Multi-protective effects of wheat embryo globulin on D-gal-induced aging mice
Aimei Lio, Xing Lyu, Jirui M, Yinchen Hou, Ming Hui, N Liu,Yi Zho, Yixing Cui, Jihong Hung,,d,*
a College of Biological Engineering, Henan University of Technology, Luoyang 471000, China
b Henan Provincial Key Laboratory of Biological Processing and Nutritional Function of Wheat, Luoyang 471000, China
c Zhengzhou Synear Food Co., Ltd., Zhengzhou 450000, China
d School of Food and Pharmacy, Xuchang University, Xuchang 461000, China
Keywords:Wheat embryo globulin Antioxidant activity D-galactose Superoxide dismutase Glutathione peroxidase
A B S T R A C T Wheat embryo globulin (WEG) has been proven to possess multiple biological activities, including antioxidative properties, immunomodulatory, and so on. Aged mouse model were established by subcutaneous injection of D-galactose (D-gal), and the effects of WEG on learning, memory, and antioxidant capacity in aging mice were explored through behavioural tests and antioxidant enzyme activities determination.Compared with the Model group, WEG improved the percentage of the platform quadrant, increased the number of crossing platforms, and enhanced the identif ication indexs. WEG also increased total antioxidant capacity (T-AOC), superoxide dismutase (SOD), and glutathione peroxidase (GSH-Px) activities in the liver and brains of aging mice, and reduced malondialdehyde (MDA) content. Pathological observations indicated that WEG protected against damage to brain in D-gal-induced aging mice. These results effectively revealed that WEG not only improved the abilities of learning and memory, and the cognitive impairment, but also delayed the aging process of the D-gal-induced mice.
1. Introduction
Aging is an extremely complex and multi-factor natural process involving physiological, cellular, psychological inf luence of an organism,which is defined as the degeneration of organism functions [1-3].Aging can lead to the decline of various tissues, organs and cells in the human body, and is associated with the pathogenesis of multiple disorders or diseases including inflammatory, diabetes,cancer, metabolic, cardiovascular, and neurodegenerative diseases,such as Alzheimer’s disease (AD) [4,5]. One of the most prominent manifestations of aging is brain dysfunction, resulting in decreased cognition such as language capabilities, memory, and ability to recognize faces, brain and evident hippocampal atrophy, and so on [4,6].The world’s population ages, according to the UN report that by 2050, the world’s population over 60 years of age will reach 2.1 billion, and the population’s aging will continue to increase [7].With the increase of the global aging population, anti-aging has become an important issue. Aging models are generally divided into two types: natural aging and accelerated aging model. The latter is more preferred because of its more convenient and shorter duration of study [8]. The accelerated aging model induced by routine and quantitative injection ofD-galactose (D-gal) is widely employed as the antiaging model, since the administration ofD-gal into animals such as mice or rats may induce symptoms similar in many ways to natural aging. For instance, it may produce brain aging similar to human brain aging including cognitive deficits, neuronal degeneration, oxidative stress [8,9]. The main source of reactive oxygen species (ROS) is the mitochondrial respiratory chain,which generates oxidative stress. Increasing evidence suggests that oxidative stress and cytotoxicity are linked to the aging process [10].In order to combat ROS and their harm influences, organism display a series of endogenous antioxidant systems. Therefore, it can be concluded that protecting the antioxidant defence system is an important way to effectively prevent aging. It is indicated that consumption of antioxidants can remove free radicals in the body and delay the aging process [11,12]. Mitochondria-targeted antioxidant (SkQ1)was indicated to suppress AD-like pathology progression [5].Brown black wolfberry rich in phenolic and flavonoid contents was depicted its anti-aging potential by enhancing the survival time,reducing the oxidative stress caused by H2O2, and increasing the antioxidant enzyme activities inDrosophila melanogasterandD-gal induced aging mice, respectively [13]. With the aging population, it is more attractive to improve the general health and prevention the degenerative diseases of aging by enhance the diet or discovering the natural nutrition with antioxidant activities.
Wheat embryo, one of the by-products of wheat processing, not only consists of various nutrients necessary for life activities, but also contains a variety of micro-physiological active substances [14].Wheat embryo globulin (WEG) is the main functional active ingredient in wheat germ, and has been proven to display a variety of biological activities, including antioxidant properties, antihypertensive capacity, alleviation of acute alcohol-induced liver injury, and immunomodulatory effects [15-18]. Ji et al. [19] established a mouse model of immunosuppression and explored the immune mechanism of WEG at the gene level, indicating that WEG increased total antioxidant capacity (T-AOC) in mice and affects the ratio of Thl/Th2 cell subsets by controlling the expression of T-bet and GATA-3 proteins. WEG can not only improve the body’s immunity, but also play a certain role in fighting inflammation. Atallahi et al. [20] studied the severity of the effects of wheat germ extract on systemic primary dysmenorrhea symptoms, which may be due to its anti-inflammatory effect. Based on the manifested antioxidant and anti-inflammation activities of WEG, as well as the bioinformatics analysis of WEG carried out by our research group, we hypothesis that WEG potentially possess anti-aging capacity and prevent from the brain dysfunction related to aging.
However, as far as we know, there are few reports on the protective effect of WEG on the brain of aging mice. SinceD-gal treatment has been highlighted to cause memory and cognitive impairment in mice, similar to natural aging,D-gal-induced senescence models are usually used in the study of senescence [21-24].Therefore, the main objective of this study was to evaluate the possible protective effect of WEG onD-gal-induced senescence in mice. In this way, the mechanism of WEG intervening senescence was further understood.
2. Materials and methods
2.1 Chemicals and reagents
WEG (1%) was obtained from Henan Cooperativity Medical Science and Technology Research Institute Ltd. (Luoyang, China).Huperzine A (Hup A) capsules (No. 180501) were purchased from Shanghai Fudan Fuhua Pharmaceutical Co., Ltd. (Shanghai, China).D-gal (No. 129G051, high purity grade, purity ≥ 99.0%) was purchased from Beijing Solarbio Science & Technology Co., Ltd.(Beijing, China). Kits for determination of the level of superoxide dismutase (SOD) (No. 20190525), glutathione peroxidase (GSH-Px)(No. 20190520), malondialdehyde (MDA) (No. 20190517), and bicinchoninic acid (BCA) protein assay kit (No. 20180313) were purchased from Nanjing Jiancheng Bioengineering Institute(Nanjing, China). Haematoxylin and eosin (H&E) reagents and total antioxidant capacity (T-AOC) (No. 20190220) were purchased from Beijing Solarbio Science & Technology Co., Ltd. (Beijing,China). Paraformaldehyde (4%) was purchased from Beijing Labgic Technology Co., Ltd. (Beijing, China).
2.2 Animals and experimental design
Seventy-two male BALB/c mice (18-22 g) were purchased from the Experimental Animal Center of Henan University of Science and Technology (Luoyang, China); Animal Certificate No. SCXK(E) 2010-0007.Before the experiment, the mice were fed with drinking water freely.Adaptive feeding after 1 week, the mice were randomly divided into 6 groups (n= 12, Table 1) [25], among them, Blank group and Hup A group were severed as the negative and positive control,respectively. During the first 4 weeks, the mice in the Blank group were administered saline by the subcutaneous injection (s.c.) in the neck, others were injectedD-gal solution (s.c.) in sterile saline(300 mg/(kg·day)), followed by the morris water maze test to validate the damage of memory and recognition induced byD-gal to mice. During the following 6 weeks, mice in Blank group were supplemented saline (s.c.) in the neck and intragastrically administered (i.g.) saline. Mice in Model group were supplied saline(i.g.) whileD-gal solution administration (s.c.). Mice in Hup A group were given Hup A (20 µg/(kg·day)) (i.g.) andD-gal solution (s.c.).Mice in WEG groups were administrated corresponding amount of WEG (i.g.) andD-gal solution (s.c.) in the neck. All animals were treated according to the regulations of Experimental Animal Center of Henan University of Science and Technology (Luoyang, China).
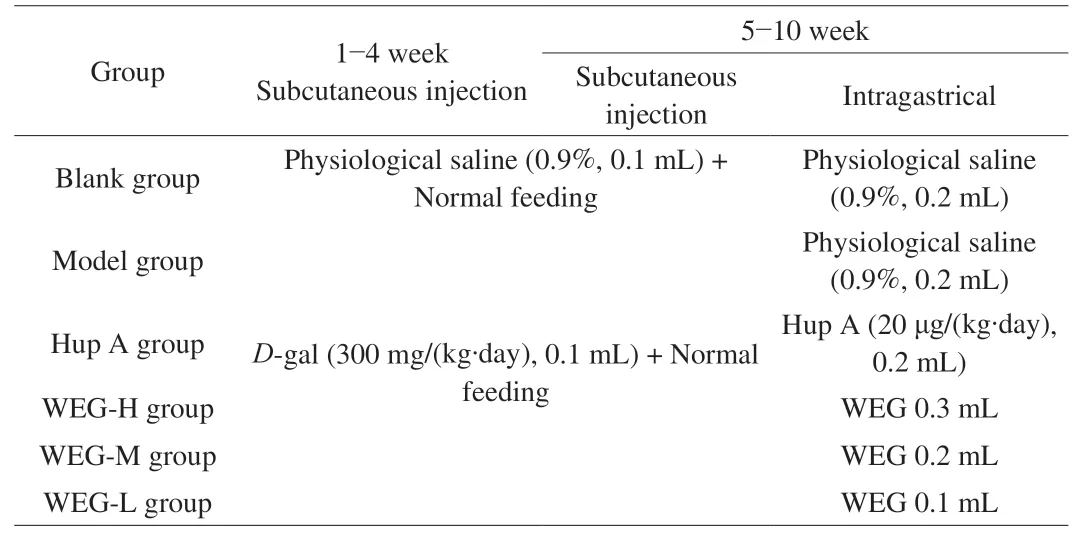
Table 1Grouping and administration of animals.
2.3 Daily physical status
The body weight and physiological changes of the mice were measured and recorded every week, including food intake, physical activity, state of urine, feces and hair [26].
2.4 Morris water maze test
The Morris water maze device includes a circular pool (120 cm diameter and 40 cm height) and a platform (8 cm diameter and 20 cm height) [11,25,27]. Four quadrants divide the pool into 4 positions and the platform is fixed in the first quadrant in the centre. Warm water is added to the pool up to 1 cm above the platform. The Morris water maze was tested for a total of 4 days. The first three days of testing were positioning navigation training, and the mice were trained 4 times each day. The mice were trained to find the movable platform within 60 s, if a mouse did not find the mobile platform within 60 s,and the mouse did not stay on the platform for 10 s, the escape latency of each mouse and the percentage of the platform quadrant were recorded.
The last day of the test was a space exploration test. First, we removed the platform from the pool and placed each mouse in any quadrant in the pool for 60 s. During the period of time when each mouse was swimming, we recorded the number of times the mouse crossed the platform and the percentage of the platform quadrant. In addition, we also plotted and analyzed the swimming trajectories of the mice [28-30].
2.5 Novel object recognition test
The experiments were performed in an opaque box (60 cm ×60 cm × 50 cm). Two identical objects were fixed in parallel position in the box at a distance of 10 cm from the nearest two sides. Mice were placed in the box away from the object and the time that the mouse explored each object with its nose or feet (distance ≤ 2 cm)was recorded. Next, one of the objects was replaced with a completely different object (based on colour and shape), and the mice were once again introduced to the box [31]. The discrimination index (DI) is currently the main criterion for assessing the curiosity of mice to regard new things. The DI is calculated as the percentage of the total time of the mice spent exploring the new object as compared to the total time they spent exploring the original object and the new object.
2.6 Serum and tissue preparation and organ indices
After the experiment, all mice were fasted for 24 h. Mice were anesthetized with ether, and blood was collected via the venous sinus.The collected blood was centrifuged for 10 min at 8 000 r/min (4 °C)and serum was collected. After blood collection, mice were sacrificed by cervical dislocation. The heart, liver, spleen, lung, kidney, and brain were removed; the tissues were washed with cold saline and the weights of each organ were recorded. All collected serum, tissues,and organs were stored at -80 °C for future use [32]. The organ index calculation formula is expressed as below:
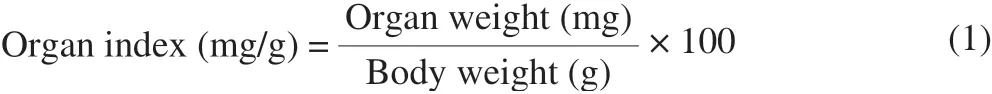
2.7 Histopathology of the brain
Memory-related functions in the brain include synaptic plasticity, hippocampal function, and some molecular and cellular mechanisms of hippocampal dependence on memory [33,34]. After the calculation of the organ index, the brains of the mice were immersed in 4% paraformaldehyde for 24 h, and then subjected to gradient ethanol dehydration, paraffin embedding, and sectioning(5 µm). The cut tissue sections were placed in warm water, then fixed on glass slides, dewaxed, and stained with H&E. All stained tissue sections were viewed under a light microscope (D2, Zeiss, Wetzlar,Germany) to determine the histopathological changes (×100 and ×200 magnifications, respectively) [35].
2.8 Determination of oxidation related biomarkers
The 10% homogenates of mouse tissues were prepare in 9%sodium chloride by a homogenizer (8 000 r/min, 10 min, 4 °C) [36].The prepared tissue supernatant was used for analysis in biochemical experiments. Oxidation related biomarkers including T-AOC, SOD,GSH-Px, and MDA in the brain and liver tissue fluids of mice were detected according to the introduction of the commercial kits.
2.9 Statistical analysis
All experiments were performed three times. The results of the final study are expressed as mean ± standard deviation (SD). A normality test was used to determine the normal distribution, and the Levene test was used to measure the homogeneity of variance. Oneway ANOVA was used for comparisons among the groups. The data were considered significantly different whenP< 0.05.
3. Results
3.1 Effect of WEG on the physical status and body weight of mice
Observation of the mice for 10 weeks showed that the mice in the Blank group had relatively stable normal diet, good appetite, sensitiveto environment and normal activities, and shiny hair (Table 2).Compared with the mice in Blank group, the mice in the Model group were dull, their activity was reduced, their diet increased, and their sensitivity to the environment was reduced may due to the injection ofD-gal. The supplement of higher dose of WEG significantly improved physical activity, brightened the hair gloss, and restored other physiological state to normal such as food intake, frequencies and amount of urine and feces to normal. Medial dose or less dose of WEG indicated slighter beneficial effect on physical status of aging mice induced byD-gal-induced in comparison to the higher-dose of WEG.

Table 2Effects of WEG on physiological state of mice.
As shown in Tables 3 and 4, the mice in the Blank group generally had relatively stable weight gain. When onlyD-gal was injected,the weight of the mice changed, the Blank group grew slowly, while the other 5 groups grew faster, although the changes of body weight was not significant, indicating thatD-gal can reduce the activity of the mice and verify the successful model establishment (Table 3);After drug treatment, the weight gain of mice in each group changed(Table 4). In comparison to the weight gain of control group, the weight gains of the mice treated with high, medium, and low dose of WEG were lower, while the ones of the model group and Hup A group were relatively higher, with no statistic difference. On the whole, there were significant differences in the growth values among the groups, indicating that WEG can provide energy for mice and improve their mobility.
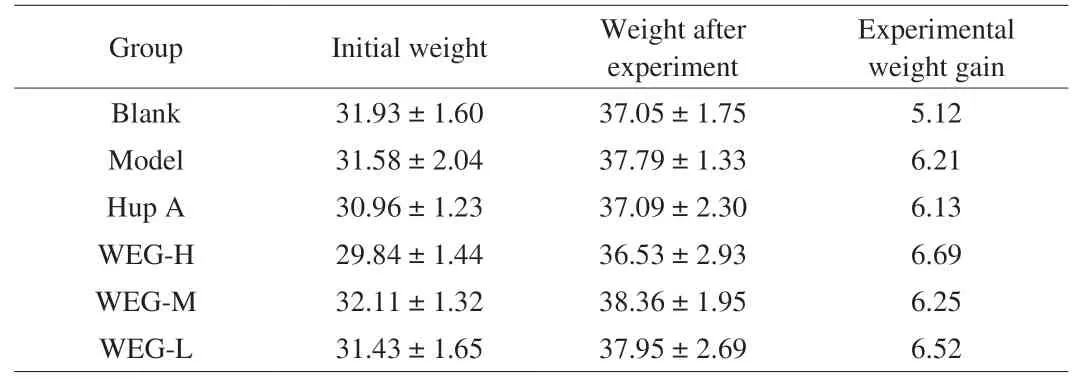
Table 3Effect of D-gal on body weight (g) in mice.

Table 4Effects of WEG on body weight (g) in D-gal-treated mice.
3.2 WEG ameliorates spatial learning and memory in D-gal-induced aged mice
During the Morris water maze test, the results of positioning navigation training are shown in Figs. 1A and B. On the first day of training, there was no difference in escape latency and percentage of swimming time in the platform quadrants in all groups of mice.With increasing training time, the escape latency of all groups of mice decreased at different rates, and the percentage of swimming time in the platform quadrants of all groups of mice increased at different rates. We selected the first 3 days of evaluation as the result of positioning navigation training. Compared with the Blank group,D-gal significantly prolonged the escape latency of mice (bothP< 0.05), and significantly shortened the percentage of swimming time in the platform quadrant of mice (bothP< 0.05). However,WEG significantly prolonged the percentage of swimming time in the platform quadrant of model mice in the WEG-M group (P< 0.05) and significantly reduced the escape latency of the mice in the WEG-M group (P< 0.05).
In the spatial exploration test on the 4thday, we removed the platform and performed the experiments (Figs. 2A, B, D). Compared with the Blank group, the percentage of swimming time in the platform quadrant of the Model group was significantly reduced(P< 0.05), and the number of cross-platform times was significantly reduced (P< 0.01). Compared with the Model group, the percentage of swimming time in the platform quadrant of the WEG group significantly increased in the WEG-L group (P< 0.05), and the number of cross-platform swims also increased significantly in the WEG-H group (P< 0.05), and in the WEG-M and WEG-L group (P< 0.01).
The results of the novel object recognition test are shown in Fig. 2C. Compared with the Blank group, the DI value of the Model group was significantly reduced (P< 0.01). Compared with the Model group, WEG increased the DI ofD-gal-induced aging mice in the WEG-L group (P< 0.05).
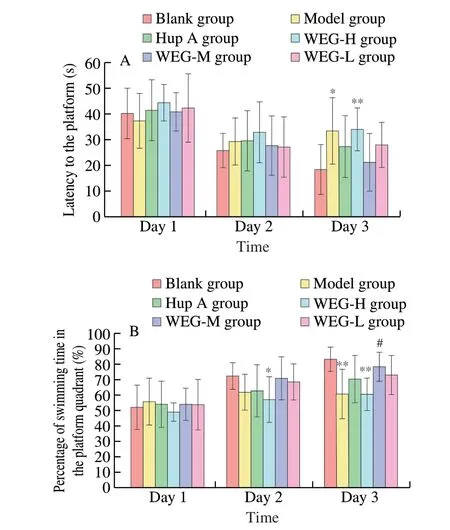
Fig. 1 Forest plot in positioning navigation training during Morris water maze test. The effect of WEG on escape latency in spatial performance (A)and the percentage of swimming time in the platform quadrant (B). All data are expressed as mean ± SD, n = 8. *P < 0.05 or **P < 0.01 vs Blank group;#P < 0.05 or ##P < 0.01 vs Model group.

Fig. 2 Effect of WEG on spatial learning and memory impairment in D-gal-induced aging mice. The percentage of swimming time in the platform quadrant (A),and the number of cross-platform times (B) in the probe trial during Morris water maze test, the DI of mice in the novel-object recognition test (C), and the swimming trajectory after the platform was removed during the probe test (D). D1-D6. Blank group, Model group, Hup A group, WEG-H group, WEG-M group,WEG-L group, respectively. All data are expressed as mean ± SD, n = 8. *P < 0.05 or **P < 0.01 vs Blank group; #P < 0.05 or ##P < 0.01 vs Model group.
3.3 Effects of WEG on organ indicators
Compared with the Blank group, the heart index of the Model group mice increased significantly (P< 0.05), and the liver index and spleen index increased significantly (P< 0.01) (Table 5).Compared with the Model group, the liver index of WEG group mice significantly decreased in the WEG-M group and in the WEG-L group(P< 0.05, andP< 0.01, respectively), and the WEG group’s spleen index significantly decreased in the WEG-L group (P< 0.05).

Table 5Effects of WEG on organ index in D-gal-treated mice.
3.4 Analysis of histopathological alterations
Histopathological analysis is mainly used to evaluate the protective effect of WEG onD-gal induced senile brain injury. As shown in Fig. 3, brain cells, as well as nuclei and cytoplasm of mice were clearly observed after H&E staining. The nucleus and cytoplasm of the Blank group (Fig. 3A) were clearly visible, and the nucleus was full and round. Compared with the Blank group, the nucleus of the Model group (Fig. 3B) was relatively blurred, and most of the nucleus appeared to be condensed. Compared with the Model group, the Hup A group (Fig. 3C), and the WEG group (Figs. 3D-F), a small number of cells had nuclear shrinkage, the whole nucleus and cytoplasm were clearly visible, the nucleus was enlarged, and the staining was darker.The pathology of the WEG-L group (Fig. 3F) was closest to the Blank group.
As shown in Fig. 4, neurons in the CA1 region of the hippocampus of mice in the Blank group (Fig. 4A) were closely arranged and orderly. Compared with the Blank group, the hippocampal neurons in the Model group (Fig. 4B) were loosely organized and sparsely arranged, and the number of normal neurons was significantly reduced, and they had different cell morphology. The morphology of neurons in the CA1 region of the hippocampus of mice in Hup A group (Fig. 4C) and WEG group (Figs. 4D-F) was basically normal,but the number of cell layers was less than that in the Blank group and the cell arrangement was slightly loose. WEG reduced the neuron damage caused byD-gal, which indicated that WEG can reduce the neuron damage caused byD-gal injection in the CA1 region of the hippocampus of mice.
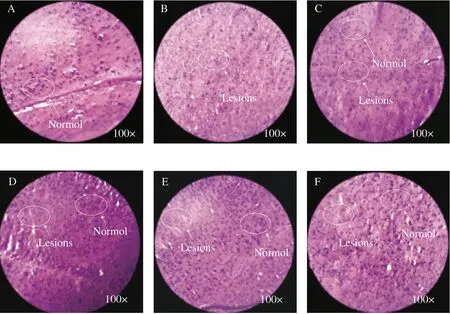
Fig. 3 Effect of WEG treatment on brain histopathological alterations (H&E staining, magnification 100×). (A) Blank group, (B) Model group, (C) Hup A group,(D) WEG-H group, (E) WEG-M group, (F) WEG-L group. Haematoxylin is a blue-purple basic dye, which stains the nucleus. Eosin is a pink acid dye that dyes the cytoplasm of most cells red.
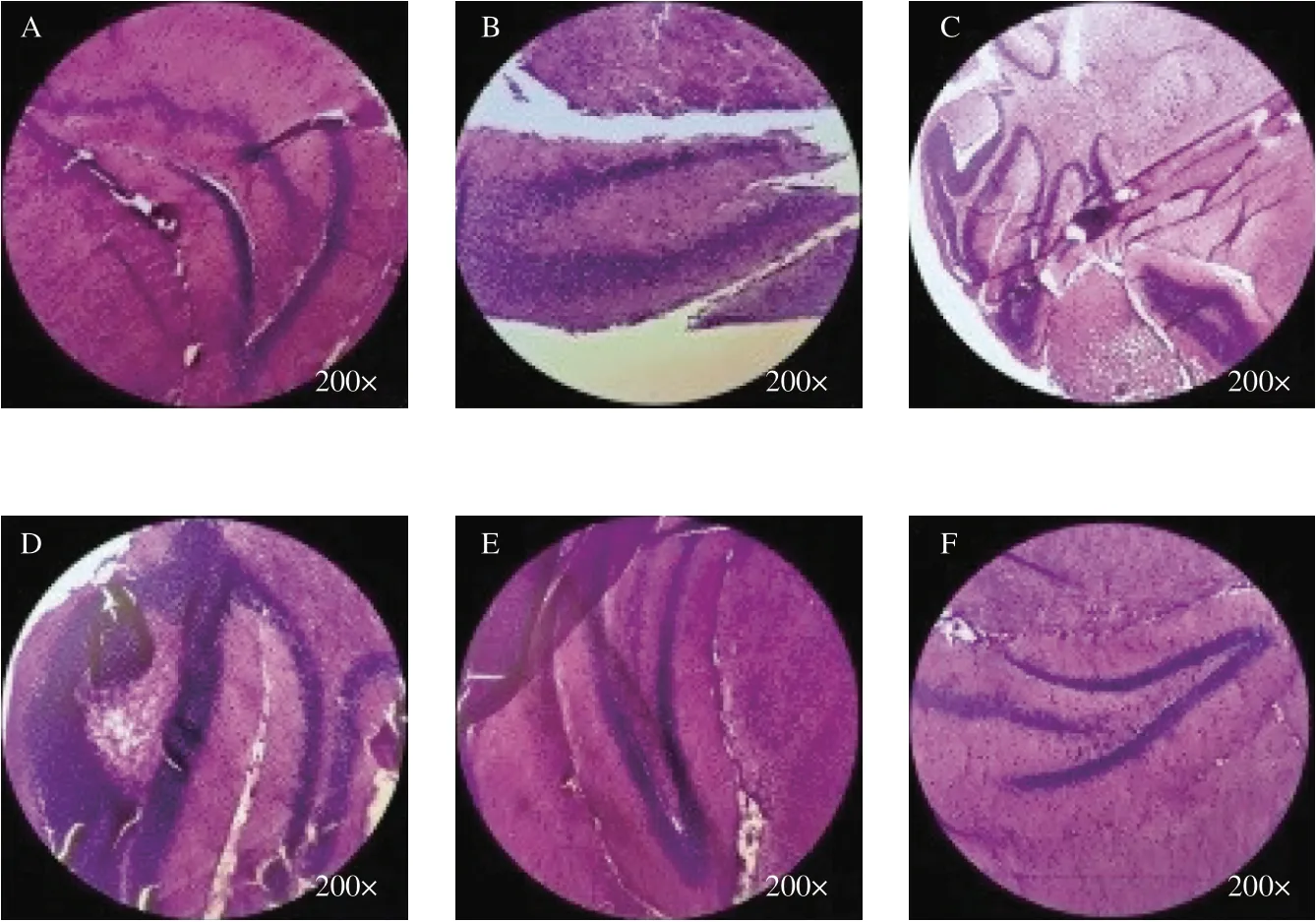
Fig. 4 Effect of WEG treatment on the CA1 region of the hippocampus (H&E staining, magnification 200×). (A) Blank group, (B) Model group, (C) Hup A group, (D) WEG-H group, (E) WEG-M group, (F) WEG-L group.
3.5 Effect of WEG on biochemistry indices in the brain and liver
According to previous studies, antioxidant activity plays a vital role in the process of biological aging. The effect of WEG on the level of antioxidants in the brain tissue (Figs. 5A-D) and liver tissue(Figs. 6A-D) ofD-gal-induced aging mice were determined. As shown in Fig. 5A, GSH-Px in the Model group was significantly lower than that in the Blank group (P< 0.05), and GSH-Px in the WEG-H group was significantly higher (P< 0.01). Compared with the Model group, the GSH-Px levels in the WEG-H group, the WEG-M group and the WEG-L group were all increased, and the differences between the WEG-H group and the WEG-L group were extremely significant (P< 0.01), and the differences between the WEG-M group and the WEG-L group were significant (P< 0.05).As shown in Fig. 5B, the MDA content in the model group was significantly increased compared with that in the Blank group(P< 0.05). Compared with the Model group, MDA content in the WEG-M group significantly decreased (P< 0.05), among which MDA content in the WEG-H group and the WEG-L group was relatively higher. The effect of WEG on SOD in brain tissue were shown in Fig. 5C. Compared with the Blank group, SOD levels in the Model group was significantly reduced (P< 0.01). Compared with the Model group, SOD levels of Hup A group, WEG-H group,WEG-M group, and WEG-L group were all significantly increased.Except Hup A group, there were significant differences (P< 0.05),and the other groups had extremely significant differences(P< 0.01). As shown in Fig. 5D, T-AOC in brain tissue of mice in the remaining 5 groups was significantly reduced compared with the Blank group (P< 0.01).D-gal can accelerate tissue aging, especially in the brain. Compared with the Model group, the T-AOC of the WEG-M group and the WEG-L group were both increased, with extremely significant difference (P< 0.01) and significant difference(P< 0.05) between the WEG-M group and the WEG-L group,respectively.
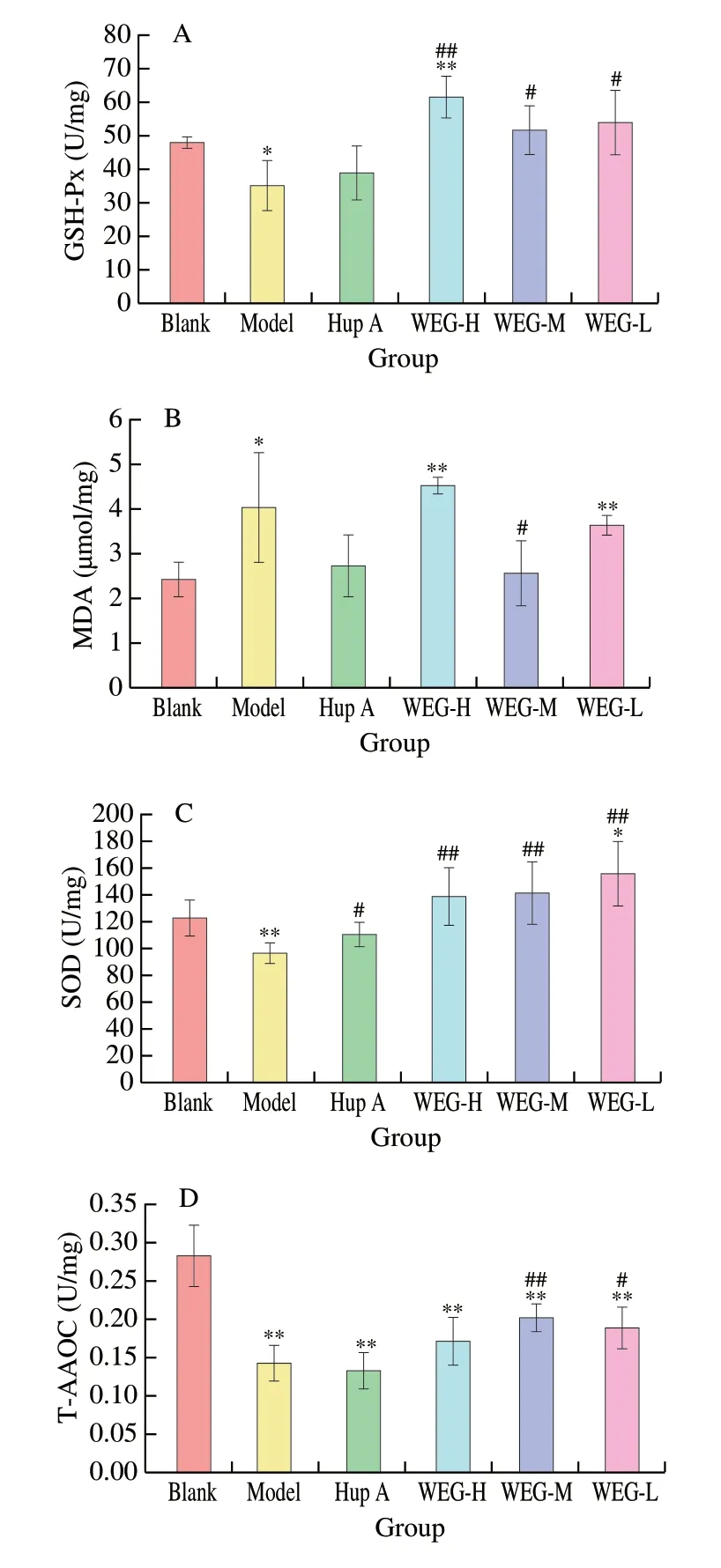
Fig. 5 Effects of WEG on oxidative stress and activity of anti-oxidative enzyme in the brain of mice. (A) GSH-Px, (B) MDA, (C) SOD and (D) T-AOC.Date are presented as mean ± SD from each group (n = 6). *P < 0.05 and**P < 0.01 vs Blank group, #P < 0.05 and ##P < 0.01 vs Model group.
The effect of WEG on GSH-Px level in liver were shown in Fig. 6A. Compared with the Blank group, GSH-Px in Model group showed no significant difference, but GSH-Px in Hup A group and WEG-H group was significantly increased (P< 0.01). Compared with the Model group, the GSH-Px in Hup A group was significantly increased (P< 0.01), and the GSH-Px in WEG-M group and WEG-L group were decreased (P< 0.01). As shown in Fig. 6B, there was no significant difference in MDA content in each group. Compared with the Model group, MDA in the WEG-M group and the WEG-L group tended to decrease. The effect of WEG on SOD activity in liver tissues were shown in Fig. 6C. Compared with the Blank group,SOD levels in the Model group was significantly reduced, with a very significant difference (P< 0.01). Compared with the Model group, SOD levels in the WEG-L group, the WEG-M group and the WEG-L group were significantly increased (P< 0.05). As shown in Fig. 6D, T-AOC in the liver tissues of mice in the Model group was significantly lower than that in the Blank group (P< 0.01). Compared with the Model group, T-AOC of WEG-H group, WEG-M group,WEG-L, and Hup A group, were all significantly increased (P< 0.05,andP< 0.01, respectively).
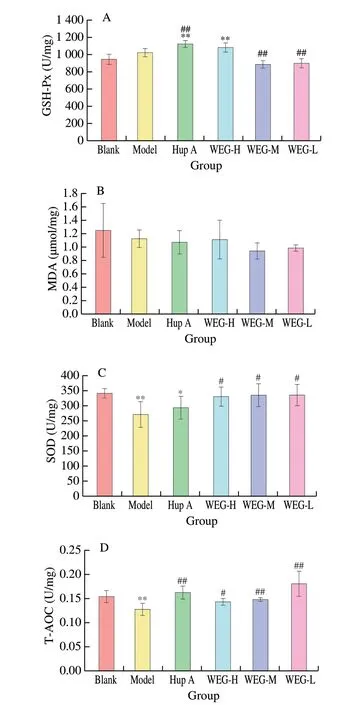
Fig. 6 Effects of WEG on oxidative stress and activity of anti-oxidative enzyme in the liver of mice. (A) GSH-Px, (B) MDA, (C) SOD, and (D) T-AOC.Date are presented as mean ± SD from each group (n = 6). *P < 0.05 and**P < 0.01 vs Blank group, #P < 0.05 and ##P < 0.01 vs Model group.
4. Discussions
The imbalance between ROS produced by endogenous or exogenous process and antioxidant defenses usually takes place in so far as aging, which results in the dysfunction of tissue and organ [9]. A large number of studies have shown that the injection ofD-gal induced accelerated aging to mice due to the enhancement of oxidative stress resulting from the excessive production of ROS and imbalance between ROS and antioxidant system. The oxidative stress was evidenced by the obvious increase in the level of MDA,decreased level of T-AOC, as well as a general decrease in the activity of key antioxidant enzymes involved in the antioxidant defence system. Except increased oxidative stress,D-gal probably cause mitochonrial dysfunction, mitochondrial DNA mutations, neuronal apoptosis, inflammation in the brain of the mice, correspondingly lead to neurodegeneration or cognitive deficits [37,38]. Therefore, the model ofD-gal induced aged mice has received great attention in the study of memory disorders [21,29,39-42]. In the biological oxidation process of the body, a large amount of free radicals are produced. The balance of free radicals in the body is accomplished by a number of antioxidant defense systems, including GSH-Px, SOD, catalase (CAT),reduced GSH, and vitamin C. Their synergistic effect converts excess free radicals in the body into oxygen molecules and water molecules,thereby acting as an antioxidant [10]. In this study, theD-gal-induced aging model was used to evaluate the therapeutic effect of WEG on aging-induced cognitive dysfunction.
A large number of peptides and proteases are the main source of human T-AOC [42-45]. WEG is the main active protein in wheat germ. It has been considered as a traditional medicine-homologous food in China with various beneficial activities for human health [46].Chemical antioxidant activity of WEG were previously evidenced via the 2,2’-azinobis(3-ethylbenzothiazoline-6-sulfonic acid ammonium salt (ABTS) free radical scavenging ability, Fe2+chelating ability,and hydroxyl radical scavenging activity in our lab, which were not significantly influenced during the WEGin vitrodigestion [47]. In present work, we explored the beneficial effects of WEG on spatial learning, memory impairment, and antioxidant ability in aging mice induced byD-gal. Supplement of WEG especially higher dose of WEG significantly increased the activities of SOD, the level of GSH-Px and T-AOC, and decreased the content of MDA. SOD and GSH-Px present the activity of enzymatic antioxidant system to inhibit radical formation or free radical damages [48,49]. T-AOC acts as an important nonenzymatic antioxidant. As the product of lipid peroxidation, the level of MDA services the marker of free radical activity [50]. WEG has increased the antioxidant enzymes capacityin vivo, and the main mechanism may be to effectively repair the damage caused byD-galin vivoby clearing excess free radicals, regulating the excess produce of ROS, managing the dynamic balance of oxidation-reduction reaction, or suppressing the accumulation of the product of lipid peroxidation in the accessed aging body, which in turn cause damages to the neurons. Paraffin sections of the brain tissue of mice vividly and intuitively reflected the degree of lesions in the brain of mice, which confirmed the protective effects of WEG against theD-gal inducing aging. The protection may be also related to the beneficial effect of WEG on anti-inflammation and improvement of immune system of mice. This possibly involves the nuclear factor erythroid 2-related factor 2 (Nrf2) which plays a crucial part in the activation of the protective genes that are antioxidant and anti-inflammatory [51].Additionally, the protection of WEG fromD-gal induced aging in mice passably perform through the modulation of gut microbiota after affording WEG, since the supplement of fermented wheat germ (FWG) increased the abundance of Bacteroidetes(B) while decreased the Firmicutes (F) in the intestinal flora in theD-gal-induced aging mice The enhancement of the ratio of the B/F is good for health and anti-aging [52]. The exact mechanism of WEG on protection ofD-gal induced aging need further investigation in future since there is close and complex relationship among the antioxidant, inflammation system, and gut flora. For instance,in vitroantioxidant activity was also observed fermented wheat germ extract (FWGE) obviously inhibited the ROS production, and the rate of lipid peroxidation regarding lipopolysaccharide (LPS) induced inflammatory response,owing to bioactive compounds including benzoquinone derivates [53].The beneficial effect of WEG on anti-aging revealing the potential effect of WEG to prevent against AD, possibly attributed to some special proteins in WEG, such as W5HB91, W5GM69, P00068, P94058,and W5C5F1, which are indicated to be involved in the metabolic pathway of AD by Kyoto Encyclopedia of Genes and Genomes(KEGG) analysis. Among them, protein W5HB91 potentially plays a key role in preventing the production of toxic Aβ in amyloid precursor protein (APP), which require further studies in future.
5. Conclusion
Through this experimental study, we concluded that WEG improves cognitive impairment in aging mice caused byD-gal,improves the learning and memory capabilities ofD-gal-induced aging model mice, and delaysD-gal-induced aging. Biochemical indexes of T-AOC, GSH-Px, SOD, and MDA indicate that WEG has strong antioxidant capacityin vivo. These data indicated that WEG has anti-aging effects. This study revealed that WEG can be used as a potential drug to delay aging. The molecular mechanism of its anti-aging effect needs further study.
Conflict of interest
The authors declare that they do not have conflict of interest with
the work submitted.
Acknowledgements
This research was funded by Zhongyuan Scholars in Henan Province (192101510004), Major Science and Technology Projects for Public Welfare of Henan Province (201300110300),and Innovation Demonstration Special Project of Henan Province(201111110100). This work was also financially supported by Key Project Foundation of Natural Science Research of Universities of Henan Province in China (20A550004), and Fundamental Research Funds for the Henan Provincial Colleges and Universities in Henan University of Technology (HAUT) and High-Level Talents Research Fund of HAUT (2018QNJH13, and 2018BS012). We also appreciate Editage (www.editage.cn) for English language editing.
Ethical approval
All treatment and maintenance of animals were performed in accordance with Experimental Animal Centre of Henan university of science and Technology (Luoyang, China; Animal certificate number:SCXK(E) 2010-0007).
- 食品科学与人类健康(英文)的其它文章
- Emerging natural hemp seed proteins and their functions for nutraceutical applications
- A narrative review on inhibitory effects of edible mushrooms against malaria and tuberculosis-the world’s deadliest diseases
- Modulatory effects of Lactiplantibacillus plantarum on chronic metabolic diseases
- The role of f lavonoids in mitigating food originated heterocyclic aromatic amines that concerns human wellness
- The hypoglycemic potential of phenolics from functional foods and their mechanisms
- Insights on the molecular mechanism of neuroprotection exerted by edible bird’s nest and its bioactive constituents

