. melanogasterCanton-S, which was obtained from the Institute of Biochemistry and Cell Biology in Shanghai. All commercial kits used in the experiment were purchased from Nanjing Jiancheng Bioengineering Co., Ltd. (Nanjing, China).Chromatographic grade methanol and acetonitrile were purchased from J.T.BAKER Co., Ltd. (Shanghai, China). DMSO-d6and CD3OD were purchased from SIGMA-ALDRICH Co., Ltd. (Shanghai,China).
2.2 Preparation of PHE
In short,P.hepialiwas inoculated onto the plate poured with a sterile solid PDA medium, and incubated in a 27 °C incubator for 7 days to make it rejuvenate successfully. The rejuvenatedP.hepialiwas inoculated into sterilized rice and fermented at room temperature for 30 days. Then, poured into methanol (1:10,m/V) and soaked in ultrasonic for 2 h, and obtained the extract was after vacuuming filtration. The extracts were extracted three times withn-butanol and then brought together to obtain the extracts, which were spin-dried under reduced pressure to obtain PHE.
2.3 DPPH free radical scavenging activity
Dissolving PHE with distilled water, PHE solutions of different concentrations were prepared. Referring to the detection method in reference and making appropriate adjustments, the DPPH free radical scavenging ability test was carried out [17,18]. In brief, the 100 µL sample was mixed with a 100 µL DPPH (0.1 mmol/L, ethanol) and incubated at 25 °C in darkness for 0.5 h. Then the absorbance of each group was measured at 517 nm. Ethanol was used to replace DPPH as the reference group and ethanol was used to replace the sample as the blank control group. The DPPH free radical scavenging activity of the sample was calculated according to the following formula:

WhereAiandAjare the absorbance of the sample group and the reference group, respectively.A0is the absorbance of the blank control group.
2.4 ABTS free radical scavenging activity
The ABTS free radical scavenging activity assay was performed using the prefabricated PHE with appropriate adjustment according to the determination method in reference [19,20]. The specific method was as follows: ABTS solution of 7 mmol/L and K2S2O8solution of 2.45 mmol/L were mixed 1:1 and incubated at 25 °C in darkness for 0.5 h. Then, before use, methanol was used to adjust the absorbance of the mixture at 734 nm to 0.70 ± 0.05 (working fluid). The 100 µL sample was mixed with 100 µL working solution and incubated for 10 min at 25 °C under dark conditions. Then the absorbance of each group was measured at 734 nm. Methanol reagent was used to replace the same volume of working fluid as the reference group. Methanol reagent was used to replace the samples as the blank control group. ABTS free radical scavenging activity of the sample was calculated as follows:

WhereAPandACare absorbances of the sample group and the reference group respectively.Amaxis the absorbance of the blank control group.
2.5 Diet preparation and Drosophila feeding
TheDrosophilabasal diet consisted of 7.0% cornmeal, 1.3% soybean meal, 1.0% agar, 1.8% glucose, 1.8% sucrose and 0.3% yeast.In the bargain, 0.1% methyl paraben, 1.0% propionic acid and 1.0% Tween-20 emulsifier were added to prevent the diet from spoiling [21]. 10.0% lard was added to the basic diet as a high-fat diet. 3 mL of diet was prepared in each vial to feed theDrosophila,and the prepared food was stored at 4 °C. AllDrosophilawere fed in a dark/light cycle for 12 h in a temperature and humidity cabinet at 25 °C and relative humidity of 60.0%.
2.6 Lifespan analysis
The method ofDrosophilalifespan analysis was inspired by that reported in the study [15]. After the collected newly hatchedDrosophilawere anesthetized with CO2, they were separated according to male and female. TheDrosophilawere randomly split into 10 groups, with 5 groups of males and females (60 per group,20 per tube).Drosophilafed on a basic diet was served as a non-lard control (NCT), andDrosophilafed a high-fat diet was served as a lard control (LCT).Drosophilafed on a high-fat diet containing 1, 5 and 10 µg/mL PHE (PHE1, PHE5, PHE10) were used as experimental groups (the added amount of PHE was determined according to its IC50value of DPPH free radical scavenging activity). All groups were transferred to corresponding vials containing a fresh diet every 3 days. The number ofDrosophilathat died was recorded at the same time every day. To demonstrate that the increase in lifespan was independent of dietary restriction,Drosophilabody weights were assessed on days 7 and 14, respectively.
2.7 Climbing ability test
Drosophilatends to climb in an enclosed environment, and we can evaluate the motor ability ofDrosophilaby its crawling ability [22]. We can test their crawling ability by the number ofDrosophilaclimbing a fixed distance in a certain period. Feeding 20Drosophilain each group of the diet for 7 and 14 days, respectively,then transferred to an empty vial and marked at a fixed position.Then, shaking the vial to make all theDrosophilafall to the bottom of the bottle, and the number ofDrosophilathat climbed upwards by more than 7 cm within 20 s was recorded. The crawling ability ofDrosophilawas expressed as the percentage ofDrosophilathat climbed more than 7 cm.
2.8 In vivo antioxidant activity
Drosophilawas fed in each group for 7 and 21 days, respectively.Drosophilawere anesthetized with CO2and then frozen and sacrificed at -20 °C.Drosophila100 mg was mixed with 0.9 mL normal saline, and theDrosophilawere homogenized, and centrifuged at 10 000 r/min for 10 min. The total protein content inDrosophilawas determined according to the kit instructions, and the activities of T-SOD and CAT, as well as the contents of MDA and PCO inDrosophila, were determined by diluting the concentration of tissue homogenate according to the instructions.
2.9 Separation and purification of PHE
Making appropriate adjustments to the methods of reference [23,24]. In a nutshell, the PHE was dissolved in methanol and mixed with silica gel, and then the silica gel was used to fill the column and the mixed sample was loaded. The sample was gradient eluted with methanol and dichloromethane, and then the eluent was collected and detected by TLC, and similar fractions were combined and concentrated. Finally, the DPPH free radical scavenging activity of the collected fractions was detected. After that, the most active fraction was selected for gel column chromatography. After filling the gel, the sample was slowly poured into the gel and eluted with methanol. The eluent was collected and the previous steps were repeated. The selected fractions with the strongest antioxidant activity were separated and purified by high-performance liquid chromatography, and sufficient samples were collected to prepare for NMR detection.
2.10 Identification of main antioxidant compound and new compound from PHE
After the deuterium reagent DMSO-d6was used to dissolve the sample to be the subject, the solution was transferred to the NMR tube, and then the sample was tested. The test contents were: 1D NMR (1H-NMR,13C-NMR, and DEPT-135) and 2D NMR (HSQC,HMBC). And the antioxidant activity of the obtained chemical compounds was detected using the same method as section 2.3.
2.11 Statistical analyses
The results were expressed as mean ± SD for independently performed experiments. The data of the antioxidant activityinvitrowere analyzed by one-way ANOVA using SPSS 23.0. All data were reported as mean ± SD of 3 independent experiments. The experimental results were statistically analyzed using GraphPad Prism8.0 software,t-test and a log-rank test were used, andP< 0.05 was considered statistically significant.
3. Results and discussion
3.1 Antioxidant activity of PHE in vitro
Excessive accumulation of free radicals in the organism will cause damage to proteins, nucleic acids, and lipids in the organism,which will lead to the destruction of the structure of cells and even the mutation of cells [25]. In this study, the scavenging activity of PHE on DPPH and ABTS free radicals was tested [26]. The result was displayed in Fig. 1. The scavenging activity of PHE on free radicals showed an obvious concentration-dependent manner. The IC50values of PHE on DPPH free radicals and ABTS free radicals scavenging activity were 14.3 and 6.5 µg/mL, respectively. The results showed that PHE had excellent antioxidant activityinvitro.

Fig. 1 The (A) DPPH and (B) ABTS free radicals scavenging activity of PHE. Data are represented as mean ± SD. Different lowercase letters indicate significant differences at the 0.05 probability level. Statistical significances were analyzed by one-way ANOVA.
3.2 The effect of PHE on the lifespan of Drosophila on high-fat diet
Studies have demonstrated that the accumulation of free radicals can damage cells, tissues, and organs by attacking biological macromolecules such as nucleic acids, causing oxidative stress and accelerating human aging [27]. As shown in Fig. 1, PHE had excellent antioxidant activity against free radicalsinvitro, and we speculated that PHE also had antioxidant activityinvivo. This conjecture was confirmed by survival experiments ofD. melanogaster. The results showed (Table 1, Fig. 2) that compared with the NCT group, when 10% lard was added to the diet of theDrosophilain the LCT group,the mean lifespan of male and femaleDrosophilawas greatly reduced, and the reduction reached to 33.6% (P< 0.01) and 49.6%(P< 0.01). This indicated that excessive fat intake in the daily diet can significantly shorten the lifespan ofDrosophila, which was similar to the results of other studies [28,29]. Amazingly, when PHE was added to the high-fat diet, the lifespan ofDrosophila, especially females, was significantly increased and showed a concentration dependence. The mean lifespan of the femaleDrosophilain the PHE10 group increased from 19.1 days to 32.9 days (P< 0.01),with the 50% survival days increased from 15.7 days to 34.3 days(P< 0.05), and the maximum lifespan extended from 44.7 days to 52.7 days (P< 0.05). Correspondingly, the maleDrosophilamean lifespan in the PHE10 group was prolonged from 17.8 days to 23.3 days (P< 0.05), with the 50% survival days increased from 20.0 days to 26.2 days (P< 0.05), and the maximum lifespan extended from 28.8 days to 33.7 days (P< 0.05). Studies have shown that dietary restriction (DR) significantly increases the lifespan ofDrosophila[30]. The test results showed (Fig. S1) that there was no significant change in the bodyweight of the maleDrosophilaexperimental group compared with the NCT group. However, in femaleDrosophila, at day 14, the LCT group lost significant body weight and recovered significantly when PHE was added to the diet.The results of lifespan analysis suggested that PHE could markedly prolong the lifespan ofDrosophila, indicating that PHE had an effective protective effect on oxidatively damaged by a high-fat diet,especially on femaleDrosophila. The weight test results eliminated the effect of DR on lifespan extension inDrosophilaand were consistent with the results of increased lifespan inDrosophila.
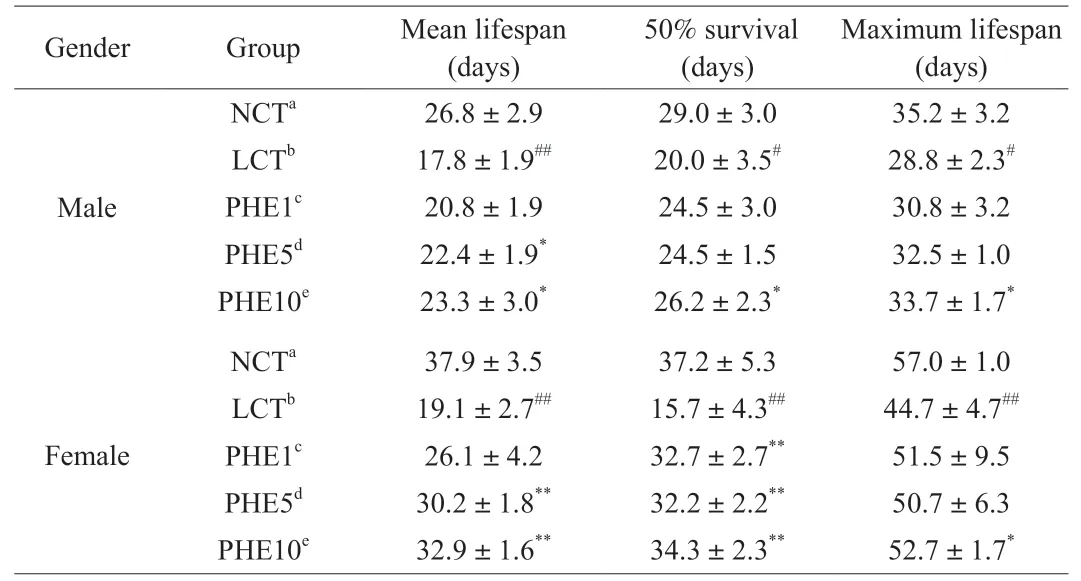
Table 1Effect of different diets on the lifespan of Drosophila.

Fig. 2 Survival curves of (A) male and (B) female Drosophila on different diets. PHE1, PHE5, and PHE10 represent 10% lard diets containing 1, 5, and 10 µg/mL of PHE, respectively. LCT group compared with the NCT group of the corresponding gender (##P < 0.01). PHE1, PHE5, and PHE10 group compared with the LCT group of the corresponding gender (ns P > 0.05,*P < 0.05, and **P < 0.01).
3.3 The effect of PHE on the climbing ability of Drosophila
ForDrosophila, aging goes hand in hand with a slowdown in metabolism and a decline in motor ability [31]. As shown in Fig. 3,on the 7thand 14thdays, the climbing ability of male and femaleDrosophilain the LCT group was significantly reduced (P< 0.01).When PHE was added to the diet, the climbing ability ofDrosophilawas significantly improved, especially in the PHE10 group. On the 14thday,the climbing ability of male and femaleDrosophilain the PHE10 group was increased by 77.2% (P< 0.01) and 48.1% (P< 0.01),respectively, while on the 7thday, the climbing ability of male and femaleDrosophilawas only increased by 17.1% (P< 0.05)and 10.7% (P< 0.05). At the same time, it could be observed in the experiment that under the high-fat diet, the femaleDrosophilawas more obese than the maleDrosophila, and their actions were more sluggish.

Fig. 3 Effect of PHE on the climbing ability of Drosophila. (A) 7th day,(B) 14th day. Data are represented as mean ± SD. ##P < 0.01 compared with the NCT group of the corresponding gender. *P < 0.05 and **P < 0.01 compared with the LCT group of the corresponding gender.
3.4 The effect of PHE on antioxidant enzyme activity
ROS is a common by-product of the process of cell metabolism.When external conditions stimulate the generation of oxidative stress reaction, the production of free radicals overwhelms the antioxidant capacity of the organism, resulting in various diseases including aging [32]. Antioxidant enzymes can achieve the dynamic balance of free radicals in the organism by scavenging excess free radicals in the organism, which is a significant oxidative stress defense system of the organism [33]. This experiment studied the effect of supplementing different doses of PHE on the activities of CAT and T-SOD inDrosophila. As shown in Fig. 4, whether it was female or maleDrosophila, the activities of CAT and T-SOD enzymes in their bodies were reduced with the aging ofDrosophila,and this situation was more obviously in the LCT group. Adding PHE to the diet could significantly increase the activity of the CAT enzyme in both male and femaleDrosophila. Particularly in femaleDrosophila, when PHE supplemental level reached 10 µg/mL (PHE10 group), CAT activity in femaleDrosophilawas increased by 62.7%(P< 0.01) and 91.1% (P< 0.01) compared with the LCT group on the 7thand 21stday, respectively. On the 7thday, adding PHE to the diet did not significantly improve the T-SOD enzyme activity inDrosophila, but on the 21stday, the T-SOD enzyme activity of male and femaleDrosophilain the PHE10 group was increased by 12.2%(P< 0.05) and 23.6% (P< 0.05), respectively. The experimental results showed that the supplementation of PHE could effectively improve the activities of antioxidant-related enzymes inDrosophilawith a high-fat diet, notably in femaleDrosophila.

Fig. 4 Effects of PHE on CAT (A, 7th day; B, 21st day) and T-SOD (C, 7th day;D, 21st day) levels in Drosophila. Data are represented as mean ± SD. #P < 0.05 and ##P < 0.01 compared with the NCT group of the corresponding gender.*P < 0.05 and **P < 0.01 compared with the LCT group of the corresponding gender.
3.5 The effect of PHE on the level of oxidation products
The process of oxidative damage and aging of the body is usually accompanied by lipid oxidation and protein carbonylation [34].Malondialdehyde (MDA) is the final product produced by the lipid peroxidation reaction of free radicals in the organism, which aggravates the damage of cell membranes, and can cause cross-linking and polymerization of large molecules such as proteins and nucleic acids and is cytotoxic [35]. Protein carbonylation (PCO) is a process in which proteins undergo oxidative damage and eventually convert to carbonyl products [36]. In this study, the protective effect of PHE on oxidative damage inDrosophilawas explored by the determination of the levels of MDA and PCO inDrosophila. The result was shown in Fig. 5. For femaleDrosophila, when PHE was supplemented to 10 µg/mL (PHE10 group), MDA level was decreased by 51.4%(P< 0.01) and 40.3% (P< 0.05) on the 7thand 21stday, respectively,compared with LCT group. Under the same condition, MDA level in maleDrosophilawas decreased by 34.6% (P< 0.05) and 27.8%(P< 0.05). PHE also drastically reduced the PCO level inDrosophila.For femaleDrosophila, on the 7thand 21stday, the PCO level of the PHE10 group decreased by 46.9% (P< 0.05) and 39.9% (P< 0.05),respectively. The PCO level of the maleDrosophilain the PHE10 group was significantly reduced by 37.6% (P< 0.05) and 29.7%(P< 0.05) on the 7thand 21stdays. These results proved that PHE could slow down lipid oxidation and protein carbonylation inDrosophila.
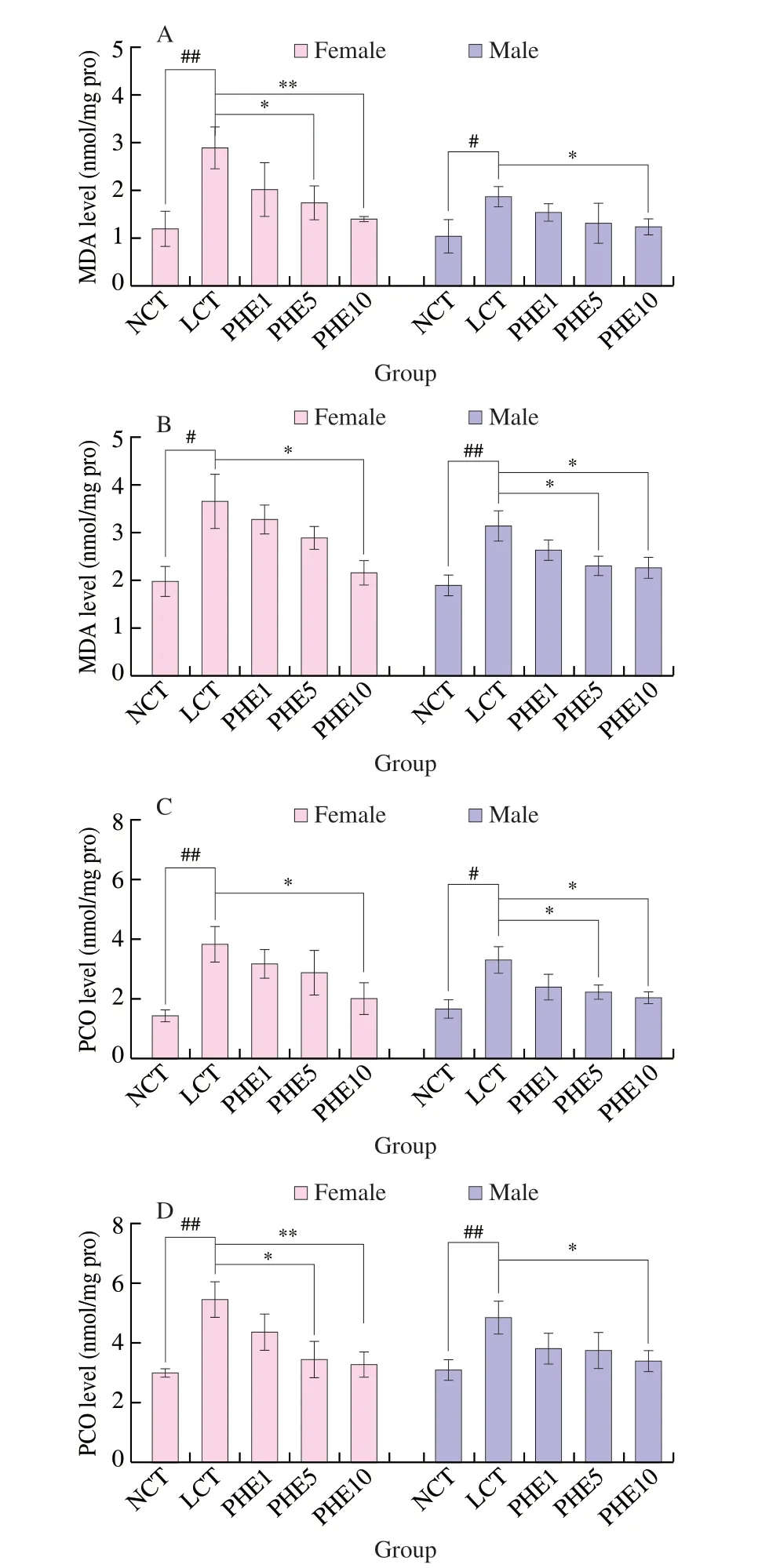
Fig. 5 Effects of PHE on MDA (A, 7th day; B, 21st day) and PCO (C, 7th day;D, 21st day) levels in Drosophila. Data are represented as mean ± SD. #P < 0.05 and ##P < 0.01 compared with the NCT group of the corresponding gender. *P < 0.05 and **P < 0.01 compared with the LCT group of the corresponding gender.
3.6 Separation and purification of PHE
The above results have proved that PHE has excellent antioxidant activity. To explain the basis of antioxidant active substances of PHE and find the main active fractions, the method of antioxidant activity tracking was used to find the main active compounds. The separation and purification process of PHE was shown in Fig. S2, and the antioxidant activity of each fraction was presented in Fig. S3. After gradient elution of PHE on the silica gel column, it was found that the antioxidant activity of fractionEwas the strongest (Fig. S3A). Then, the fractionEwas eluted with a gel column, and the antioxidant activity of each fraction was detected after re-separation. It was found that theE2fraction had the strongest antioxidant activity, with the IC50value of 4.8 µg/mL on DPPH free radicals scavenging activity (Fig. S3B). The final separation and purification of fractionE2were performed by HPLC to obtain compounds1,2, and3(Fig. 6).

Fig. 6 HPLC of compounds 1 (A) and 2, 3 (B).
3.7 Identification of main antioxidant compound and new compound
Compound1was isolated as crimson oil, and its molecular formula C22H36N4O8was determined by HRESIMS (Fig. S4), which indicated 7 degrees of unsaturation. Combining NMR data (Tables 2,Figs. S5-S9), it could be concluded that compound1contained 4 carbonyl carbons (δC168.4, C-1;δC167.1, C-7), 2 methoxy groups (δC54.3,δH3.82 (s),CH-2), 10 methylenes (δC30.9,δH1.69, m, CH-3;δC22.6,δH1.60, m,CH-4;δC46.0,δH3.36 (t,J= 6.6 Hz), CH-5;δC44.3,δH2.24,(t,J= 6.6 Hz), CH-10;δC59.7,δH3.53 (t,J= 6.6 Hz), CH-11),4 olefinic carbon signals (δC116.7,δH6.24 (s), CH-8;δC151.5, CH-9),2 methyl delta (δC18.6,δH2.02 (s), CH-12). These data were in complete agreement with that of the reference [37], so compound1was identified as dimerumic acid (PubChem CID: 6443767, Fig. 7).
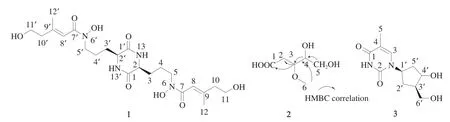
Fig. 7 The structure of compounds 1-3.
Compounds2and3cannot be separated by common chromatographic methods, and they were identified using 1D and 2D NMR. The molecular formula of compound2was deduced to be C6H12O5by interpretation of NMR data, suggesting one degree of unsaturation. The NMR data (Table 3, Figs. S10-S14) displayed that compound2contained 1 carboxyl group (δC176.3, C-1),2 oxygenated methines [(δC85.3,δH4.45 (td,J= 3.0, 1.0 Hz), CH-4;δC78.1,δH4.01 (dt,J= 5.5, 1.5 Hz), CH-3)], 1 oxygenated methylene(δC61.8,δH3.56, m, CH2-5), 1 methoxy (δC56.2,δH3.26, s, CH3-6),1 methylene (δC35.3,δH2.84 (dd,J= 15.0, 6.0 Hz),δH2.42 (dd,J= 15.0, 1.5 Hz), CH2-2). The HMBC correlations (Fig. S14) of H-2/C-1,C-3, and C-4, and H-3/C-1, C-2, C-4, C-5, and C-6, and H-4/C-2,C-3, and C-5 and H-6/C-3 completed the structure of compound2and named 4,5-dihydroxy-3-methoxypentanoic acid.
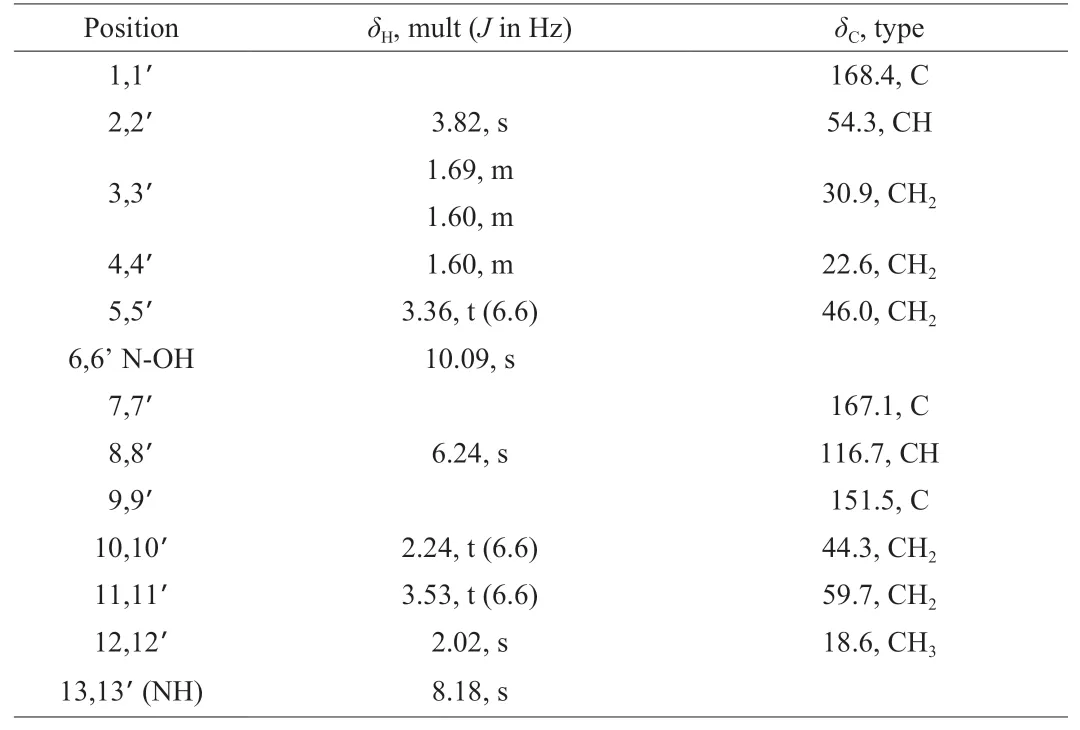
Table 21H NMR (600 MHz) and 13C NMR (150 MHz) data for compound 1 in DMSO-d6.
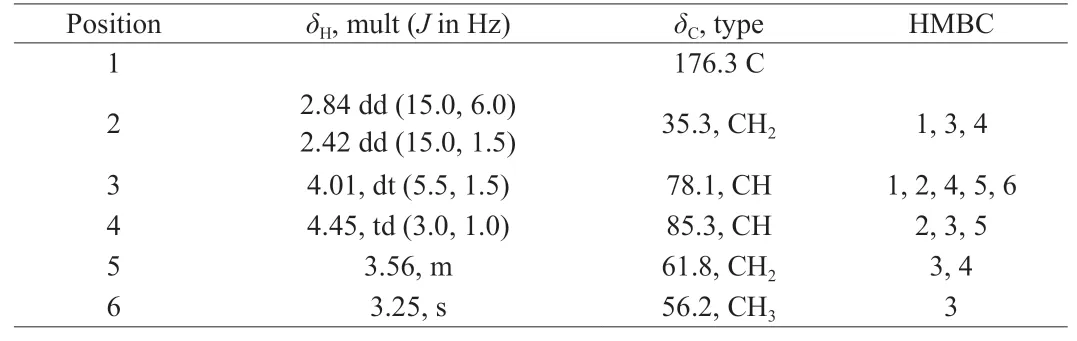
Table 31H NMR (600 MHz), 13C NMR (150 MHz) and HMBC data for compound 2 in DMSO-d6.
Compound3contained 2 carbonyl groups (δC164.2, C-1;δC150.9, C-2), 2 olefinic carbons (δC136.6,δH7.70 (d,J= 1.2 Hz), CH-3;δC109.8, C-4), 3 methine groups (δC84.2,δH6.17 (td,J= 5.5, 1.2 Hz),CH-1’;δC70.9,δH4.23 (m), CH-3’;δC87.7,δH3.76 (q,J= 3.0 Hz),CH-4’), 2 methylene groups (δC40.0,δH2.05 (m), CH2-2’;δC84.2,δH3.56 (m), CH2-5’), 1 methyl group (δC12.7,δH1.77 (d,J= 0.6 Hz), CH3-5).These data are completely consistent with the literature data [38], so compound3was identified as thymidine (PubChem CID: 5270603, Fig. 7).
The antioxidant activity of compounds1-3was tested. Compound1showed excellent DPPH free radical scavenging activity with IC50of 3.4 µg/mL, which is equivalent to the activity of EGCG (positive control, IC50of 1.3 µg/mL). Unfortunately, the IC50value of compounds2and3on DPPH free radical scavenging activity was greater than 1.5 mg/mL (Fig. S3C). These results showed that the main antioxidant compound in PHE was compound1(dimerumic acid, which accounts for 3%). Dimerumic acid, mainly found inMonascus, had been shown to have anti-inflammatory, anti-tumor, and other biological activities,and its antioxidant activity was stronger than resveratrol [39].In addition, a large amount of dimerumic acid was isolated fromP.hepialifor the first time in this article.
4. Conclusions
In this study,n-butanol extract ofP.hepialirice medium (PHE)was selected forinvivoanti-aging activity and chemical study,because of its excellent antioxidant activityinvitro. The diet-induced a high fatD. melanogasterexperiment showed that PHE could prolong the mean lifespan, 50% survival days, and maximum lifespan.At the same time, adding PHE to the diet could significantly improve the climbing ability ofDrosophila, reduce the levels of MDA and PCO, and increase the activities of T-SOD and CAT inDrosophila.These studies suggest that PHE has anti-aging activity and can improve the antioxidant enzyme system by eliminating or decreasing the level of peroxide products. The studies also demonstrated that PHE was better for female than maleDrosophilain resisting oxidative damage caused by a high-fat diet. The results of antioxidant activity tracking showed that there was a large amount of dimerumic acid in PHE, which was the main material basis for PHE to exert antioxidant activity and it was isolated fromP.hepialifor the first time. From what has been discussed above, PHE has the potential to be used as antioxidant and anti-aging agents in the food and healthcare industries.
Declaration of interest statement
All authors declare that no conflict of interest exists.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (42006094), and by Fujian Natural Science Foundation (2019J05032).
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://doi.org/10.1016/j.fshw.2022.10.015.
- 食品科学与人类健康(英文)的其它文章
- Emerging natural hemp seed proteins and their functions for nutraceutical applications
- A narrative review on inhibitory effects of edible mushrooms against malaria and tuberculosis-the world’s deadliest diseases
- Modulatory effects of Lactiplantibacillus plantarum on chronic metabolic diseases
- The role of f lavonoids in mitigating food originated heterocyclic aromatic amines that concerns human wellness
- The hypoglycemic potential of phenolics from functional foods and their mechanisms
- Insights on the molecular mechanism of neuroprotection exerted by edible bird’s nest and its bioactive constituents

