Lonicera caerulea polyphenols inhibit fat absorption by regulating Nrf2-ARE pathway mediated epithelial barrier dysfunction and special microbiota
Yuhua Wang, Ningxuan Gao, Anra Nito-Vloz Lingxi Zhou, Xiyun Sun,Xu Si, Jinlong Tian, Yang Lin, Xinyao Jiao*, Bin Li,*
Keywords:Lonicera caerulea berries Polyphenols Gut microbiota Oxidative stress Intestinal epithelial barrier
A B S T R A C T Scope: High-fat diet (HFD) induces imbalance in the small intestine environment, where fat digestion and absorption mainly take place. This study aimed to elucidate the mechanisms by which Lonicera caerulea polyphenols (LCP) might inhibit fat absorption, from the perspective of small intestine microbiota and epithelial barrier integrity. Methods and results: Male Sprague-Dawley rats were given HFD with or without co-administration of LCP for 8 weeks. The results showed that LCP supplementation signif icantly decreased the levels of serum triglycerides (TG), total cholesterol (TC), and low-density lipoprotein cholesterol(LDL-C), and increased the contents of fecal sterols, in HFD rats. LCP also inhibited the dysfunction of the small intestine epithelial barrier, via alleviating the oxidative stress activated by Nrf2-ARE pathway,and by modulating the expressions of pro-inflammatory factors such as tumor necrosis factor-α (TNF-α),interleukin-6 (IL-6), cyclooxygenase-2 (COX-2), nuclear factor kappa-B p65 (NF-κB p65) and inducible nitric oxide synthase (iNOS) in the small intestine. Additionally, LCP administration restored the balance in small intestine microbiota and increased the abundance of the specif ic bacteria, such as Lactobacillus, involved in fat absorption. Conclusion: Our results demonstrated that LCP may be beneficial to inhibit fat absorption. The mechanism seems to be associated with the protection of the epithelial barrier integrity and the modulation of specif ic bacteria in the small intestine.
1. Introduction
In recent years, the fat content in human diet has increased dramatically, and in many countries high-fat diet (HFD) has even become one of the main causes of obesity and cardiovascular disease.The increased intake of fat can induce oxidative stress in the intestine,which directly affects the function of intestinal barrier, activates a series of inf lammatory reactions, and leads to an imbalanced gut microbiota composition [1]. Studies have shown that the damage of the intestinal barrier, which includes the epithelial barrier and the microbial barrier,is associated with some chronic diseases, such as lipid metabolism disturbance, liver disease, and inflammation, etc. [2]. The excessive digestion and absorption of fat has been connected to lipid metabolism disturbance, overweight and obesity caused by HFD. These diseases have become a major challenge in terms of chronic disease prevention,and have imposed a heavy economic burden on societies [3].
Several studies have demonstrated that the intake of vitamin C (VC)and orlistat can reduce HFD-induced oxidative stress, and inhibit the digestion and absorption of fat, respectively [4]. However, the long-term or incorrect supplementation of VC or orlistat can cause several side effects such as increased gastric acid production [5], poor oral utilization of nutrients, and formation of kidney stones, among others [6].
Polyphenolic substances, which exist in cereals, berries and other plant-derived foods, have been reported to exhibit inhibitory effects on the digestion and absorption of fats via modulating fat transport-associated proteins [7]. Intestinal microbiota, such as the bile acid and short chain fatty acid producing bacteria, as well as intestinal permeability, are directly implicated in the digestion and absorption of fats [8]. Therefore, it is necessary to further study,from the perspective of the physical and microbial intestinal barriers,the mechanisms by which polyphenols inhibit fat digestion and absorption.
Lonicera caeruleaL., a member ofLoniceraegenus in the family of Caprifoliaceae, is an important economic crop in Russia, China and Japan [9]. It is rich in polyphenols, especially cyanidin-3-O-glucoside which exhibits high antioxidant activity, and many biological activities including anti-microbial, anti-inflammatory,anti-carcinogenic, and anti-obesity [10-15]. Our previous study found thatL.caeruleapolyphenols (LCP) inhibited the translocation of lipopolysaccharides by modulating the colonic microbiota and the intestinal epithelial barrier [1]. Kim et al. [15] found thatL.caeruleaberry extracts exhibited anti-obesity effects in HFD mice through the activation of AMP-activated protein kinase. Wu et al. [16] found that supplementation with LCP could modulate gut microbiota, especially the ratio of Firmicutes to Bacteroidetes in HFD-fed mice. Studies have shown that polyphenols can suppress lipid absorption and weight gain [8,17],moreover, fat absorption mainly occurs in the small intestine and it is directly affected by intestinal permeability and microbiota [18-21].Therefore, we speculate that the ability of LCP to inhibit fat absorption might be attributed to its regulatory effect over the small intestine epithelial barrier, and particularly over the gut microbiota.
In order to prove this hypothesis, the effects of LCP on the small intestine epithelial barrier and microbiota, were investigated in HFD-fed rats. Serum levels of triglycerides (TG), total cholesterol (TC)and low-density lipoprotein cholesterol (LDL-C), as well as markers of oxidative stress, Nrf2-ARE and other related pathways were evaluated, while microbiota analysis was focused on fat-absorption related bacteria. The results are expected to lay the ground for the utilization of LCP as a prebiotic, to inhibit fat absorption and to improve the intestinal epithelial barrier dysfunction and the microbiota imbalance caused by HFD.
2. Materials and methods
2.1 Materials and reagents
L.caeruleaberries were collected from Heilongjiang region of China. LCP were obtained using our previously reported method [9],and then stored at -20 °C until use. The detailed composition and content of LCP used in this study were analyzed via HPLC-ESI-MS2and the results are shown in Table 1. Twelve individual compounds were identified with cyanidin-3-O-glucoside and isochlorogenic acid as the main components. All the chemicals and reagents used in this study were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA)and Wanlei Biological Technology Co., Ltd. (Shenyang, China).
2.2 Animals and experimental design
Six-week-old male Sprague-Dawley rats (Wanlei Bio Co., Ltd.,Shenyang, China) were housed individually, and maintained under controlled conditions (temperature: (22 ± 3) °C, humidity: (55 ± 5)%)with a 12 h light/dark cycle. The experiment was carried out according to the guidelines of the Standards for Laboratory Animals of China(GB/T 35892-2018 and GB/T 35823-2018), and was approved by the Laboratory Animal Management and Ethics Committee of Shenyang Agricultural University.
After one-week acclimatization, the rats were randomly divided into 4 groups (n= 6): normal control (NC) group, model (MG) group,VC group, and LCP group. NC group was fed with a standard diet,AIN-93M diet (Research Diets, Inc., New Brunswick, NJ, USA); MG group was fed with HFD diet (63.8% AIN-93M diet + 15% swine lard +10% sucrose + 1% cholesterol + 0.2% sodium cholate + 10% egg yolk powder); VC group was fed with HFD diet and daily gavaged with VC dissolved in saline (100 mg/kg bw); LCP group was fed with a HFD diet and gavaged daily with LCP dissolved in saline(250 mg/kg bw). The dose of VC or LCP used in our experiments is identical to the dose used in previous researches [22-26]. The design of the diet for each group is shown in Table 2. The rats had continuous free access to food and water. All the animals wereweighed once every week for 8 weeks and were sacrificed at the end of the experiment via intraperitoneal injection of pelltobarbitalum natricum. Cardiac blood samples were collected into tubes, and plasma was separated from other blood components by centrifugation at 1 200 ×gfor 15 min. The small intestine was harvested, measured and photographed. Ileum contents were collected for microbiota analysis while the tissue was sliced into two sections: one section was fixed in formalin and paraffin embedded for histopathological evaluation, while the other section was immediately frozen and stored at -80 °C for further analysis.

Table 1Detailed composition and content of L. caerulea berry polyphenols.

Table 2Diet compositions used in the present study (g/100 g diet).
2.3 Biochemical analysis
Levels of glucagon-like peptide-2 (GLP-2) in the small intestine were measured using ELISA kits (Wuhan USCN Business Co., Ltd.,Wuhan, China). TC, TG, and LDL-C in serum, as well as reactive oxygen species (ROS), malondialdehyde (MDA), inducible nitric oxide synthase (iNOS), glutathione peroxidase (GSH-Px), and superoxide dismutase (SOD) in the small intestine were determined using commercially available kits (Wanlei Bio Co., Ltd., Shenyang,China) following the protocol provided by the manufacturer.
2.4 Fecal sterols analysis
Fecal samples were collected and processed for sterols extraction as described elsewhere [27]. The neutral sterols extracted in the organic phase (cyclohexane), and the acidic sterols extracted by saponification from the aqueous phase were derivatized using TMS, and detected by gas chromatography [27]. Stigmasterol and hyodeoxycholic acid were used as neutral and acidic sterol internal standards respectively.
2.5 Histopathological analysis
Small intestine paraffin embedded tissue was used for hematoxylin and eosin (H&E) staining following a standard protocol. The stained sections were observed under a microscope (DP73, OLYMPUS Co.,Ltd., Tokyo, Japan) and photographed using 200× magnification.Signs of inflammatory damage in the intestinal architecture, including changes in the population of goblet cells, superficial epithelial cells and presence of inflammatory cells infiltration were evaluated.
2.6 RNA extraction and quantitative real-time PCR (RT-qPCR) analysis
Total RNA extraction from the small intestine (200 mg) was performed using a RNA extraction kit (BioTeke Co., Ltd., Beijing,China) according to the instructions provided by the supplier. RNA concentration was determined using an ultraviolet spectrophotometer(Thermo Scientific, Waltham, MA, USA). Transcriptor First Strand cDNA Synthesis Kit (Roche Co., Ltd., Basel, Switzerland) was used to synthesize the single-stranded cDNA from the extracted RNA (0.1 µL). The cDNA was subjected to RT-qPCR analysis in an ExicyclerTM 96 (Bioneer Co., Ltd., Daejeon, Korea). The reaction mixture was prepared as 1 µL cDNA, 0.5 µL forward primer(10 µmol/L), 0.5 µL reverse primer (10 µmol/L), 10 µL SYBR Green mastermix (SY1020, Solarbio Life Science Co., Ltd., Beijing, China),and double-distilled water to a total volume of 20 µL. The reaction conditions were 94 °C for 5 min, 94 °C for 10 s, 60 °C for 20 s,72 °C for 30 s, followed by 40 cycles of 72 °C for 150 s, 40 °C for 90 s,then melting from 60 °C to 94 °C, and 25 °C for 60-120 s. The primer sequences are shown in Table 3.
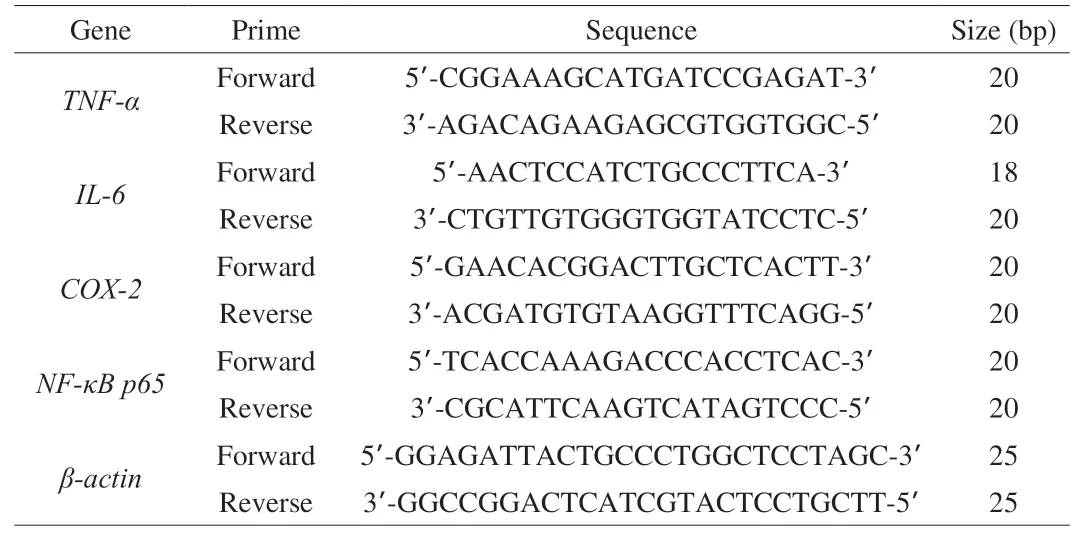
Table 3Primes used in the quantitative RT-qPCR analysis.
2.7 Western blot analysis
Whole proteins from the small intestine (200 mg) were extracted using the Whole Cell Lysis Assay kit (BioTeke Co., Ltd., Beijing,China) according to the manufacturer’s protocol. Briefly, ileum(200 mg) was cut into very small pieces and mixed with 300 µL RIPA buffer, the appropriate amounts of the protein extraction reagents A and B, and PMSF (final concentration 1 mmol/L) to prepare the tissue homogenate. Then protein concentration was measured by Bradford assay using Bradford Kit (BioTeke Co., Ltd., Beijing, China)according to the manufacturer’s protocol. Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis.40 µg of protein per sample were loaded in 8%, 10% and 12%gels, and after electrophoretic separation proteins were transferred to polyvinyl fluoride membranes (Millipore Co., Bedford, MA,USA). The membranes were blocked with 5% non-fat dry milk in TBST buffer for 1 h, followed by incubation overnight at 4 °C with the appropriate monoclonal primary antibody (detailed information in Table S1), then membranes were washed to remove non-bound antibodies and incubated with the secondary antibody (goat anti-rabbit immunoglobin G-horseradish peroxidase (IgG-HRP),1:5 000; Wanlei Bio Co., Ltd., Shenyang, China) for 45 min at 37 °C.Protein bands were detected using an enhanced chemiluminescence(ECL) western blotting detection reagent (Wanlei Bio Co., Ltd.,Shenyang, China) and visualized with a Gel Imaging System (Beijing Liuyi Biotechnology Co., Ltd., Beijing, China).
2.8 Gut microbiota analysis by 16S rRNA gene sequencing
The total DNA of the samples was extracted using DNA isolation kits (BioTeke Co., Ltd., Beijing, China) following the protocol provided by the manufacturer. The V3-V4 region of the 16S rRNA gene was amplified for Illumina library construction using TruSeq Nano DNA LT Library Prep Kit. Paired-end sequencing was performed by Illumina MiSeq using MiSeq Reagent Kit V3 (600 cycles). The low-quality sequences were removed and operational taxonomic units (OTUs) clustering was performed using QIIME data2 (Quantitative Insights Into Microbial Ecology, v1.8.0). Samples were grouped according to the diet types. The raw sequence data were submitted to the sequence read archive (SRA) database of NCBI under the accession number PRJNA594505. To assess alpha diversity,the richness of sample was characterized by Observed species,Chao1, Shannon, and Simpson indices, the diversity based on sample evolution was characterized by Faith’s Phylogenetic Diversity, the evenness of sample was characterized by Pielou’s evenness, and the coverage of sample was characterized by Good’s coverage. To assess beta diversity, weighted UniFrac distance-based principal coordinate analysis (PCoA), weighted UniFrac distance-based non-metric multidimensional scaling (NMDS), and unweighted pair-group method with arithmetic means (UPGMA) were used.To explore the differences in microbial community composition,Venn diagram and linear discriminant analysis (LDA) effect size(LEfSe) method were conducted to reveal the key species that cause the differences. PICRUSt was used to analyze the differences of functional profiles between different groups.
2.9 Statistical analysis
Data are expressed as mean ± standard deviation. Comparisons between groups were performed by one-way ANOVA followed by Tukey’spost hocmultiple comparison test. The results were considered statistically significant atP< 0.05. Data were analyzed using Graph Pad Prism 5.00 (Graph Pad Software, San Diego, CA,USA) and SPSS 17.0 (IBM Corporation, Armonk, NY, USA). The R Version 3.5.3 (The R Foundation), package ggplot2 3.3.0, vegan 2.5-6, VennDiagram 1.6.20, Python LEfse, and metagenomeSeq 3.0-2 (https://cran.r-project.org) were used to analyze the gut microbiota diversity and functional pathways in this study.
3. Results
3.1 Effects of LCP on the body weight gain, food intake, TG,TC, LDL-C and fecal sterols levels of HFD rats
The body weight of rats was measured weekly during the experiment. As shown in Table 4, there was no significant differences(P> 0.05) in the initial body weight among the 4 groups. After being fed with HFD, the body weight gain of the rats in MG group was significantly higher (P< 0.05) compared with that of NC group. LCP and VC supplemented animals exhibited significantly lower (P< 0.05)body weight compared with MG group, but the body weight gain of the rats in LCP group was not significantly different (P> 0.05) from the body weight gain observed in NC group. Additionally, there was no significant difference in the food intake among the three HFD feeding groups but it was significantly lower than the food intake of the rats in NC group (P> 0.05). These results suggested that LCP supplementation had a significantly inhibitory effect on body weight gain without affecting food intake. Compared with MG group, VC or LCP supplementation significantly decreased (P< 0.05) serum levels of TG, TC, and LDL-C, and increased fecal neutral and acidic sterols contents (P< 0.05) (Table 4), which demonstrates that LCP inhibited fat absorption while benefited fat excretion in feces in HFD-fed rats. And no significant differences in the above indices were found between VC and LCP groups (P> 0.05).
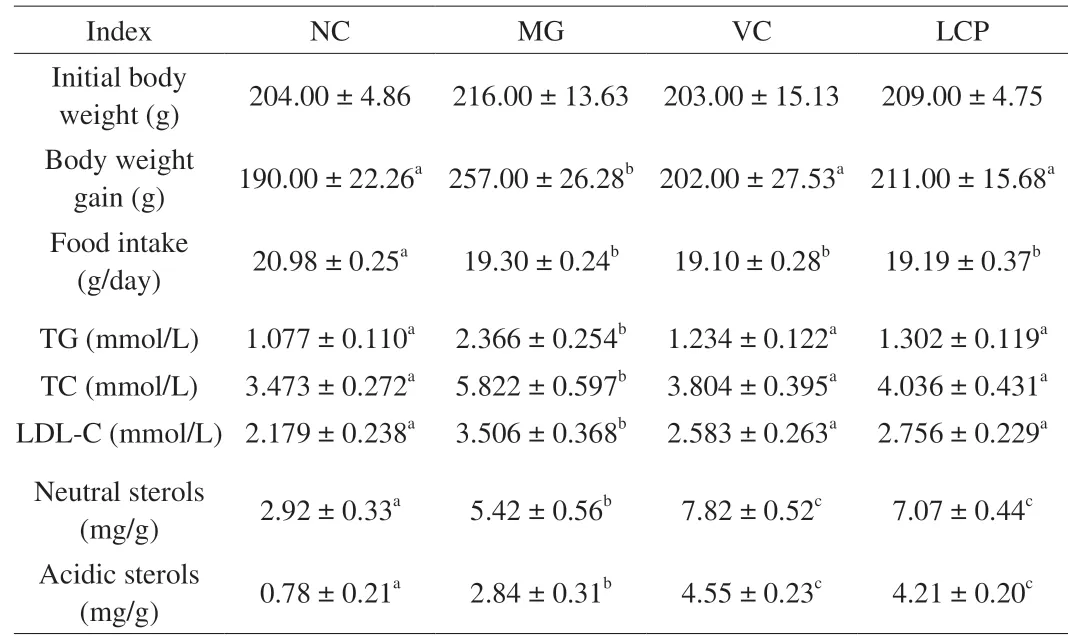
Table 4Initial body weight, body weight gain, serum TG, TC and LDL-C levels, and fecal sterols.
3.2 Inhibitory effects of LCP on oxidative stress via Nrf2 pathway in the small intestine tissue of HFD rats
ROS and MDA were measured as indicators of oxidative stress and antioxidant activity in the small intestine. The levels of these two markers in MG group were significantly higher (P< 0.05) than in NC,VC and LCP groups. Supplementation of VC and LCP significantly reduced (P< 0.05) the levels of ROS and MDA, which were similar to those in the NC group (P> 0.05) (Figs. 1A and B). In addition, the decrease in GSH-Px and SOD levels observed in the small intestine of MG group (P< 0.05), was significantly reversed by VC and LCP supplementation (P< 0.05). Moreover, there were no significant differences in GSH-Px and SOD levels (P> 0.05) among NC, VC and LCP groups (Figs. 1C and D).
Subsequently, HO-1, NQO1 and Nrf2 (cytoplasm and nucleus)protein expression levels in the small intestine were measured.HFD significantly raised (P< 0.05) the expression levels of Nrf2 in the cytoplasm compared with NC group. Both, VC and LCP supplementation, intensified the increased cytoplasmic levels of Nrf2; however, cytoplasmic expression of Nrf2 in VC group was significantly higher than that in LCP group (P< 0.05, Fig. 2A). At the nucleus level, Nrf2 expression in VC group was significantly lower than LCP group (P< 0.05, Fig. 2B), and both of them exhibited a significantly reduced expression in comparison with NC and MG groups. There were no significant differences in Nrf2 nuclear expression NC and MG groups. These results suggest that VC and LCP may promote the binding of Nrf2 to anti-oxidant response element (ARE) in the nucleus, which results in the decreased nuclear levels of Nrf2. Additionally, HO-2 and NQO1, the downstream factors of Nrf2, were significantly higher in VC and LCP groups(P< 0.05) compared with NC and MG groups, and the expression levels of HO-2 and NQO1 were significantly higher in VC group than LCP group (P< 0.05, Figs. 2C and D). The results above indicate that VC and LCP may suppress oxidative stress via modulation of the Nrf2 pathway.

Fig. 1 Effects of LCP on oxidative stress indices of ROS, MDA, GSH-Px,and SOD in the small intestine tissue. n = 6 per group. Results are expressed as the mean ± SEM. Mean values with different letters are significantly different (P < 0.05).

Fig. 2 Effects of LCP on the protein expression levels of Nrf2 (cytoplasm),Nrf2 (nucleus), HO-1 and NQO1 in the small intestine tissue. n = 6 per group.Results are expressed as the mean ± SEM. Mean values with different letters are significantly different (P < 0.05).
3.3 Effects of LCP on inflammatory cytokines in the small intestine tissue of HFD rats
The mRNA expression levels of pro-inflammatory cytokines including tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6),cyclooxygenase-2 (COX-2) and nuclear factor kappa-B p65(NF-κB p65) in the small intestine were determined. Compared with NC group, HFD significantly up-regulated the mRNA levels ofTNF-α,IL-6,COX-2andNF-κB p65(P< 0.05), but this effect was significantly suppressed by VC and LCP supplementation (Figs. 3A-D).There were no significant differences inCOX-2and iNOS levels between VC and LCP groups (P> 0.05), and for both of them iNOS level was significantly lower compared with MG group (P< 0.05)(Fig. 3E). These results indicate that LCP treatment can potentially inhibit inflammation caused by HFD-induced oxidative stress in the small intestine.
3.4 Effects of LCP on epithelial barrier function in the small intestine of HFD rats
3.4.1 Histopathological changes in the small intestinal tissue
Representative H&E stained sections of the small intestinal are displayed in Fig. 4A. The intestinal morphology in NC group did not exhibit any signs of damage or inflammation, while in MG group there was a decrease in the population of goblet cells and superficial epithelial cells, in addition to signs of inflammatory cells infiltrates.In VC group, nearly 50% of the goblet cells population were affected and the superficial epithelial cells were not recovered, accompanied by the infiltration of a small number of inflammatory cells. The morphology of the tissue in the LCP group was comparable to that one observed in the NC group, with no evidence of changes or damage in the goblet cells, superficial epithelial cells or evident inflammatory cells infiltration. These results suggest that LCP supplementation can decrease the pathological damage that HFD induces in the small intestine.

Fig. 3 Effects of LCP on the mRNA levels of TNF-α, IL-6, COX-2, NF-κB p65, and iNOS in the small intestine tissue. n = 6 per group. Results are expressed as the mean ± SEM. Mean values with different letters are significantly different (P < 0.05).

Fig. 4 Effects of LCP on small intestine epithelial barrier disruption. (A) Micrographs of H&E stained sections of the small intestine used for histopathological evaluation (×200). Representative sections were selected from 6 rats in each group. Effects of LCP on the expression levels of (B) GLP-2, (C) claudin-2, and(D) occludin in the small intestine tissue. n = 6 per group. Results are expressed as the mean ± SEM. Mean values with different letters are significantly different (P < 0.05).

Fig. 4 (Continued)
3.4.2 Changes in the expression levels of GLP-2, occludin,and claudin-2
The expression level of GLP-2 in the small intestine was determined (Fig. 4B). Compared with NC group, HFD significantly decreased the expression level of GLP-2 (P< 0.05), which was significantly reversed by VC and LCP supplementation (P< 0.05).The protein levels of claudin-2 and occludin in the small intestine were subsequently assessed. The protein expression level of claudin-2 in MG group was significantly higher than that in NC group (P< 0.05).VC and LCP supplementation significantly inhibited (P< 0.05)the increase in the expression of this protein (Fig. 4C). Regarding the protein expression level of occludin, it was significantly lower (P< 0.05) in MG group than that in NC group. VC and LCP supplementation significantly increased (P< 0.05) occludin expression compared with MG group (Fig. 4D). These results indicate that LCP can promote intestinal barrier function.
3.5 Regulatory effect of LCP over the imbalance induced by HFD in the small intestine microbiota
The Illumina MiSeq platform was used to assess the effects of LCP on the small intestine microbiota.
Interestingly, there were not significant differences for any of the indexes evaluated for alpha diversity, (Chao1, Observed species,Shannon, Simpson, Faith’s PD, Pielou’s evenness and Good’s coverage) among the four groups (Fig. 5A).

Fig. 5 (Continued)

Fig. 5 Effects of LCP on the diversity and composition of the small intestine microbiota. (A) Alpha diversity analysis. (B) Relative abundances of the gut microbiota at the bacterial phylum level. (C) Relative abundances of the gut microbiota at the bacterial genus level. Only the top 20 taxa are shown in the plots. n = 6 per group.
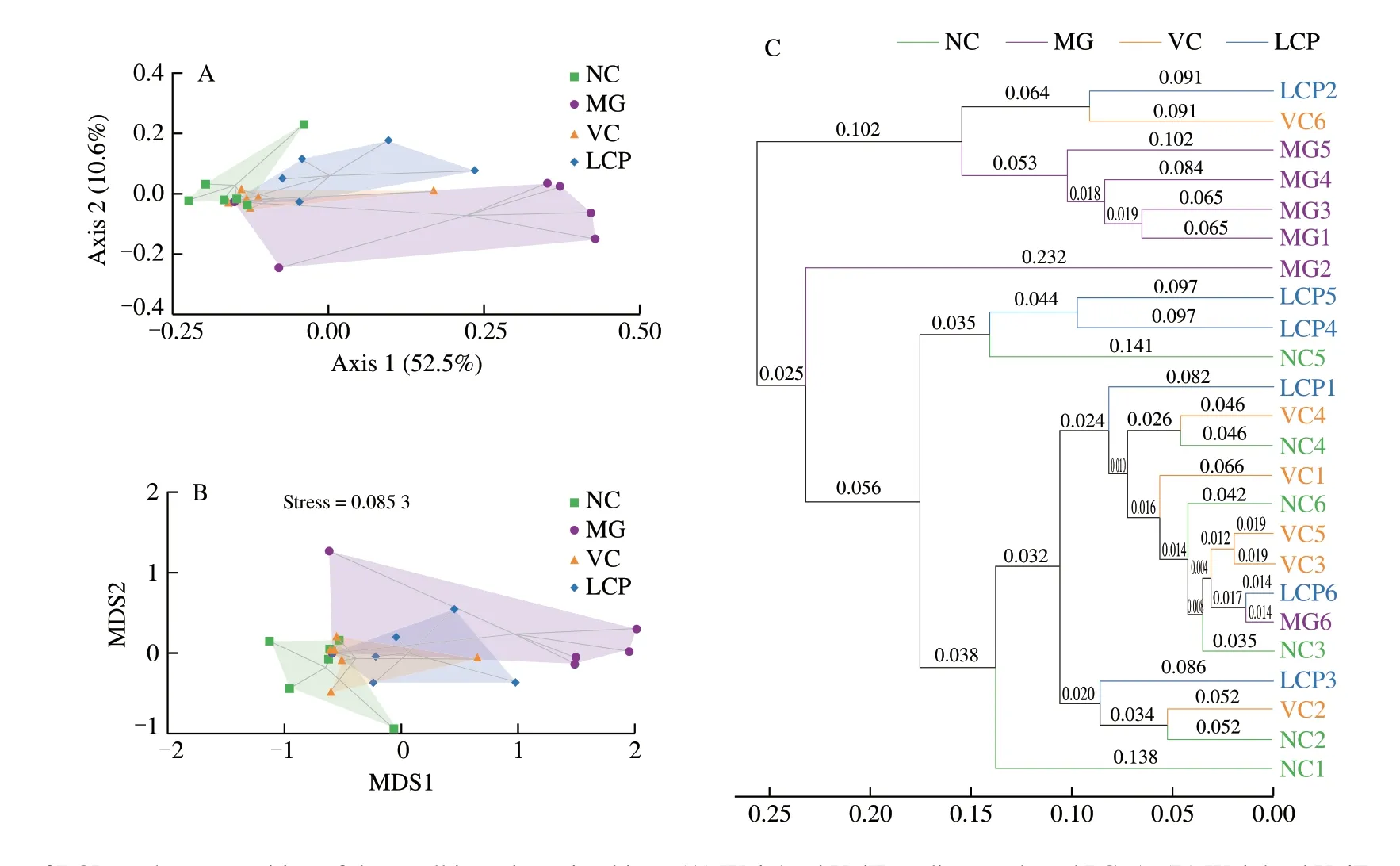
Fig. 6 Effects of LCP on the composition of the small intestine microbiota. (A) Weighted UniFrac distance-based PCoA. (B) Weighted UniFrac distance-based NMDS. (C) UPGMA. n = 6 per group.
The relative abundance of predominant taxa at phylum and genus level was compared among the 4 groups, and the changes in the microflora of the small intestine, induced by HFD were analyzed (Figs. 5B and C). There were significant differences in the composition of microbiota at all taxonomic levels among the groups. At the phylum level, MG group had significantly higher abundance of OD1 and Proteobacteria, and significantly lower abundance of Firmicutes (P< 0.05) compared with NC group. VC supplementation significantly increased the abundance of Firmicutes and decreased the abundance of Proteobacteria (P< 0.05) compared with MG group. Gut community of LCP group had a higher relative abundance of Firmicutes, and much lower relative abundance of OD1 and Proteobacteria compared with MG group. At the genus level, compared with NC group, the abundance ofBrevibacterium,
Corynebacterium,Dietzia,Leucobacter,Rhodococcus,Yaniella,Hymenobacter,Sediminibacterium,Vagococcus, [Eubacterium],Asticcacaulis,Mycoplana,Bradyrhizobium,Ochrobactrum,Agrobacterium,Sphingomonas,Alcaligenes,Oligella,Leptothrix,Acinetobacter,Psychrobacter,PseudomonasandLuteimonassignificantly increased (P< 0.05), while the abundance ofLactobacillussignificantly decreased (P< 0.05) in MG group.Supplementation with VC significantly increased (P< 0.05)the abundance ofLactobacillusand decreased (P< 0.05) the abundance ofBrevibacterium,Dietzia,Rhodococcus,Hymenobacter,[Eubacterium],Bradyrhizobium,Ochrobactrum,Sphingomonas,Alcaligenes,Oligella,Leptothrix,PsychrobacterandPseudomonas.However, compared with MG group, supplementation with LCP significantly increased (P< 0.05) the abundance ofBifidobacterium,NatronobacillusandAlkaliphilus, and significantly decreased(P< 0.05) the abundance ofBrevibacterium,Rhodococcus,[Eubacterium],Alcaligenes,OligellaandPsychrobacter. Compared with VC group, LCP group had significantly higher abundance ofGemllaandActinomycesand significantly lower abundance ofLactobacillus(P< 0.05). Collectively, these results demonstrated the effective modulation of LCP on the small intestine microbiota of HFD-fed rats.
For beta diversity, the two components of the PCoA plot explained 63.1% of the total variance (52.5% and 10.6%, respectively), and illustrated both, similarity and variance among the NC, MG, VC and LCP groups. The PCoA plot showed that HFD rats without supplements had a distinct microbiota composition that clustered separately from NC rats, but the microbiota compositions of VC or LCP groups was relatively similar to that of NC group (Fig. 6A). The weighted UniFrac NMDS analysis also led to a similar result (Fig. 6B).The microbiota composition cluster of MG group was separated from that of NC rats. There was overlapping of clusters among NC, VC and LCP groups. The weighted UniFrac based clustering tree of UPGMA showed that the similarity among NC, VC and LCP samples was higher than that between NC and MG samples (Fig. 6C).
The Venn diagram was generated to display the shared and unique species among groups. The results showed that only 309 (5.22%) of the total 5 914 OTUs were shared among the four groups, and 843 of the observed OTUs were detected after supplementation with LCP (Fig. 7A1).Lactobacillus, which is related to lipid absorption,contributed the highest percentage of the shared species among groups. LCP group had higher percentages ofLactobacillusandStrptococcusand lower percentage ofPsychrobactercompared with MG and VC groups (Fig. 7A2). Additionally, LEfSe analysis was used to identify the specific bacteria with significant differences in relative abundance among NC, MG, VC and LCP groups. The results showed that MG group had the greatest number of bacterial taxa.Candidatus_Arthromituswas specific bacteria in VC group. Notably,Bifidobacteriales, Bifidobacteriaceae andBifidobacteriumwere dominant bacteria in LCP group (Fig. 7B).
Besides taxonomic composition, the discrepancies of functional profiles between different groups were predicted using PICRUSt.The differences in functions between MG and LCP groups are shown in Fig. 8. The results showed that 31 (12 enriched, 19 depleted)functional modules were significantly altered (P< 0.05) in LCP group. LCP supplementation induced changes in functional pathways,mainly in metabolism, organismal systems, human diseases, and environmental information processing, such as starch and sucrose metabolism, IL-17 signaling pathway, central carbon metabolism in cancer, and PI3K-Akt signaling pathway. Thus, LCP intervention contributed to change microbiota functionality in the small intestine of HFD-fed rats.
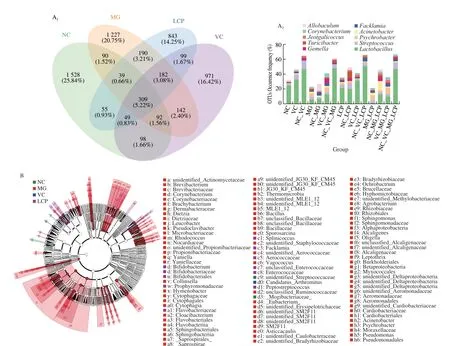
Fig. 7 Effects of LCP on the composition of the small intestine microbial community. (A) Venn diagram and statistical histogram of amplicon sequence variants(ASV)/OTU number in different regions of Venn diagram. (B) Taxonomic cladogram derived from LEfSe analysis of 16S rRNA sequences. n = 6 per group.

Fig. 8 Effects of LCP on microbial community functions predicted by PICRUSt. n = 6 per group.
3.6 Correlations between host parameters and microbial taxa
The spearmanrcorrelations between the microbial species with higher abundance and host parameters were analyzed to further explore their relationships, and the hierarchical clustering heatmap is shown in Fig. 9. The results demonstrated that 18 microbial species were at least positively or negatively correlated with body weight gain, oxidative stress, inflammation or intestinal epithelial cell barrier related markers in the small intestine tissue. Firmicutes,Lactobacillaceae,Lactobacillus, Bacilli and Lactobacillales were negatively correlated with the parameters that promote body weight gain, oxidative stress, inflammation and intestinal epithelial cell barrier dysfunction and positively correlated with the parameters that prevent body weight gain, oxidative stress, inflammation and intestinal epithelial cell barrier dysfunction. These 5 microorganisms were all within phylum Firmicutes, 4 of them (Lactobacillaceae,Lactobacillus, Bacilli, and Lactobacillales) belong to class Bacilli, 3 of them (Lactobacillaceae,Lactobacillus, and Lactobacillales) belong to order Lactobacillales, 2 of them (Lactobacillaceae andLactobacillus)belong to family Lactobacillaceae.Allobaculumwas only positively correlated with Nrf2 (nucleus) and negatively correlated with Nrf2(cytoplasm), NQO1 and HO-1. The other 12 microorganisms were positively correlated with parameters that promote body weight gain, oxidative stress, inflammation and intestinal epithelial cell barrier dysfunction, and negatively correlated with parameters that prevent these processes. Among the 12 microbial species, 5 of them(Actinobacteria, Actinomycetales, Actinomycetaceae,Actinomyces,and Actinobacteria) were within phylum Actinobacteria, 3 of them(Gemella, Gemellales and Gemellaceae) belong to phylum Firmicutes,2 of them (Proteobacteria and Gammaproteobacteria) belong to phylum Proteobacteria, one of them was phylum Fusobacteria, and one of them was phylum Bacteroidetes. Most notably,Lactobacillusand Bacilli were negatively correlated with serum TC and LDL-C contents.

Fig. 9 Heatmap of the spearman r correlations between the gut microbial species and evaluated parameters. * Indicates the associations are significant (P < 0.05).
4. Discussion
L.caeruleawas firstly reported in 1894 as a horticultural plant in Russia, and it has been domesticated since 1913 [28]. In recent years, scholars have paid more attention to the health benefits ofL.caeruleabecause of its higher phenolic and anthocyanins content in comparison to other berries such as blueberry, raspberry and black currant [29-31]. The excessive absorption of fats cause imbalance in fat metabolism and increases the risk of chronic diseases. Oxidative stress is a state of imbalance between the production and elimination of ROSinvivo, and is considered to be related to aging and diseases [32,33].Intestinal oxidative stress can cause inflammation, which leads to intestinal barrier disruption [34]. HFD can promote fat absorption and intestinal oxidative stress, leading to intestinal barrier dysfunction and microbiota imbalance. As natural antioxidants, polyphenols can alleviate oxidative stress-related injury [35]. It is generally recognized that the degradation of polyphenols in the gastrointestinal tract reduces their bioavailability. Contrarily, the catabolism of polyphenols in the gastrointestinal tract can produce bioactive phenolic metabolites,which will enhance their bioavailability and benefits both mucosal barrier and microbiota [36]. In this study, the effects of LCP on regulating the serum levels of TC, TG and LDL-C, small intestine oxidative stress, epithelial barrier function and microbiota in HFD rats were explored.
Supplementation with LCP reduced TC, TG and LDL-C concentration in serum, and restored ROS, MDA, GSH-Px and SOD levels in the small intestine, indicating that LCP can inhibit fat absorption and reduce HFD-induced oxidative stress. Heinrich et al. [37]have found that the consumption ofL.caeruleamodulated oxidative stress metabolism in erythrocytes of volunteers. Nrf2-ARE pathway plays an important role in the cellular antioxidant defense system and can up-regulate antioxidant genes, which synergistically improve cellular defense system [38]. Nrf2 is the key redox-sensitive transcription factor, which protects against oxidative stress by stimulating the transcription of antioxidants and detoxifying related genes via binding with ARE [39]. HO-1 and NQO1 are target enzymes regulated by Nrf2-ARE pathway, and have important protective effects against oxidative stress at the cellular level [40].Supplementation with LCP significantly decreased Nrf2 in nucleus but increased Nrf2 in cytoplasm, and increased HO-1 and NQO1 levels.The possible explanation is that Nrf2-Keap1 complex was dissociated by ARE activation signals, which enabled Nrf2 to translocate into the nucleus where it bound to the ARE and transcriptionally activated downstream target genesHO-1andNQO1. These suggested that LCP might counteract the oxidative stress induced by HFD via activating Nrf2-ARE pathway.
In addition, oxidative stress can lead to chronic inflammation.iNOS plays a critical role in inflammation since it can generate high quantities of nitric oxide (NO), which can damage host cells and tissues [41]. LCP supplementation demonstrated anti-inflammatory effect by decreasing not only the level of iNOS but also the expression levels of pro-inflammatory cytokines such as TNF-α, IL-6,COX-2 and NF-κB p65 in the small intestine. Wu et al. [42,43]have reported that polyphenols fromL.caeruleaberry can inhibit inflammation by reducing the expression levels of pro-inflammatory cytokines, and Firmicutes, Lactobacillaceae,Lactobacillus, Bacilli and Lactobacillales were positively correlated with the antiinflammatory cytokines. Hu et al. [44] have found that protocatechuic acid down-regulatedPrevotella9,Prevotella2,HoldemanellaandRuminococcustorquesgroups which promote the production of inflammatory cytokines, as well as up-regulatedRoseburiaandDesulfovibriowhich inhibit the production of inflammatory cytokines,indicating that polyphenols ameliorate inflammation by modulating gut microbiota.
GLP-2, an intestine-derived hormone, can promote intestinal growth, digestion, absorption, barrier function and blood circulation of nutrients in healthy animals. The increase in endogenous GLP-2 production contributes to the improvement of intestinal barrier function in obese and diabetic patients [45,46]. The level of GLP-2 in MG group was only one-tenth of the level observed in NC group,and LCP supplementation restored it to near 50% of NC group.Claudin-2 and occludin are important tight junction (TJ) proteins, and the protective effects of flavonoids on intestinal TJ proteins against oxidative stress have been reported [47,48]. LCP supplementation mitigated the upregulation of claudin-2, and increased the expression of occludin in HFD-diet rats. Bibi et al. [49] have found that raspberry can reduce pore forming TJ protein claudin-2 in dextran sulfate sodium-treated mice, andLyciumruthenicumMurray has been reported to improve occludin in dextran sodium sulfate-induced colitis mice [50]. Resveratrol can improve intestinal barrier function by regulating intestinal tight-junction proteins, such as ZO-1, claudin-1,occludin and JAM-A [51]. Although the effect of LCP on epithelial TJ proteins is partial, histological evaluation supported the beneficial effect of LCP on the barrier function of the small intestine.
In order to explore the potential correlation between microbiota,fat absorption and epithelial barrier in the small intestine of HFD rats, the changes of microbiota in different groups were analyzed.Although there were no significant differences in bacterial richness and diversity indices among the four groups, compositional and functional changes in the small intestine microbiota caused by HFD and LCP were observed. Proteobacteria increased significantly in HFD rats and decreased with LCP supplementation. Proteobacteria was positively correlated with the body weight gain, oxidative stress,inflammation and epithelial barrier dysfunction. This is consistent with the previous research, which showed that the increase of Proteobacteria was a marker of intestinal microbiota dysbiosis and a potential diagnostic signature of disease risk [52]. Firmicutes, the dominant taxa in all groups, significantly decreased in the rats fed with HFD only, and increased with LCP supplementation. In addition,Firmicutes are negatively correlated with body weight gain, oxidative stress, inflammation and epithelial barrier dysfunction. However,this result is different from a previous study, which demonstrated that Firmicutes abundance increased in the HFD group and decreased by LCP supplementation [16]. This difference might be due to the different locations in the intestine where the microbial samples came from.
Lactobacillusis widely considered as a type of probiotic [53]. Its abundance was significantly decreased in MG group but remarkably restored by supplementation with LCP. Moreover,Lactobacilluswas negatively correlated with the parameters reflecting body weight gain,oxidative stress, inflammation and epithelial barrier dysfunction in this study.Bifidobacterium, whose fermented products are beneficial for health, is also widely considered as probiotics [54]. LCP supplementation significantly increased its abundance in this study.In addition, the abundance ofAkkermansiawas the lowest in MG group but increased after LCP supplementation, which may contribute to the restoration of intestinal epithelial barrier integrity [55].Our results are consistent with those of Zhang et al. [56], who found that polyphenol-richCanariumalbumextract caused a significant increase in the proportion ofAkkermansia. Nrf2-ARE pathway related genes, includingNrf2(nucleus and cytoplasm),HO-1andNQO1, were only associated withAllobaculum, whose abundance decreased in the small intestine of HFD rats. Several studies also found that the abundance ofAllobaculumwas lowered in cecum [57,58] but elevated in the feces of HFD mice [59].LactobacillusandAkkermansiaare both specific microflora involved in the inhibition of fat digestion and absorption [60],and their abundances were improved by LCP treatment. It is suggested that the inhibitory effect of LCP on weight gain may be associated with the regulation ofLactobacillusandAkkermansia.The spearmanrcorrelation analysis showed that oxidative stress markers and pro-inflammatory cytokines were significantly associated with some microorganisms in the small intestine. Pomegranate peel polyphenols suppressed chronic inflammation and colonic tissue damage by modulating gut microbiota in HFD rats [61]. Chlorogenic acid ameliorated the HFD-induced gut microbiota dysbiosis by significantly inhibiting the growth of Desulfovibrionaceae,Ruminococcaceae, Lachnospiraceae, Erysipelotrichaceae, and promoting the growth of Bacteroidaceae, and Lactobacillaceae [62].The beta diversity analysis demonstrated that the microbial composition of HFD-fed only rats was distinct from that of NC rats.Supplementation with VC or LCP could restore the microbiota.The significant differences in functional profiles between MG and LCP groups were predicted by PICRUSt, which reveals that gut microbiota may play an important role in metabolic pathways. LCP supplementation significantly improved the function of carbohydrate metabolism such as glycolysis/gluconeogenesis, fructose and mannose metabolism, amino sugar and nucleotide sugar metabolism, and starch and sucrose metabolism. In contrast, a previous study has shown that resveratrol supplementation reversed carbohydrates metabolism in HFD-fed mice [63], which may be ascribed to the different functional factors. The inflammatory cytokine IL-17 can cause numerous chronic autoimmune and inflammatory diseases [64]. LCP supplementation significantly alleviated the transduction of IL-17 signal pathway induced by HFD. These results confirm the regulatory potential of LCP on metabolic pathways by modulating small intestine microbiota in HFD rats.
5. Conclusions
The present results showed that LCP exerted certain inhibitory effects on fat absorption and small intestinal barrier (physical barrier and microbial barrier) injury in HFD rats. The inhibitory effects of LCP on fat absorption and body weight gain may be ascribed to the regulation of small intestine epithelial barrier, and specially microbiota such asLactobacillusandBacilli. The results provide theoretical support forL.caeruleapolyphenols as the raw materials of functional products related to fat metabolism.
Conflictofintereststatement
The authors have declared no conflicts of interest.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (32001685), the Guidance Plan of Liaoning Natural Science Foundation (20180550776), and the Research Initiation Fund of Shenyang Agricultural University (880418026).
AppendixA.Supplementarydata
Supplementary data associated with this article can be found, in the online version, at http://doi.org/10.1016/j.fshw.2022.10.013.
- 食品科学与人类健康(英文)的其它文章
- Emerging natural hemp seed proteins and their functions for nutraceutical applications
- A narrative review on inhibitory effects of edible mushrooms against malaria and tuberculosis-the world’s deadliest diseases
- Modulatory effects of Lactiplantibacillus plantarum on chronic metabolic diseases
- The role of f lavonoids in mitigating food originated heterocyclic aromatic amines that concerns human wellness
- The hypoglycemic potential of phenolics from functional foods and their mechanisms
- Insights on the molecular mechanism of neuroprotection exerted by edible bird’s nest and its bioactive constituents

