Ingredients from integral valorization of Isabel grape to formulate goat yogurt with stimulatory effects on probiotics and benef icial impacts on human colonic microbiota in vitro
Frnyeli Arújo Silv*, Rit de Cássi Rmos do Egypto Queirog, Evndro Leite de Souz,Glenise Bierhlz Voss, Mri Mnuel Estevez Pintdo, Mrgrid Angéli d Silv Vsonelos
a Department of Nutrition, Federal University of Pernambuco, Recife 50670-420, Brazil
b Department of Nutrition, Health Sciences Center, Federal University of Paraíba, João Pessoa 58050-585, Brazil
c Universidade Católica Portuguesa, CBQF-Centro de Biotecnologia e Química Fina-Laboratório Associado, Escola Superior de Biotecnologia, Porto 4169-005, Portugal
Keywords:Goat dairy product Grape Benef icial microorganisms Gut microbiota Modulatory effects
A B S T R A C T Isabel grape (IG) products have high contents of phenolic compounds and f iber recognized for their positive impacts on microorganisms associated with health benefits to host. This study evaluated the effects of goat yogurts formulated with ingredients from IG integral valorization on the growth and metabolism of different probiotic strains, as well as on the population of selected bacterial groups and metabolic activity of human colonic microbiota in vitro. Goat yogurts with IG ingredients (IGI) stimulated the growth of tested Lactobacillus and Bifidobacterium probiotic strains during a 48-h cultivation, as well as decreased the pH values and enhanced the organic acid production. Goat yogurts with IGI increased the population of Lactobacillus spp. and Bifidobacterium spp. during a 24-h in vitro colonic fermentation. A stable Firmicutes:Bacteroidetes ratio close to 1 was found in media with goat yogurt formulations during the colonic fermentation, being similar to the effect caused by fructooligosaccharides. Goat yogurt formulations with IGI caused increased production of short-chain fatty acids and sugar consumption during colonic fermentation.Goat yogurts with IGI should be a valuable strategy for development of novel added-value foods with benef icial effects on gut microbiota and human health.
1. Introduction
The increasing attention to health and quality of life by consumers has increased the demand for natural products with functional properties and positive effects on human health. The development of new foods with natural ingredients has been a dietary strategy to health promotion [1]. Goat milk has been used in different technological treatments to formulate a variety of fermented dairy products with superior quality characteristics and good acceptance [2,3].Goat and cow milk have similarities in composition in terms of protein, fat and lactose contents; however, some differences (e.g.,amino acid composition, physicochemical properties of casein micelles, aggregation between proteins and lipids and smaller fat globules and chain length of fatty acids) confer a low allergenicity and high digestibility to goat milk [3], which are characteristics receiving increasing interest by consumers [4].
The diet exerts a great inf luence on the composition and metabolic activity of gut microbiota [5,6], being recognized as an important influential factor for the development of chronic diseases linked to altered gut microbiota diversity [7,8]. The increasing importance attributed to gut microbiota modulation has stimulated the search for compounds capable of influencing positively the gut microbiota,resulting in benefits to host health [9-11].
Similar to dietary fibers, phenolic compounds are recognized for their ability to provide benefits to gut microbiota [5,7,12]. Most of the dietary phenolic compounds are not absorbed in the small intestine,reaching the colon where undergo intense biotransformation by colonic microbiota [11]. This transformation should generate more active and bioavailable forms of phenolic compounds, besides of increasing the populations and stimulating the metabolic activity of beneficial groups forming the gut microbiota [12,13].
The Isabel grape (Vitis labruscaL.) is one of the grape cultivars most cultivated in Brazil, being mainly used for in natura consumption and juice production [2]. The integral use of Isabel grape to elaborate a low-calorie preparation and a flour obtained from derived solid by-products (seeds and peels) has resulted in products with high contents of fiber and anthocyanins with antioxidant activities [14],besides of being an approach linked to the emerging zero-residues strategy adopted by fruit agro-industrial sector [15].
The incorporation of these ingredients derived from Isabel grape integral valorization into goat yogurt should be a strategy to add value to this product and increase the intake of phenolic compounds by consumers [2,3,16]. Studies have found positive effects of grape fiber [17,18] and phenolic compounds [5,12,19] on probiotics,as well as on composition and metabolic activity of human gut microbiota [18,20]. However, investigations to measure the impacts of phenolic-rich ingredients incorporated in goat dairy products on probiotics and human gut microbiota are still limited [20].
This study hypothesized that goat yogurts formulated with phenolic- and fiber-rich ingredients from integral valorization of Isabel grape could exert stimulatory effects on probiotics, as well as cause changes in human gut microbiota during anin vitrocolonic fermentation. For this, the viable counts and metabolic activity of different probiotics in media with goat yogurts formulated with a low-calorie preparation from Isabel grape and a flour prepared from its solid by-products, as well as the changes in bacterial populations and metabolic activity of human gut microbiota during anin vitrocolonic fermentation were measured over time.
2. Materials and methods
2.1 Production of ingredients from Isabel grape integral valorization
Grape cv. Isabel (V. labruscaL.) at commercial maturation stage(peel with uniform dark purple coloration), agave syrup and xylitol were purchased in local supermarket (João Pessoa, PB, Brazil). A whole low-calorie Isabel grape preparation was formulated with 7.5 g/100 g of agave syrup, 7.5 g/100 g of xylitol (low calorie natural sweeteners used to achieve a healthy formulation) and 10 mL/100 g of water to the grape. The mixture was heated (92.5 °C, 3 min)in a water bath, cooled in an ice bath, crushed with a previously domestic blender for 2 min and filtered with a 20 mesh sieve. The mild-heat treatment for a short time was applied to facilitate the migration of phenolic compounds from peel to the grape must, as well as to deactivate enzymes account for degradation of phenolic compounds [2].The residual solids retained in the filtration of Isabel grape preparation were dried in an air circulation oven up to reach constant weight ((60 ± 2) °C, approximately 18 h), the resulting product was crushed with a domestic blender and sieved with a 28 mesh sieve to obtain a flour (moisture: 6%-7%) [14]. Domestic blender and mesh sieve used to prepare the Isabel grape ingredients were sanitized before use with immersion in a sodium hypochlorite solution (150 ppm, 15 min).
2.2 Production of goat yogurt formulations and characterization
Goat milk was obtained from a Rural Producer Cooperative(Monteiro, PB, Brazil). Milk was pasteurized at 65 °C for 30 min and kept under refrigeration ((5.0 ± 0.5) °C) for a maximum period of 12 h. The starter culture (YF-L903,Streptococcus salivariussubsp.thermophilusandLactobacillus delbrueckiisubsp.bulgaricus) was obtained from Christian Hansen (Belo Horizonte, MG, Brazil). To prepare the yogurt, pasteurized goat milk was mixed with xylitol(5 g/100 mL), heated ((90 ± 2) °C/10 min), cooled to (45 ± 2) °C,added of starter culture (0.5 g/L) and allowed to ferment ((45 ± 2) °C,4 h) in an incubator (MA 415 BOD Incubator, Marconi Ltda., São Paulo, SP, Brazil). Subsequently, fermented goat milk was cooled to (10.0 ± 0.5) °C and homogenized to break the clot with a sterile glass stick.
Goat yogurt was separated into three different formulations:(i) goat yogurt with 20 g/100 mL of grape preparation (YP), (ii) goat yogurt with 20 g/100 mL of Isabel grape preparation and 2 g/100 mL of Isabel grape by-product flour (YPF); and (iii) control goat yogurt(YC) without Isabel grape ingredients. After the addition of Isabel grape ingredients, yogurts were homogenized, packed in polyethylene bottles and stored under refrigeration ((5.0 ± 0.5) °C) [2].
Samples of goat yogurt formulations with grape ingredients were evaluated for dietary fiber [21]. Goat yogurt formulations were also analyzed for phenolic compound profile, where the yogurt samples(5 g) were weighed in a polyethylene tube, mixed with 5 mL of methanol/water (85:15,V/V) and subjected to ultrasound treatment for 30 min. The resulting mixture was centrifuged (4 000 ×g, 15 min,4 °C) and supernatant was collected. Extraction was repeated two times under the same conditions using the residue. The supernatants were combined, subjected to concentration in a rotary evaporator(Fisatom 802, São Paulo, SP, Brazil), the extract was resuspended in 2 mL of methanol and filtered with a 0.45 µm syringe filter (PTFE).
The identification of phenolic compounds was done with high performance liquid chromatography (HPLC) technique using an Agilent 1260 Infinity liquid chromatography LC system (Agilent Technologies, Santa Clara, CA, USA) coupled to a diode array detector (DAD) (model G1315D). Data were processed with OpenLAB CDS ChemStation Edition software (Agilent Technologie,USA). A Zorbax Eclipse Plus RP-C18column (100 mm × 4.6 mm,3.5 µm) and a Zorbax C18pre-column (12.6 mm × 4.6 mm, 5 µm)(Agilent, USA) were used. The mobile phase was composed of 0.1 mol/L phosphoric acid, pH 2.0 (A) and methanol with 0.5%phosphoric acid (B). The temperature was 35 °C and injection volume was 20 µL of sample previously diluted in phase A and filtered through a 0.45 µm membrane (Millex Millipore, Barueri, SP, Brazil).Solvent flow rate was 0.8 mL/min. Gradient used in separation was 0-5 min: 5% B; 5-14 min: 23% B; 14-30 min: 50% B; 30-33 min:80% B. Compounds were detected at 220, 280, 320, 360 and 520 nm and identification and quantification were done by comparison with external standards [22-24]. Results of total dietary fiber and phenolic contents are shown in Table 1.

Table 1Fiber and phenolic contents (mg/100 g) of goat yogurt with Isabel grape preparation alone or combined with Isabel grape by-product flour.
2.3 In vitro gastrointestinal digestion of goat yogurt formulations
The yogurt formulations (YC, YP and YPF) were submitted to anin vitrogastrointestinal digestion performed in three different phases simulating the oral, gastric and intestinal digestion, followed by dialysis to simulate intestinal absorption [25]. The system simulated pH, temperature, peristaltic movements and specific enzyme juices at each stage. Initially, 100 mL of the yogurts formulation had their pH adjusted to 6 with 1 mol/L NaHCO3. To simulate the oral phase,1.2 mL of artificial saliva prepared withα-amylase 100 U/mL(Sigma-Aldrich, St. Louis, MO, USA) diluted in 1 mmol/L CaCl2was added and incubated for 2 min ((37 ± 1) °C; 200 r/min). After that, 1 mol/L HCl was used to adjust the pH to 2. To simulate the gastric phase, a 25 mg/mL pepsin solution (Sigma, USA) prepared in 0.1 mol/L HCl was added in a proportion of 0.05 ml/mL of sample, followed by incubation for 120 min ((37 ± 1) °C, 130 r/min). 1 mol/L NaHCO3was used to adjust the pH to 6. For simulation of small intestine step,2 g/L of pancreatin (Sigma, USA) + 12 g/L of bile salts (Sigma, USA)diluted in 1 mol/L NaHCO3were added and incubated for 120 min((37 ± 1) °C, 45 r/min). To simulate intestinal absorption, a semipermeable dialysis membrane with molecular weight of 3.5 kDa (Spectra/Por®3, Spectrum Europe, Netherlands) submerged in 0.01 mol/L NaCl ((5.0 ± 0.5) °C) was used. After 15 h, the dialysis fluid was replaced and dialysis followed for additional 2 h. The final content of dialysis membrane was freeze-dried using a bench top lyophilizer (model L-101, LIOTOP, São Carlos, SP, Brazil) to obtain a powder, which was stored under refrigeration ((5.0 ± 0.5) °C) in polyethylene bags with metallic cover up to use in experiments.
2.4 Evaluation of the effects of goat yogurt formulations on probiotic growth and metabolism
2.4.1 Probiotic strains and cultivation media
Three strains of well-known probiotics, namelyLactobacillus acidophilusLa-05 (Chr. Hansen, Hørsholm, Denmark),Lactobacillus caseiLAFTI L-26 (DSM Food Specialties, Sydney, Australia) andBifidobacterium animalissubsp.lactisBb-12 (Chr. Hansen) [26-28]were used as test microorganism. Furthermore, theseLactobacillusandBifidobacteriumspecies are part of the human gut microbiota [29,30]. Before use in assays, each strain was inoculated in de Man, Rogosa and Sharp broth (MRS) (HiMedia, Mumbai, India)at 37 °C for 20-24 h, centrifuged (4 500 ×g, 15 min, 4 °C), washed and resuspended in saline solution (8.5 g/L of NaCl; FMaia, Belo Horizonte, MG, Brazil) to obtain a suspension with viable cell count of approximately 7 (lg (CFU/mL)) when plated on MRS agar [28].Each strain was tested separately in experiments.
To evaluate the effects of goat yogurt formulations on the growth of probiotic strains, MRS broth with a modified carbon source was used as basal medium [31]. To monitor the growth of probiotics,different sole carbon sources were used, to cite: 20 g/L of glucose(non-prebiotic ingredient), 20 g/L of fructooligosaccharides (FOS, a well-known prebiotic ingredient; Galena Ltd., Campinas, SP, Brazil)and 20 g/L of digested goat yogurt sample (YC, YP and YPF).
Each probiotic strain (20 g/L; viable cell count of approximately 7 (lg (CFU/mL))) was inoculated in sterile flask with 10 mL of each culture medium with the examined carbon source, being followed by agitation with a Vortex and incubation at (37 ± 1) °C.
2.4.2 Enumeration of probiotic viable counts
The viable counts of probiotic strains were enumerated in different cultivation time periods (zero-just after inoculation and after 12, 24 and 48 h). On each incubation period, a 1 mL aliquot of the cultivation medium was serially diluted (1:9) in sterile saline and 10 µL of each dilution was plated on MRS agar. The plates were incubated at (37 ± 1) °C for 48 h. At the end of the incubation period,the visible colonies were enumerated and results were expressed as lg (CFU/mL). ForBifidobacterium, the medium was supplemented withL-cysteine (0.5 g/L; Sigma-Aldrich, St. Louis, MA, USA)and incubation was done under anaerobic conditions (AnaeroGen,Basingstoke, England).
2.4.3 Assessment of probiotic metabolic activity
The metabolic activity of the probiotic strains was evaluated in different incubation periods (zero-just after inoculation and after 12, 24 and 48 h) by determination of pH values and contents of organic acids in cultivation medium. The pH was measured with a digital potentiometer (Q400AS, Quimis, São Paulo, SP, Brazil). The samples were filtered through a 0.22 µm syringe filter and analyzed for contents of organic acids (lactic, citric, succinic and acetic acids)using a HPLC system composed of a Knauer K-1001 pump (Berlin,Germany), an ion exchange Aminex HPX-87H column (300 mm ×7.8 mm) (Bio-Rad, Hercules, CA, USA) maintained at 40 °C. The detection was performed with a refractive index detector and a UV-vis detector (Knauer, Berlin, Germany). The mobile phase was 13 mmol/L sulfuric acid at a flow rate of 0.6 mL/min. The running time was 30 min, injection volume was 20 µL and each sample was injected in duplicate. Data were collected and analyzed using Clarity software.The peaks were detected and quantified using calibration curves(0.2-20 mg/mL) of each organic acid [10,32].
2.5 Assessment of the effects of goat yogurt formulations on bacterial populations and metabolic activity of human colonic microbiota
2.5.1 Fecal inoculum (FI) and in vitro colonic fermentation
Human feces were collected from 5 healthy selected human donors (A-E), being 3 men and 2 women aged between 25 and 35 years, in sterile flasks kept under anaerobic conditions up to further use (maximum 2 h after collection). Donors confirmed to have no restrictive diet (e.g., vegetarian), no food intolerance or severe food allergy and no previous use of prebiotic supplements, probiotics or antibiotics in the last three months. Additionally, all donors have signed an informed consent form. FI was prepared by dilution of individual fecal matter in physiologically reduced saline solution(RPS) consisting of 0.5 g/L of cysteine-HCl (Merck, Darmstadt,Germany) and 8.5 g/L of NaCl (LabChem, Zelienople, PA, USA),with a final pH value of 6.8, in an anaerobic workstation (Don Whitley Scientific, West Yorkshire, United Kingdom) (10% of CO2,5% of H2and 85% of N2).
The basal cultivation medium was prepared with 5 g/L of trypticase-free soy broth without dextrose (Fluka Analytical, St. Louis,MO, USA), 5 g/L of bactopeptone (Becton Dickinson Biosciences,New Jersey, NY, USA), 0.5 g/L of cysteine-HCl (Merck, Germany),1% (V/V) of saline A (100 g/L NH4Cl (Merck, Germany), 10 g/L of MgCl2·6H2O (Merck, Germany), 10 g/L of CaCl2·2H2O (Carlo Erba,Chaussée du Vexin, France)), 1% (V/V) of mineral solution (ATCC,Virginia, USA), 0.2% (V/V) of saline B (200 g/L of K2HPO4·3H2O(Merck, Germany)) and 0.2% (V/V) of 0.5 g/L resazurin solution (Sigma-Aldrich, USA) prepared in distilled water with pH adjusted to 6.8 [33].
Basal cultivation medium was dispensed in airtight glass anaerobic bottles and sealed with aluminum caps before sterilization in an autoclave. The medium was distributed in several tubes, being used only the medium as the negative control (C-), the medium with precipitated yogurt formulations obtained at the end of thein vitrogastrointestinal digestion (YC, YP and YPF, 20 g/L) or with fructooligosaccharides (FOS, 20 g/L) (Megazyme, Bray, Ireland)as the positive control (C+). The FI was added at a concentration of 20 g/L at (37 ± 1) °C, being followed by a 24 h incubation without shaking. Samples were collected at 0 (just after inoculation), 8, 12 and 24 h of fermentation and pH values were measured with a pH meter (MicropH 2002, Crison, Barcelona, Spain) equipped with a pH electrode (52-07, Crison). The experiments were done in an anaerobic booth (Don Whitley Scientific, United Kingdom) with 5% of H2, 10% of CO2and 85% of N2. Experiments were done in compliance with institutional guidelines. Collected aliquots were centrifuged (4 000 ×g, 10 min, 4 °C) and supernatants were used to evaluate the production of organic acids and pellets were used to extract the genomic DNA.
2.5.2 Bacterial DNA extraction
Genomic DNA was extracted and purified from stool samples using the NZY Tissue gDNA Isolation kit (Nzytech, Lisbon, Portugal)as previously described [33]. Pellets collected from samples in each time of incubation were washed with Tris EDTA buffer (pH 8.0),vortexed and centrifuged (4 000 ×g, 10 min, 4 °C), a process that was repeated until the supernatant was colorless. Then, 180 µL of a freshly prepared lysozyme solution (10 mg/mL lysozyme in NaCl-EDTA solution; 30 mmol/L NaCl and 10 mmol/L EDTA) were added and incubated (1 h, (37 ± 1) °C) with periodic shaking to guarantee the total break down of the bacterial cell wall to improve DNA extraction efficiency. Afterwards, 350 µL of NT1-buffer were added to samples,which were vortexed and allowed to rest (95 °C, 10 min). After, the samples were centrifuged (11 000 ×g, 10 min, 4 °C), supernatants(200 µL) were mixed with 25 µL of proteinase K and allowed to rest(70 °C, 10 min). The remaining steps followed the manufacturer instructions. After extraction, the DNA purity and quantification were evaluated with a NanoDrop spectrophotometer (Thermo Scientific,Wilmington, DE, USA).
2.5.3 Real-time PCR for measurements of bacterial populations of colonic microbiota
Real-time PCR was performed using a CFX96 Touch detection system (Bio-Rad Laboratories, Inc., Hercules, USA). PCR reaction mixtures (10 µL) had 5 µL of 2× iQTM SYBR Green Supermix(Bio-Rad Laboratories), 2 µL of ultrapure water and 1 µL of sample DNA (balanced to 20 ng/µL) and 1 µL of forward and reverse primers(100 nmol/L) marking the 16S rRNA gene. The primers (STABvida,Lisbon, Portugal) used are listed in Table 2 along with specific annealing temperatures. The conditions used were: beginning of heating at 95 °C for 10 min, followed by 45 cycles of denaturation(95 °C, 10 s), annealing and extension (72 °C, 15 s). Standard curves were constructed using serial dilutions (2-6 (lg (copies /μL) number of 16S rRNA) of bacterial genomic DNA (DSMZ, Braunschweig,Germany) (Table 2). As a quality control of PCR, analysis of melting curve was done for each PCR with temperatures ranging from 60 °C to 97 °C. Data were processed and analyzed with LightCycler software (Roche Applied Science) and presented as mean values of quadruplicate PCR analyzes.
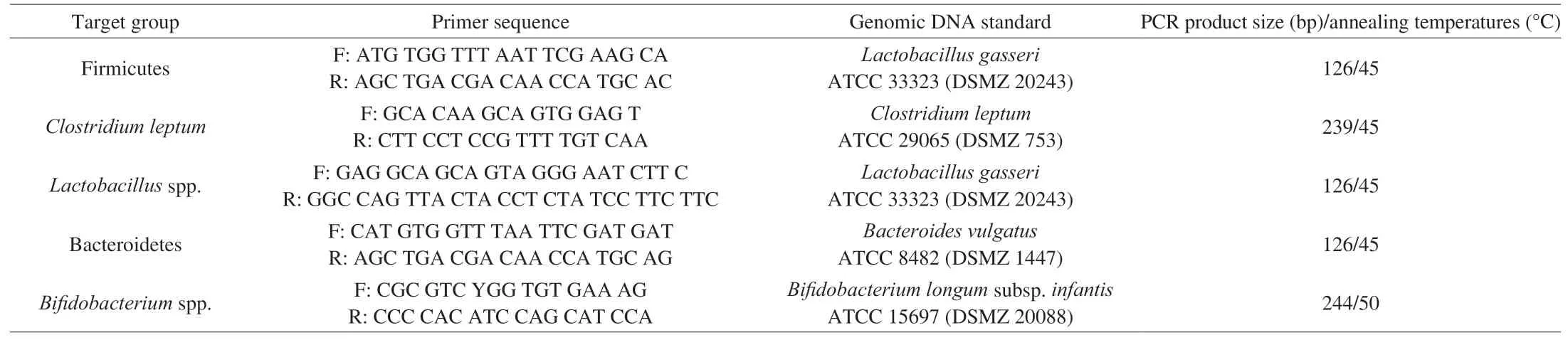
Table 2Sequences of primers targeting bacterial groups and real-time PCR conditions (adapted from Campos et al. [34]).
The differences of samples with yogurt formulations with Isabel grape ingredients or FOS in relation to percentage found for negative control were calculated with the equation:

where SMC is the average number of copies of examined sample at a respective incubation time (8, 12 or 24 h) and CMC is the average number of copies found for negative control at the same incubation time. Positive percentage values indicate an increase in number of copies of examined target microbial group when compared to negative control sample at the respective incubation time [34]. The ratio of Firmicutes to Bacteroidetes (F:B) was obtained by dividing the number of copies of Firmicutes by number of copies of Bacteroidetes [34].
2.5.4 Measurements of metabolic activity of colonic microbiota
Samples were evaluated for contents of sugars (glucose, fructose and lactose) and organic acids (lactic, succinic, acetic, propionic and butyric acids) during colonic fermentation. The analyses were done with HPLC technique as detailed in section 2.4.3. The results were expressed as the average of the values obtained for samples corresponded to the 5 donors.
2.6 Statistical analysis
The experiments were done in triplicate with three independent repetitions and results were expressed as mean ± standard deviation.Data were submitted to an analysis of variance (ANOVA) followed by Tukey test or to Student’sttest to identify significant differences(P< 0.05). Statistical analyses were done with XLstat software version 2018.5 (Addinsoft, Paris, France).
3. Results and discussion
3.1 Effects of goat yogurt formulations on probiotic viable counts
The viable counts of probioticL. acidophilusLa-05,L. caseiLAFTI L-26 andB. lactisBb-12 in media with digested goat yogurt formulations (YC, YP and YPF), glucose and FOS during 48 h of cultivation are shown in Fig. 1. All the examined probiotics had viable counts as high as > 8 (lg (CFU/mL)) with 24 h of cultivation in media with YC, YP, YPF, glucose and FOS, being followed by a reduction in their viable counts with 48 h of cultivation, with the exception ofL. acidophilusandL. caseiin media with FOS. However, the viable counts of all the examined probiotics were always of > 7 (lg (CFU/mL))with 48 h of cultivation. In agreement with these results, an early study [35] reported thatLactobacillusspecies commonly reach maximum growth with approximately 24 h of cultivation. It has been also reported thatB. lactiscommonly reaches maximum growth between 8-16 h of cultivation and growth could keep constant up to 48 h of cultivation [35]. However, in this study,B. lactisBb-12 showed the highest viable counts with approximately 12 h of cultivation, being followed by a reduction in viable counts up to 48 h of cultivation, especially in media with goat yogurt samples. The disagreement among these results could be possibly related to specific metabolic characteristics of the strain used in this study(i.e.,B. lactisBb-12), affecting its ability to use the nutrients available in cultivation media with examined goat yogurt formulations over time [36,37].
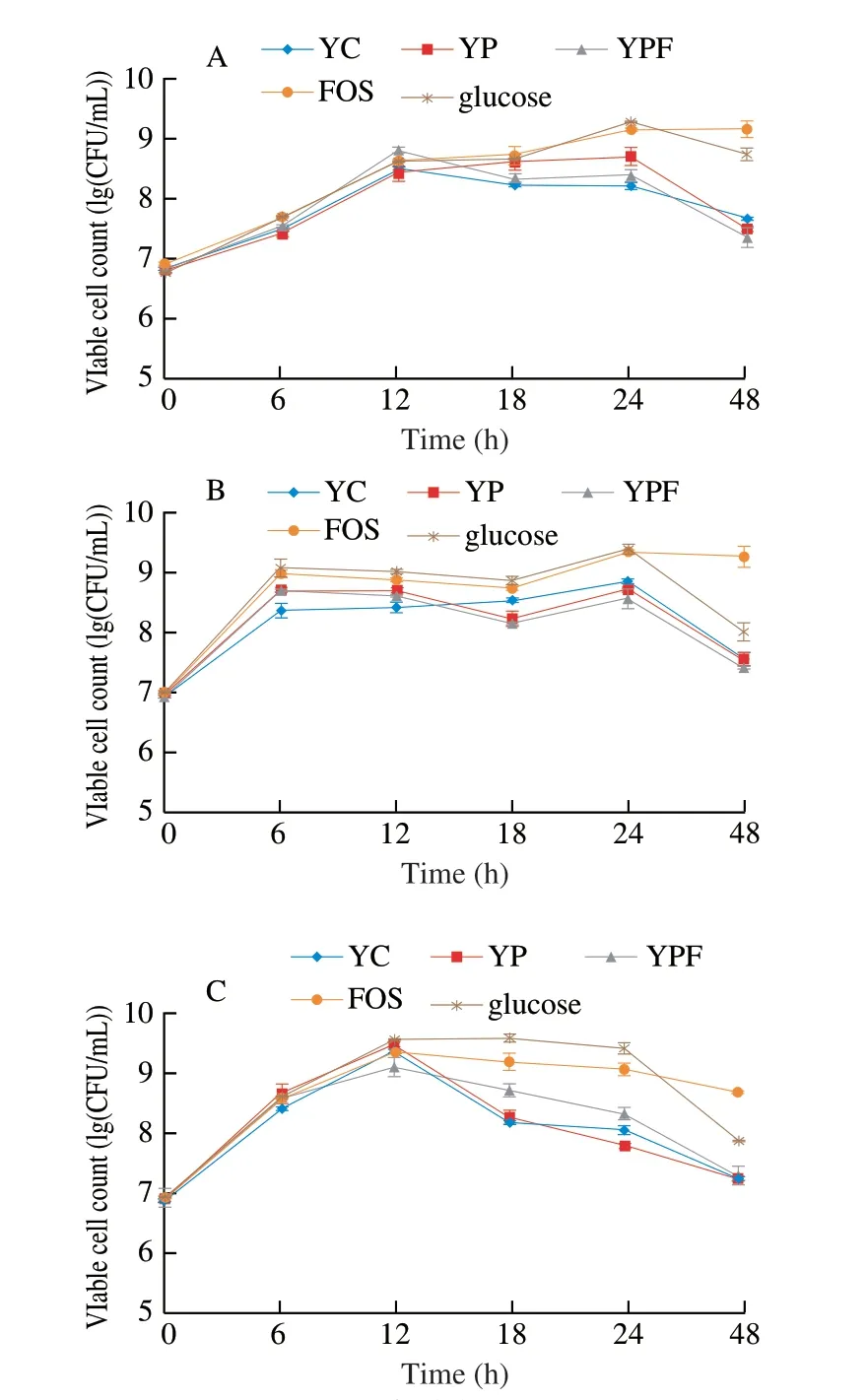
Fig. 1 Viable cell counts of L. acidophilus La-05 (A), L. casei L-26 (B) and B. animalis subsp. lactis Bb-12 (C) during 48 h of cultivation.
The viable counts of examined probiotics were higher in media with FOS and glucose when compared to media with YC, YP and YPF at the end of the 48 h cultivation. Examined probiotics had increases in viable counts varying 2.0-2.5 (lg (CFU/mL)) in media with FOS and glucose with 48 h of cultivation when compared to viable counts found on time zero. This behavior could be linked to a preference of examined probiotics to use glucose as the main carbon source [31,38]. However, YC, YP and YPF also caused increases(1-2 (lg (CFU/mL)) in viable counts of examined probiotics with 48 h of cultivation, reinforcing their capability of stimulating the growth of these microorganisms. Carbohydrates, such as lactose found in YC, YP and YFP, as well as fructose and fibers found in YP and YPF, could be also used as fermentable substrates to promote the growth of examined probiotics [4,38,39].
3.2 Effects of goat yogurt formulations on metabolic activity of probiotics
Decreases (P< 0.05) in pH values of cultivation media with different tested sole carbon sources were found throughout the measured cultivation period regardless of the inoculated probiotic(Table 3), which indicate intense probiotic metabolic activity in these media [40]. The lowest pH values with 48 h of cultivation were found in media with FOS and glucose regardless of the inoculated probiotic.Regarding the cultivation media with goat yogurt formulations, the lowest pH values were overall found in media with YP and YPF,being followed by media with YC.

?
Lactic and acetic acid contents increased in media during the 48 h of cultivation regardless of the added carbon source and inoculated probiotic (Table 3). Lactic acid was the organic acid found in the highest contents in different cultivation when compared to other measured organic acids. The highest contents (P< 0.05) of lactic acid with 48 h of cultivation were found in media with glucose inoculated withL. acidophilusandB. lactis, which should be linked to the lowest pH values found in these media when compared to media with goat yogurt formulation or FOS.
The highest contents (P< 0.05) of acetic acid were found overall in media with YC, YP and YPF inoculated withB. lactis. The contents of acetic acid were higher (P< 0.05) in media with YC, YPF and glucose inoculated withL. acidophiluswhen compared to medium with FOS. The contents of acetic acid were higher (P< 0.05) in media with YC, YP and YPF inoculated withL. caseiwhen compared to medium with glucose at the end of the 48 h cultivation, but were smaller (P≥ 0.05) when compared to medium with FOS. Lactic and acetic acids are recognized as the main end-products of glucose and fructose metabolism byLactobacillusandBifidobacteriumspecies and increases in their contents should be associated with intense metabolism of inoculated probiotics in cultivation media [31,40].
In general, the cultivation media with goat yogurt formulations had higher initial contents of citric acid (P< 0.05) when inoculated withL. acidophilus,L. caseiorB. lactis. Contents of citric acid decreased with 48 h of cultivation in different media regardless of the added carbon source or inoculated probiotic. Succinic acid was not found in media with FOS or glucose. Contents of citric acid varied during the 48 h of cultivation in media with different goat yogurt formulations. Nevertheless, succinic acid contents decreased(P< 0.05) at the end of the 48 h cultivation in medium with YPF regardless of the inoculated probiotic. Citric and succinic acids are naturally found in grape and derived products [35], but they can be also produced by lactic acid bacteria under different fermentation conditions [31].
The decreases in pH values and increases in organic acid contents are parameters linked to metabolism of inoculated probiotics in cultivation media [36]. The association of results found for pH values and organic acids contents in media with YC, YP and YPF in parallel to the increases in viable counts of inoculated probiotics reinforce the growth-stimulatory effects exerted by examined goat yogurt formulations on these microorganisms [36,37]. The differences in the capability of decreasing the pH values and producing organic acids in cultivation media among the tested probiotics could be related to different metabolic characteristics of the examined species or strains affecting their ability to use the nutrients available in cultivation medium to increase their populations and produce different metabolites [36,37].
3.3 Effects of goat yogurt formulations on populations of target bacterial groups of colonic microbiota
The investigation of the main groups of human gut microbiota was used to evaluate the effects of the samples on the growth and metabolic activities of microbial groups during fermentation.The composition on average copies numbers of faecal microbiota evaluated by real time PCR are shown in Table 4. Among the groups evaluated are Firmicutes, Bacteroidetes and Actinobacteria, 3 of the 4 dominant phyla in the human gut, and results obtained were in accordance with those found in healthy human volunteers’ faeces,e.g.Lactobacillusspp., which is usually found in lower numbers in normal gut microbiota, as similarly described previously [33,34].
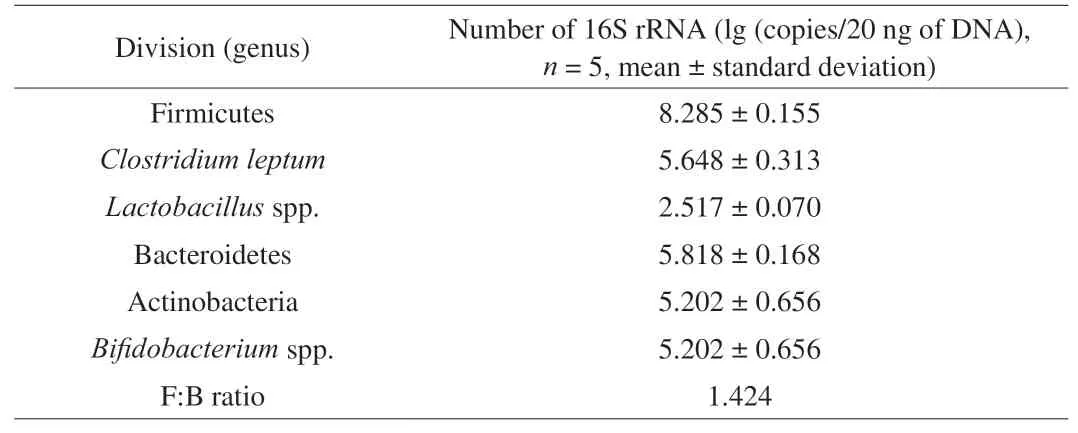
Table 4Faecal microbiota composition of volunteer participants.
The results of the relative difference of examined (target)bacterial groups when compared to negative control found in cultivation media with YC, YP, YPF and FOS after 8, 12 and 24 h of colonic fermentation are shown in Fig. 2 and the statistical results are expressed on Table 5. Goat yogurt formulations caused significant increases (P< 0.05) inLactobacillusspp. population during the 24 h of colonic fermentation when compared to negative control. On the other hand, FOS did not cause increases (P≥ 0.05) inLactobacillusspp.population after 8 and 12 h of colonic fermentation relative to negative control and only a small increase was found after 24 h of colonic fermentation. The increases in populations ofLactobacillusspp.(137%-182%) relative to negative control caused by YC, YP and YPF were higher than those caused by FOS (0-26%) after either 8,12 or 24 h of colonic fermentation, standing out the increases caused by YP (142%-182%). YC, YP, YPF and FOS caused increases inBifidobacteriumspp. populations in the range of 15%-25% relative to negative control after 24 h of colonic fermentation.
Based on values of DNA copies of examined bacterial groups found in cultivation media with YC, YP, YPF, FOS and negative control (Table 5), goat yogurt formulations and FOS caused similar increases (P≥ 0.05) in populations ofBifidobacteriumspp. during the 24 h of colonic fermentation, which were significantly higher(P< 0.05) than those found to negative control. FOS caused increases(P≥ 0.05) in population ofLactobacillusspp. only after 24 h of colonic fermentation, while YPF, YP and YC caused increase in this population already from 8 h of colonic fermentation onward (Fig. 3).

Fig. 2 Relative differences to negative control of population of different selected bacterial groups part of the intestinal microbiota in media with FOS, YP, YPF and YC during 24 h of colonic fermentation. (A) Lactobacillus spp., (B) Bifidobacterium spp., (C) Clostridium leptum, (D) Firmicutes, (E) Bacteroidetes.

Fig. 3 Number of DNA copies of real time PCR of different selected bacterial groups part of the intestinal microbiota in media with FOS, YP, YPF, YC and Cduring 24 h of colonic fermentation. (A) Lactobacillus spp., (B) Firmicutes, (C) Clostridium leptum, (D) Bacteroidetes, (E) Bifidobacterium spp.

Fig. 3 (Continued)
These results could be associated with the characteristics ofLactobacillusspecies of being more restrictive to fermentative substrates when compared toBifidobacteriumspecies, besides of being more demanding for some specific nutrients, such as amino acids and peptides [26], available in goat yogurt formulations. Yogurt has shown typically excellent protein composition [4], which may have contributed to the remarkable positive effects caused by YPF,YP and YC onLactobacillusspp. populations. The combination of proteins and carbohydrates could contribute to intestinal health since nitrogen from dietary protein is essential for carbohydrate metabolism and gut microbiota growth [6].
The phenolic compounds (2.99 and 4.37 mg/100 g) and fibers(25.07 and 76.70 mg/100 g) found in YP and YPF, respectively(Table 1), may have contributed to the increases in populations ofLactobacillusspp. andBifidobacteriumspp. in fermentation mediawith these yogurt formulations. Phenolic compounds and fibers have been associated with stimulatory effects onLactobacillusandBifidobacteriumin human gut microbiota [5,16,26].Lactobacillusspp. andBifidobacteriumspp. are bacterial groups related to a variety of benefits on host intestinal health [26]. Many bacterial groups forming the human gut microbiota, includingBifidobacteriumandLactobacillus, exhibitβ-glucosidase activity involved in catabolism of phenolic compounds [41]. Dietary fibers can be also metabolized by these bacterial groups with production of short chain fatty acids (SCFAs)and induction of positive modulation of gut microbiota [8,26].

Table 5Values (lg (copies/20 ng of DNA), n = 5, mean ± standard deviation) of distinct gut bacterial populations obtained by real time PCR during a 24-h colonic fermentation.
The Firmicutes phyla comprises theLactobacillusspp. andClostridium leptumgroups [25]. YC, YP, YPF and FOS caused slight increases (4%-17%) in population ofC. leptumrelative to negative control during the 24 h of colonic fermentation (Fig. 2 and Table 5).Based on data of DNA copies (Table 5), no difference (P≥ 0.05)was found between initial and final populations ofC. leptumin negative control as well as in media with YC, YP and FOS.C. leptumis a major inFirmicutesphyla forming 16%-25% of total gut microbiota [25], besides of being associated with inflammatory bowel disease inhibition [5].
The YC, YP, YFP and FOS caused few alterations (0-12%) inFirmicutespopulation relative to negative control (Fig. 2) and no significant increase (P≥ 0.05) in this population was found during the 24 h of colonic fermentation (Table 5). Even with the high positive impacts that goat yogurt formulations had onLactobacillusspp.population during the colonic fermentation, these effects were not extended to Firmicutes probably becauseLactobacillusis one of the minor groups (approximately 2%) of human gut microbiota [26].
The YC, YP, YPF and FOS caused a small decrease (4%-25%) inBacteroidetespopulation relative to negative control after 12 and/or 24 h of colonic fermentation (Fig. 2). However, based on data of DNA copies (Table 5), FOS and negative control caused increases(P< 0.05) in populations ofBacteroidetesduring the 24 h of colonic fermentation, whereas YC, YP and YPF caused no difference (P≥ 0.05)between initial and final populations of this phyla. These results are important becauseBacteroidescomprises the main group ofBacteroidetesphyla and some species of this group have been linked to harmful effects to host health, such as enhanced toxin formation and pathogenicity [26,42]. Grape fiber and phenolic compounds have well-known positive effects on gut microbiota [18-20]. Although the YPF exhibited higher values of fiber and phenolic compounds when compared to YP, both formulations had similar effects on colonic microbiota. These results indicate that differences in fiber and concentration of determined phenolic compounds between YP and YPF were not enough to cause significant differences on measured bacterial populations.
The results of DNA copies obtained for Firmicutes and Bacteroidetes were used to determine the F:B ratio after 8, 12 and 24 h of colonic fermentation (Fig. 4). Firmicutes and Bacteroidetes are the most abundant phyla comprising approximately 90% of total human colonic microbiota [34]. Maintenance of the relative abundance of Firmicutes and Bacteroidetes phyla should be more important than stimulatory effects causing increases of their populations [9,43]. The F:B ratio found typically in healthy individuals has been nearly to 1 and sharp increase (e.g., to 20:1) or decrease has been associated with obesity and weight loss, respectively [44,45]. The examined goat yogurt formulations, especially YP and YPF, caused a maintenance of F:B ratio near to 1 during the 24 h of colonic fermentation, which could be important results indicating that these formulations were capable of maintaining Firmicutes and Bacteroides populations with few overall impacts on F:B ratio.

Fig. 4 F:B ratio values evaluation during 24 h of colonic fermentation in media with FOS, YP, YPF, YC and C-.
The differences in populations of measured bacterial groups during the 24 h of colonic fermentation when compared to negative control provide a good representation of the colonic microbiota behavior when influenced by a food sample or ingredient [34], which indicated that examined goat yogurt formulations had overall positive effects on human gut microbiota composition. Additionally, the specific stimulatory effects onLactobacillusspp. andBifidobacteriumspp.populations indicate the selective stimulatory properties of examined goat yogurt formulations on human colonic microbiota, which should be one of the criteria required to characterize a prebiotic effect [11].
3.4 Effects of goat yogurt formulations on metabolic activity of colonic microbiota
Results of pH values and sugar and organic acid contents in media with YC, YP, YPF, FOS and negative control after 8, 12 and 24 h ofcolonic fermentation are shown in Table 6. There was a decreasing trend in pH values during the 24 h of colonic fermentation in media with examined goat yogurt formulations and FOS, while no alteration(P≥ 0.05) was found in negative control. The pH reduction in colon can be considered indicative of bacterial fermentation associated with production of SCFAs [9]. This relationship could be corroborated by increases in contents of organic acids during the colonic fermentation in media with examined goat yogurt formulations and FOS.

Table 6Values of pH, sugars and contents of organic acids during fermentation.
Fructose was found in similar contents (P≥ 0.05) in media with YP, YFP and FOS. The media with YC, YP and YPF had high contents of lactose, being the highest initial lactose contents found in medium with YC (P< 0.05). These results should be expected because the presence of Isabel grape ingredients in YP and YPF should decrease the concentration of milk compounds (e.g., lactose)in these yogurt formulations [3]. Glucose was found in media with goat yogurt formulations possibly due to the breakdown of lactose by bacteria. These sugars were almost or totally metabolized during the 24 h of colonic fermentation, confirming their use as substrates by colonic microbiota. Fructose, glucose and lactose were not found in negative control.
Lactic and acetic acids were found in higher contents in media with goat yogurt formulations and FOS, being found overall similar contents (P≥ 0.05) in media with YP and YPF. Increases in contents of lactic and acetic acids were found during the colonic fermentation.Lactic and acetic acids are important products from fermentative metabolism ofLactobacillusandBifidobacterium[8,36] and increases in their contents could be associated with positive impacts exerted by goat yogurt formulations on these bacterial groups. Lactic acid is an intermediate product from carbohydrate metabolism and its use by gut microbiota occurs generally for production of other organic acids [31,40].
Many microorganisms could interact with each other and are mutually dependent because the final products of metabolism of one microorganism could become a substrate for another,characterizing a cross-feeding metabolism [46]. Some organic acids are intermediate products of fermentation process, such as lactic and succinic acids, and are readily used in cross-feeding process [10,39].Lactic acid can be used for acetic acid synthesis by sulfate-reducing bacteria. Succinic acid can be used for propionic acid synthesis,mainly by Bacteroidetes and some species forming Firmicutes phyla. Butyric acid can be synthesized using acetic acid and, to a lesser extent, using lactic acid by someClostridiumspecies in media with low glucose concentration [26,34,39].
The colonic fermentation media had succinic, propionic and butyric acids in lower contents when compared to other measured organic acids, which were only found with 8 or 12 h of fermentation.It occurred probably because succinic, propionic and butyric acids can be formed from metabolic pathways using acetic and lactic acids as precursors. Acetic, butyric and propionic acids are SCFAs, which could be absorbed by epithelial cells and metabolized by the host [39],being recognized for their association with mechanisms that provide
health benefits [11]. Butyric and propionic acids are associated with
regulation of intestinal physiology and immune function, while acetic acid acts as a substrate in lipogenesis and gluconeogenesis [7].The only organic acids found in negative control were acetic,propionic and butyric acids, although in lower contents (P< 0.05)when compared to other fermentation media. Production of SCFAs is directly related to the extent of substrate fermentation. Presence
of SCFAs in colon has been linked to beneficial health effects, such
as improved immune and anti-inflammatory responses, increased nutrient bioavailability and inhibition of enteric pathogens [11,36,39].
The possible main limitations of this study could be the characteristics of the goat yogurt samples submitted to simulated
gastrointestinal digestion because the difficulties to closely simulate
the very complex physicochemical and physiological events during human gastrointestinal digestion; the use of a FI probably more representative of the microbiota of human colonic lumen with mostly strictly anaerobic bacteria when compared to mucosal surface microbiota mostly formed by facultative anaerobic bacteria; and the lack of the measurements of some additional microbial groups found as part of gut microbiota (e.g.,Ruminococcusspp.) [47,48]. However,the experimental protocol used in this study has shown consistent results in early investigations to evaluate the effects of different foods or derived-substances on gut microbiota [10,33,34].
4. Conclusion
The results showed that YC, YP and YPF stimulated the growth and metabolic activity ofLactobacillusandBifidobacteriumprobiotic strains, which was evidenced by their capability of inducing increased viable counts of these probiotics, decreased pH values and increased organic acid production in cultivation media over time.Examined goat yogurt formulations were fermented by human colonic microbiota resulting in increased populations ofLactobacillusspp.andBifidobacteriumspp., decreased pH and increased production of SCFAs during colonic fermentation. Furthermore, examined goat yogurt formulations had greater positive effects on population ofLactobacillusspp. when compared to FOS, especially the goat yogurts with Isabel grape ingredients. These results indicate that goat yogurt formulated with fiber- and phenolic rich-ingredients from integral valorization of Isabel grape should be a strategy for production of foods with positive effects on gut microbiota and human health,besides of being an approach linked to zero waste agro-industrial and food production practices. Further studies could be carried out to evaluatein vivothe effects of the ingestion of the formulated goat yogurts with ingredients from integral valorization of Isabel grape on gut microbiota and other parameters linked to intestinal health or selected metabolic disorders.
Conflict of interests
The authors declare no conflicts of interest.
Acknowledgements
The authors thank CAPES-Brazil (1734/2015) and PROPESQ-UFPE(23076.049914/2017-47) for their financial support and CNPq-Brazil for providing a scholarship to the first author.
- 食品科学与人类健康(英文)的其它文章
- Emerging natural hemp seed proteins and their functions for nutraceutical applications
- A narrative review on inhibitory effects of edible mushrooms against malaria and tuberculosis-the world’s deadliest diseases
- Modulatory effects of Lactiplantibacillus plantarum on chronic metabolic diseases
- The role of f lavonoids in mitigating food originated heterocyclic aromatic amines that concerns human wellness
- The hypoglycemic potential of phenolics from functional foods and their mechanisms
- Insights on the molecular mechanism of neuroprotection exerted by edible bird’s nest and its bioactive constituents

