Combinatorial co-expression of xanthine dehydrogenase and chaperone XdhC from Acinetobacter baumannii and Rhodobacter capsulatus and their applications in decreasing purine content in food
Chenghu Wng*, Rn Zhng Yu Sun You Wen Xioling Liu Xinhui Xing
a College of Light Industry and Food Engineering, Guangxi University, Nanning 530004, China
b Key Laboratory of Industrial Biocatalysis, Ministry of Education, Institute of Biochemical Engineering, Department of Chemical Engineering,Tsinghua University, Beijing 100084, China
Keywords:Co-expression Low purine food Uric acid Xanthine dehydrogenase XdhC
A B S T R A C T This study investigated the combinatorial expression of xanthine dehydrogenase (XDH) and chaperone XdhC from Acinetobacter baumannii and Rhodobacter capsulatus and their applications in decreasing purine content in the beer, beef and yeast. Naturally occurring xdhABC gene clusters of A. baumannii CICC 10254 and R. capsulatus CGMCC 1.3366 as well as two refactored clusters constructed by exchanging their xdhC genes were overexpressed in Escherichia coli and purif ied to near homogeneity. RcXDH chaperoned by AbXdhC showed nearly the same catalytic performance as that by RcXdhC, except for the decreased substrate aff inity.While the AbXDH co-expressed with RcXdhC displayed enhanced acidic adaptation but weakened catalytic activity. All the XDHs degraded purines in beer, beef and yeast extract effectively, indicating potential applications in low-purine foods to prevent hyperuricemia and gout. The study also presents a method for exploiting the better chaperone XdhC and novel XDHs by functional complement activity using existing XdhCs such as RcXdhC.
1. Introduction
Xanthine dehydrogenase (XDH, EC 1.17.1.4) catalyzes the successive oxidation of hypoxanthine to xanthine to uric acid, with concomitant reduction of nicotinamide adenine dinucleotide (NAD)to NAD + hydrogen (NADH) [1]. XDH is widely distributed in all 3 domains of life and can be converted into xanthine oxidase (XOD,EC 1.17.3.2), which catalyzes the same reaction as XDH. However,XOD utilizes oxygen as an electron acceptor, by post-translational modifications such as limited proteolysis and cysteine oxidation in some species [2]. XDH and XOD, which are collectively called as xanthine oxidoreductase (XOR), play key roles in purine metabolism and have potential applications in producing low-purine foods,synthesis of nucleoside drugs, and so on [3,4].
Active XDH reportedly contains three redox domains, that include two distinct iron-sulfur clusters ([2Fe-2S] clusters), a f lavin adenine dinucleotide (FAD), and a sulfurated molybdenum cofactor (Moco),respectively [5,6]. The integrity of the 3 redox centers is a prerequisite for catalytic activity, and especially the efficient synthesis and insertion of sulfurated Moco is the bottleneck of producing an active XDH. Sulfurated Moco is synthesized from molybdenum by a series of helper proteins, including molybdenum insertase, chaperone XdhC and Moco sulfurase (Nifs4) [7]. XdhC binds stoichiometric amount of Moco, interacts with anL-cysteine desulfurase such as NifS4 for the sulfuration of Moco, protects sulfurated Moco from oxidation, and transfers it to XDH [7,8]. It is also suggested that XdhC aids proper folding of the target protein after Moco insertion and the assembly of a functional Mo-pyranopterin center in XDH [9,10].
Several microbial and animal XDHs have been cloned and expressed in different hosts [11-13]; however, few XdhC chaperones have been characterized experimentally except forRhodobacter capsulatus,Pseudomonas aeruginosa, andAcinetobacter baumannii[12,14,15].Sometimes the XDH and XdhC genes are clustered in one operon and transcribed in the same direction. However, they are distributed in different regions; thus, identifying XdhC, such asComamonas acidovoransis difficult [9]. Furthermore, XdhC proteins are not conserved and are usually not present in thexdhoperon or completely absent in the genome of some species, impeding the identification and development of XDHs by simply overexpressing the naturally occurringxdhoperons.
Previous studies have shown that functionally activeC. acidovoransXDH can be obtained by the co-expression ofC. acidovorans xdhwithP. aeruginosa xdhC, or with an unannotated XdhC homolog inEscherichia coliJM109(DE3);however, no further comparative cross-activation studies by exchanging XdhCs have been carried out [15,16]. In our previous work, we successfully identified and characterized thexdhgene clusters (consisting ofxdhA,xdhB,andxdhC) ofR. capsulatusCGMCC 1.3366 andA. baumanniiCICC 10254, which share 40.2%, 30.9% and 30.9% amino acid sequence identity for XdhA, XdhB, and XdhC, respectively; these values were lower than those of XdhA (57.5%) and Xdh B (66.2%) betweenC. acidovoransandP. aeruginosa[9,15]. This study, we systematically investigated the combinatorial co-expression of XDH and chaperone XdhC fromA. baumanniiandR. capsulatusand their applications in decreasing purine content in foods.
2. Materials and methods
2.1 Materials
Plasmid pTRAN, which contains thexdhABCgene cluster ofA. baumanniiCICC 10254, and pTrc-RcXH3, which includes the gene clusterxdhABCofR. capsulatusCGMCC 1.3366, were described in our previous work [14,17].E. coliDH5α (BioMed, Beijing,China) was aerobically cultured at 37 °C in Luria-Bertani medium.The PrimeSTAR DNA polymerase was bought from TAKARA company (Dalian, China) and the other enzymes were purchased from New England Biolabs Ltd. (Beijing, China). Nucleotide primers were synthesized by Sangon Biotech Company (Shanghai, China).Nickel-nitrilotriacetic acid (Ni-NTA) agarose was purchased from Qiagen (Beijing, China). The chemical reagents (Sinopharm Group Co., Ltd., Beijing, China) used were of analytical purity.
2.2 Gene cloning and construction of the recombinant co-expression plasmids
The recombinant plasmids were constructed by the Gibson assembly method [18]. The template plasmids pTRAN and pTrc-RcXH3 were prepared using plasmid extraction kits (Promega,Madison, WI, USA). Fragment I with a length of 964 bp containing theR. capsulatus xdhCgene (939 bp) was PCR amplified by using the pTrc-RcXH3 as a template with the forward primer AbRc-gS1 and reverse primer AbRc-gA2 (Table 1). Fragment II with a length of 8 046 bp composed ofA. baumanniiXDHxdhABgenes
and pTrc99A sequence was PCR amplified by using the pTRAN as
a template with the forward primer AbRc-gS2 and reverse primer AbRc-gA1 (Table 1). Fragment III, 1 023 bp in length and consisting of theA. baumannii xdhCgene (984 bp), was PCR amplified using the pTRAN as a template with the forward primer RcAb-gS1 and reverse primer RcAb-gA2 (Table 1). Fragment IV with a length of 7 890 bp composed ofxdhABgenes encodingR. capsulatusXDH
and pTrc99A was PCR amplified by using the pTRAN as a template
with the forward primer RcAb-gS2 and reverse primer RcAb-gA1(Table 1). The underlined parts of the primers complement to the template plasmids, and the bold italic parts are complementary pairs between fragments. Fragments I and II were assembled to yield the expression plasmid pTrc-AbRc, and fragments III and IV were assembled to produce the expression plasmid pTrc-RcAb by using the Gibson Assembly Cloning Kit (NEB #E5510). DNA sequencing

Table 1Primers and their nucleotide sequences used in this study.
confirmed the recombinant plasmids.
2.3 Overexpression and purification of the recombinant enzymes
According to previous studies [12,14,17], the recombinant plasmids pTrc-AbRc and pTrc-RcAb together with the previously constructed pTRAN and pTrc-RcXH3 were transformed into competentE. coliDH5α cells to overexpress the recombinant XDHs, which were purified by Ni-NTA chromatography. Purified proteins were transferred into 50 mmol/L Tris-HCl buffer (pH 7.5)using ultrafiltration spin columns with a molecular weight cut-off of 10 000 Da (Millipore) and assayed by sodium dodecylsulfate-polyacrylamide gel electrophoresis (SDS-PAGE) for purity.Protein markers (No. PR1920) were purchased from Beijing Solaria Science & Technology Co., Ltd. (Beijing, China). Protein concentration was determined using the Bradford method with bovine serum albumin as the standard.
2.4 Enzymatic assays
According to previous study, the enzymatic activity, the effects of pH and temperature, and kinetic parameters of the purified recombinant XDHs were studied [14]. One unit of XDH activity was
defined as the amount of enzyme that converts 1 µmol of xanthine to uric acid per min under the assay conditions.
2.5 Computational analysis
Two homology models forA. baumanniiXdhC (AbXdhC) andR. capsulatusXdhC (RcXdhC) were constructed using the unpublished and unannotated crystal structure ofMycobacterium tuberculosisRv0376C homologue fromMycobacterium smegmatis(PDB ID: 2we7; www.pdb.org) [6], which have the highest homology with AbXdhC (22.12%) and RcXdhC (25.08%) deposited in the Protein Data Bank (PDB), using the online Swiss-model server (http://www.swissmodel.expasy.org). Similarly, the models for XdhC fromP. aeruginosaPA1522 (PaXdhC, 32.06% to 2we7) andP. acidovorans(PeXdhC, 25.1% to 2we7) were constructed. An unpublished structure ofBacillus haloduransXdhC-like protein(PDB ID: 3ON5) showed the second-highest sequence identity with AbXdhC (19.9%), RcXdhC (20.6%), and 2WE7 (21.7%) acquired from the PDB. Structural superimposition and structure-based sequence alignment were implemented using the Swiss-PDB Viewer(SPDBV, version 4.1) [19]. The graphical representation (sequence logo) of amino acid sequence conservation of XdhC was constructed using the online WebLogo server with default parameters (http://weblogo.berkeley.edu/logo.cgi). All structural representations were depicted using Pymol software (The PyMol Molecular Graphics System, DeLaon Scientific LLC, San Carlos, CA, USA).
2.6 Statistical analysis
The mean ± standard deviation values were calculated from triplicate experiments. Statistical analyses were performed using Microsoft Excel (Microsoft Co., Redmond, WA, USA). One-way ANOVA and pairedt-test methods with two-tailedPvalues were carried out to analyze the statistical significance, and differences were considered statistically significant atP< 0.05.
3. Results and discussion
3.1 Construction of chimeric gene clusters
In our previous studies, the XDHs ofA. baumanniiCICC 10254(AbXDH) andR. capsulatusCGMCC 1.3366 (RcXDH) were functionally expressed by co-expressing their naturally occurring XdhC chaperones in translational coupling gene cluster format under thePtrcpromoter inE. coli[14,17]. Two chimericxdhABCgene clusters were constructed by exchangingxdhCgenes in the twoxdhABCgene clusters to systematically investigate the expression efficiency of low-homologous XDHs with heterologous XdhC(Fig. 1A). The two refactoredxdhABCgene clusters were arranged in the following same manner as the naturally occurring ones: 1) three complete open reading frames (ORFs) encodingxdhA,xdhB, andxdhCwere preceded by typical ribosome binding sites and transcribed in the same direction; 2) the stop codon ofxdhAORF overlapped the start codon ofxdhBORF, and the stop codon ofxdhBORF covered the start codon ofxdhCORF, ensuring translational coupling to guarantee the synthesis of equimolar amounts of 3 proteins [17]. As shown in Fig. 1B, the chimeric gene clusters of theA. baumannii xdhAB,R. capsulatus xdhC,R. capsulatus xdhAB, andA. baumannii xdhCwere also inserted downstream thePtrcpromoter of pTrc99A,yielding recombinant plasmids pTrc-AbRc and pTrc-RcAb.
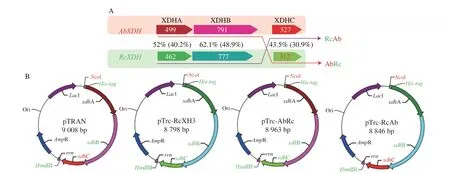
Fig. 1 Schematic diagram of combinatorial co-expression of XDH and XdhC of A. baumannii and R. capsulatus. (A) Proteins encoded by gene cluster for each co-expression. The red frame, green frame, red arrow, and purple arrow indicate the compositions of AbXDH, RcXDH, AbRc, and RcAb, respectively. The arrow boxes stand for the two subunits, XdhA and XdhB of XDH, and chaperone XdhC, with their protein length and sequence homology (identity) values labeled on and between arrow boxes. (B) Plasmid maps for co-expressions. pTRAN, pTrc-XH3, pTrc-AbRc and pTrc-RcAb are pTrc99A inserting gene clusters encoding AbXDH, RcXDH, AbRc and RcAb under the Ptrc promoter, respectively.
3.2 Expression of XDH in E. coli and purification of the recombinant enzyme
Under the control of thePtrcpromoter, two chimericxdhABCgene clusters and the two naturally occurring clusters ofA. baumanniiandR. capsulatuswere successfully overexpressed inE. coliDH5α.After purification by one-step Ni-NTA chromatography, all the recombinant proteins showed two distinct bands on SDS-PAGE,consistent with the theoretical molecular weight of XDHA and XDHB calculated from the gene sequences (Fig. 2). Furthermore, AbXDH and AbRc (A. baumanniiXDH co-expressed withR. capsulatus xdhC) displayed the same protein bands of 56 kDa (AbXDHA) and 87 kDa (AbXDHB) on SDS-PAGE, whereas RcXDH and RcAb(R. capsulatusXDHco-expressed withA. baumannii xdhC) showed two distinct bands corresponding to 50 kDa (RcXDHA) and 85 kDa (RcXDHB), respectively. We did not observe protein bands corresponding to chaperone XDHC (37 kDa for AbXdhC, 33 kDa for RcXdhC), and the phenomena were consistent with previous reports that showed that XdhC was functionally indispensable but neither stable nor an essential component of active XDH [12,14,16,17].

Fig. 2 SDS-PAGE of purified recombinant XDHs. Lane M, molecular weight marker; Lane 1, RcXDH; Lane 2, RcAb; Lane 3, AbRc; Lane 4, AbXDH.
3.3 Characterization of recombinant XDHs
All 4 purified XDHs displayed a typical bell-shaped pH-activity relationship. AbRc and AbXDH showed the same pH-activity pattern with maximum activity at approximately pH 8.5 and > 80% relative activity between pH 7.0 and 9.0, while RcAb shifted the pH-activity profile to acidic values with maximum activity at pH 7.0 and > 80%relative activity between pH 5.0 and 7.5 in comparison to RcXDH,which showed maximum activity at about pH 8.0, and > 80% relative activity between pH 7.0 and 9.0 (Fig. 3A). Furthermore, all the enzymes except for RcAb maintained nearly intact catalytic activity after treatment in the pH range 5.0-11.0 for 24 h at 25 °C (Fig. 3B),which showed a narrower pH range of stability and maintained more than 80% maximum activity between pH 6.0 and 9.5. The results of AbRc, AbXDH, and RcXDH are comparable to those of previous studies of AbXDH and RcXDH co-expressed with their naturally occurring XdhC inE. coli. At the same time, the shifts in pH-activity and stability profiles caused by heterologous chaperone XdhC of RcAb have not yet been reported [14,17].
Moreover, all the XDHs showed optimal activity at 40 °C and maintained more than 60% relative activity between 35 and 55 °C(Fig. 3C). AbXDH and AbRc were more thermostable than RcXDH and RcAb, as demonstrated by their half inactivation temperaturevalues of (65.48 ± 1.13), (68.32 ± 0.95), (57.77 ± 1.42),(44.50 ± 1.67) °C, respectively (Fig. 3D). The 2.84 °C improvement in AbRc thermostability compared to AbXDH and a 13.27 °C decrease in the thermostability of RcAb compared to RcXDH suggests that RcXdhC is a better chaperone than AbXdhC to help produce robust XDHs. Thus, it is the only case in which the quantitative chaperone performance of XDHCs for cross-inactivation are compared, and the results necessitate the choice of XDHC for better XDH production and new XDH exploitation.
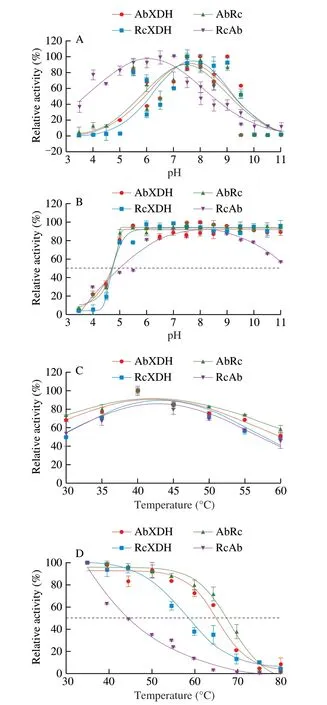
Fig. 3 Effects of pH and temperature on the activity and stability of purified recombinant XDHs. (A) pH-activity profile; (B) pH-stability relationship;(C) Temperature-activity dependence; (D) Temperature-stability profile. The effects of pH on XDH activity and stability were investigated at 25 °C in 50 mmol/L sodium acetate (pH 3.5-5.8), sodium phosphate (pH 5.8-8.0),Tris-HCl (pH 7.5-9.0), and sodium carbonate buffers (pH 9.0-11.0). pH-stability studies were carried out by incubating enzymes (0.05 mg/mL) in the same 50 mmol/L buffers (pH 3.5-11.0) for 18 h at 25 °C, and then testing their residual enzymatic activities. The effect of temperature on XDH activity was examined in 50 mmol/L Tris-HCl buffer (pH 8.0) from 30 °C to 60 °C with a 5 °C step. The temperature-stability relationship was determined by measuring the residual XDH activity after incubation at different temperatures (35-80 °C)for 30 min in 50 mmol/L Tris-HCl buffer (pH 8.0). All values are averaged from triplicate measurements. Error bars represent standard deviation.
When characterized with xanthine and NAD, all 4 purified XDH enzymes displayed typical Michaelis-Menten kinetics under the assay conditions of 25 °C and pH 8.0. As shown in Fig. 4, with the help of RcXdhC,A. baumanniiXDH (AbRc) showed apparent Michaelis constant (Km) for xanthine of (334.60 ± 75.28) µmol/L and turnover number (kcat) of (88.4 ± 17.9) s-1, in comparison to (43.8 ± 7.9) µmol/L and (86.8 ± 15.6) s-1of AbXDH aided by its own endogenous chaperone. The significant 6.64-fold decrease in substrate affinity for xanthine (Fig. 4A) but a slightly improved turnover number (1.84%)resulted in a significant 6.5-fold decrease in catalytic efficiency(from 1.98 × 106L/(mol·s) to 0.26 × 106L/(mol·s)) (Fig. 4D).RcAb showedKm,kcat, andkcat/Kmvalues of (66.0 ± 18.7) µmol/L,(11.0 ± 2.6) s-1, and 0.17 × 106L/(mol·s) for substrate xanthine, while that of RcXDH were (43.5 ± 10.0) µmol/L, (69.2 ± 15.1) s-1and 1.59 × 106L/(mol·s), respectively. The results corresponded to 52% (P> 0.05,Fig. 4A), 5.29-fold (P< 0.005, Fig. 4C), and 8.54-fold decreases in substrate affinity, turnover number, and catalytic efficiency. The specific activity for the 4 XDHs (Fig. 4B) showed the same statistical difference as their turnover numbers (Fig. 4C). The kinetic parameters of AbXDH and RcXDH here were comparable to our previous reports,in which theKm,kcat, andkcat/Kmvalues were (25.3 ± 1.3) µmol/L,(69.3 ± 0.9) s-1, and 2.74 × 106L/(mol·s) for AbXDH and(68 ± 20) µmol/L, (93 ± 11) s-1and 1.37 × 106L/(mol·s) for RcXDH,despite the different test conditions [14,17]. AbRc and AbXDH also belong to the most active, thermostable, and alkaline-tolerant XDH enzymes reported to date and have potential industrial applications [14].
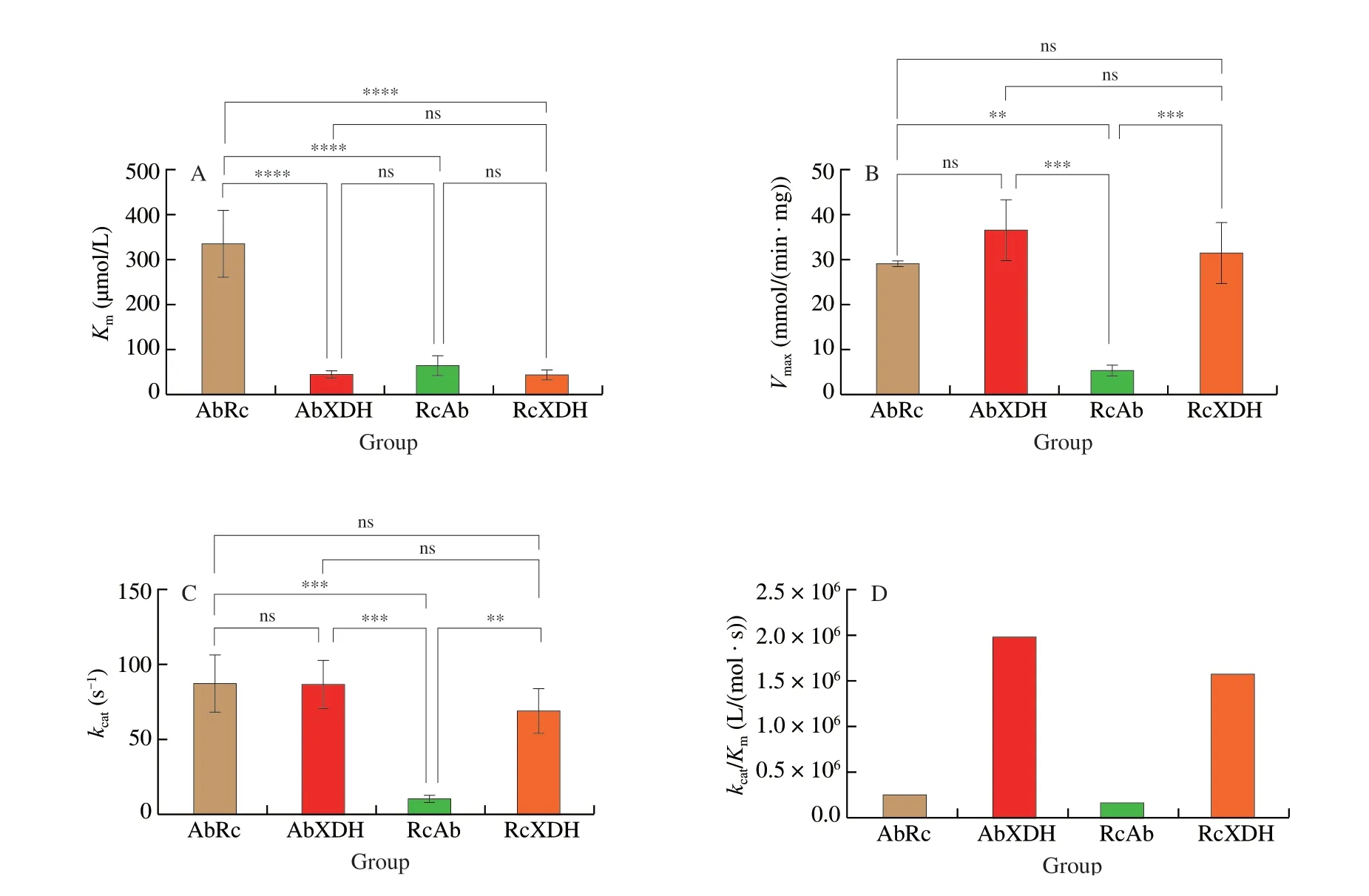
Fig. 4 Statistical comparisons of kinetic parameters of the purified recombinant XDHs. (A) Apparent Michaelis-Menten constant (Km) on xanthine. (B) Specific activity on xanthine. (C) Turnover number (kcat) on xanthine. (D) Catalytic efficiency (kcat/Km) on xanthine. One-way ANOVA and paired t test methods with two-tailed P values were carried out to analyze the statistical significance, and differences with P < 0.05 were considered statistically significant. The ns, **, ***,and **** symbols indicate no significant, significant (P < 0.05), strong significant (P < 0.005), and very strong significant (P < 0.0005), respectively.
3.4 Use of XDHs to decrease xanthine and hypoxanthine contents in foods
The 4 purified XDHs were assayed in decreasing the xanthine and hypoxanthine contents in beer, beef, and yeast extract. As shown in Figs. 5 and 6, all the XDHs efficiently degraded the xanthine and hypoxanthine in the 3 foods to different degrees after incubation with 0.5 U/mL XDHs at pH 8.5 at 25 °C for 5 h. Almost all the XDHs produced more in beer (Fig. 6A) than in yeast extract(Fig. 6B), while the lowest uric acids in the beef powder (Fig. 6C),which is opposite to the order of decrease in xanthine contents in the 3 foods. Accordingly, hypoxanthine and inosine contents, which are the precursors of xanthine in beer and yeast extract, are reduced but they increased in beef powder. The elimination of hypoxanthine and xanthine and the production of uric acid were inconsistent,dissatisfying the stoichiometric relationship of the reaction by XDH.For example, in beer, RcAb and RcXDH produced 450.63 and 510.69 µmol/L uric acid, while only decreasing the xanthine by 5.17 and 39.11 µmol/L, and reduced the hypoxanthine by 0.43 and 5.15 µmol/L, respectively (Figs. 5I, L, and 6A). Furthermore, AbRc produced 504.29 µmol/L uric acid and decreased 17.80 µmol/L xanthine and 0.65 µmol/L hypoxanthine, AbXDH produced 229.03 µmol/L uric acid but decreased 150.60 µmol/L xanthine and 0.73 µmol/L hypoxanthine (Figs. 5C, F, and 6A). The underlying reasons may be complex purine transformations, including the degradation of inosine monophosphate (IMP) to inosine, inosine to hypoxanthine, deamination of adenosine monophosphate to IMP under the assay conditions, and the random degradation of hypoxanthine and xanthine by XDHs [20,21]. As exemplified by the phenomena
that inosine in beer after enzymatic incubation by all the 4 XDHs was decreased by (134.41 ± 35.54) µmol/L (Figs. 5C, F, I, L, and 6A),in yeast extract (53.24 ± 18.97) µmol/L (Figs. 5A, D, G, J, and 6B),while in beef powder the inosine increased by (68.81 ± 13.55) µmol/L in all the XDHs except RcAb which decreased by 66.77 µmol/L(Figs. 5B, E, H, K, and 6C). The efficient purine lowering of XDHs in foods is supported by a previous study that reported that the incubation with an enzyme mixture containing 0.67 U/mL Arxula adeninivorans XDH and 0.083 U/mL of urate oxidase, adenine deaminase, and guanine deaminase at approximately pH 8.5 at 40 °C for 4 h reduced the xanthine and hypoxanthine contents in yeast extract by respective 0.85 and 0.88 mmol/L [22]. Considering the equimolar stoichiometric relationships of oxidation of hypoxanthine to xanthine and xanthine to uric acid by XDH catalysis, we can use the total decrease amount of xanthine and hypoxanthine and increase in uric acid to quantify and compare the purine-lowering abilities of XDHs in this study. As shown in Fig. 6D, all 4 XDHs showed similar purine-lowering patterns in the foods, of which the order of degradation efficiency of purine contents was beef powder > beer > yeast extract. RcXDH and RcAb showed nearly the same purine-lowering ability with a respective average of (485.48 ± 237.06) and (487.10 ± 198.14) µmol/L,while the AbRc displayed (593.87 ± 241.45) µmol/L more than the (484.85 ± 224.90) µmol/L of AbXDH (Fig. 6D). Efficient purine-lowering co-expressed XDHs provided an alternative to combine with other purine-degrading enzymes to develop new strategies to prevent hyperuricemia and gout [22-25].
3.5 Computational modeling analysis
Previous studies have suggested the equivalent functions of an E. coli XdhC-like homolog on R. capsulatus XDH and P. aeruginosa XdhC on C. acidovorans XDH, however, no opposite cross-activation by RcXdhC on E. coli XDH or C. acidovorans XdhC on P. aeruginosa XDH has been reported to date, and the corresponding XdhC-like homolog and C. acidovorans XdhC have not been characterized at the genetic or protein level [12,15]. For the first time, this study investigated the combinatorial co-expression of XDH and XdhC from A. baumannii and R. capsulatus, and showed that heterologous XdhCs with 30.9% sequence identity could effectively chaperone XDHs with approximately 30.9% (XDHA)-40.2%(XDHB) sequence identity. Because no crystal structures of XdhC have been resolved thus far, AbXdhC and RcXdhC were further explored by constructing computational homology models by using the Swiss-model server with the PDB ID: 2WE7 as a template, which is an unannotated crystal structure of M. tuberculosis Rv0376C homolog from M. smegmatis and shares the highest sequence identity with AbXdhC (22.12%) and RcXdhC (25.08%) [6,19,26]. As shown in Fig. 7, AbXdhC and RcXdhC adopt a three-domain overall fold similar to that of 2WE7, showing nearly identical C-terminal domain (domain III in Fig. 7A) and N-terminal domain (domain I in Fig. 7A). Domain I contains a set of 3 anti-parallel β-sheets and a cysteine residing 3 amino acid residues from the amino terminus of the second β-sheet (β5 in Fig. 7C), which are the conserved cysteines proposed to be involved in sulfide delivery and Moco sulfuration in the XdhC family. However, the loop containing the conserved cysteine is the most variable area with little sequence homology
(Fig. 7C) and is highly flexible, as observed in the crystal structures
of 2WE7 and 3ON7 [6]. The loops containing the conserved cysteine of AbXdhC and RcXdhC adopted two distinct conformations;the former showed nearly the same spatial positions as PaXdhC and PeXdhC, which are located opposite to RcXdhC and 3ON5(Fig. 7B). Such flexibility of the linking loop harboring conserved cysteine may enable the conformational changes of XdhC to play multiple chaperone roles and underlie the functional complement of AbXdhC and AbXdhC for the combinatorial co-expression of
A. baumannii and R. capsulatus XDHs.
4. Conclusion
For the first time, we have described the combinatorial co-expression of XDH and chaperone XdhC fromA. baumanniiandR. capsulatusand their applications in decreasing purine content in foods. BothA. baumanniiXdhC andR. capsulatusXdhC efficiently mediated the functional expression of XDHs fromA. baumanniiandR. capsulatus.R. capsulatusXDH chaperoned byA. baumanniiXdhC showed the same catalytic performance as that ofR. capsulatusXdhC, except for decreased substrate affinity. TheA. baumanniiXDH co-expressed withR. capsulatusXdhC displayed acidic adaptation with weakened catalytic activity. All the recombinant XDHs effectively catalyzed the transformation of hypoxanthine to xanthine and xanthine to uric acid in beer, beef, and yeast extract, indicating potential applications in reducing purine levels in food to prevent hyperuricemia and gout. Computational structural and multiple sequence alignment analyses revealed the conserved cysteine and the flexibility of the loop harboring the conserved cysteine betweenA. baumanniiXdhCandR. capsulatusXdhC; however, the mechanism underlying the chaperone performance needs further investigation. Furthermore, the study presents a method for exploiting the better chaperone XdhC and novel XDHs by functional complement using existing XdhCs such asR. capsulatusXdhC.
Declaration of competing interest
The authors declared no potential conflict of interest.
Acknowledgements
We would like to thank Editage (www.editage.cn) for English language editing. This work was funded by the National Natural Science Foundation of China (21868003), the Guangxi Natural Science Foundation (2018AD19022, 2017GXNSFAA198265), and the Nanning Science and Technology Development Project (2017014).
- 食品科学与人类健康(英文)的其它文章
- Emerging natural hemp seed proteins and their functions for nutraceutical applications
- A narrative review on inhibitory effects of edible mushrooms against malaria and tuberculosis-the world’s deadliest diseases
- Modulatory effects of Lactiplantibacillus plantarum on chronic metabolic diseases
- The role of f lavonoids in mitigating food originated heterocyclic aromatic amines that concerns human wellness
- The hypoglycemic potential of phenolics from functional foods and their mechanisms
- Insights on the molecular mechanism of neuroprotection exerted by edible bird’s nest and its bioactive constituents

