Effects of specif ic doses of E-beam irradiation which inactivated SARS-CoV-2 on the nutrition and quality of Atlantic salmon
Huilin Yu, Junhui Zhang, Yan Zhao, Honghao Li, Yixuan Chen, Jiajin Zhu*
Department of Food Science and Nutrition, Zhejiang University, Hangzhou 310058, China
Keywords:E-beam irradiation Atlantic salmon SARS-CoV-2 Physicochemical properties
A B S T R A C T The contamination of Atlantic salmon with severe acute respiratory syndrome coronavirus 2 ( SARS-CoV-2)has impeded the development of the cold-chain food industry and posed possible risks to the population.Electron beam (E-beam) irradiation under 2, 4, 7, and 10 kGy can effectively inactivate SARS-CoV-2 in cold-chain seafood. However, there are few statistics about the quality changes of salmon exposed to these irradiation dosages. This work demonstrated that E-beam irradiation at dosages capable of killing SARS-CoV-2 induced lipid oxidation, decreased vitamin A content, and increased some amino acids and ash content. In addition, irradiation altered the textural features of salmon, such as its hardness, resilience, cohesiveness,and chewiness. The irradiation considerably affected the L*, a*, and b* values of salmon, with the L* value increasing and a*, b* values decreasing. There was no significant difference in the sensory evaluation of control and irradiated salmon. It was shown that irradiation with 2-7 kGy E-beam did not significantly degrade quality. The inactivation of SARS-CoV-2 in salmon is advised at a dose of 2 kGy.
1. Introduction
Atlantic salmon is one of the most sought-after seafoods in the world due to its high nutritional value, appealing look, and distinctive flavor. It is universally favored as a type of high-fat, high-protein,and high-carotenoid fish with plentiful unsaturated fatty acids such as DHA and EPA, various amino acids conforming to the optimal model, and abundant astaxanthin with a powerful antioxidant capacity. It is reported that the global production of Atlantic salmon in 2021 reached 2 423 000 t, and the value of global salmon sales in 2018 amounted to 13.75 billion euros [1,2]. However, with the explosion of the COVID-19 in 2019, frozen foods, including salmon, have been shown as carriers for SARS-CoV-2 transit, raising concerns about the safety of frozen seafood. SARS-CoV-2 is an enveloped, non-segmented, positive-sense RNA virus that targets respiratory and gastrointestinal epithelial cells in humans, resulting in more than 47 million infections and 1.2 million deaths worldwide since its emergence [3-5]. In China, there were 8 incidences of SARSCoV-2 contamination in aquatic products between the beginning of July 2020 and the middle of August 2020 [6]. SARS-CoV-2 has reportedly been discovered in food samples, packaging materials,shipping containers, and wastewater. Not only has the extensive dispersion of SARS-CoV-2 in imported fish increased the global threat of SARS infection, but it has also severely impeded the development of the salmon sector. It was reported that the COVID-19 led to a 20% decline in the export of Scottish salmon, resulting in a reduction of £168 million by 2020 [7]. The pandemic has made China and the United States the two nations most hit by the import issue. Morrison [8] noted that after China suspended salmon imports from European sources due to the identification of SARS-CoV-2 in an imported salmon cutting board at a Beijing market, the entire volume of Norway’s salmon sector declined by 5%. Although the World Health Organization (WHO) has indicated that SARS-CoV-2 is unlikely to infect humans through contaminated food, researchers are nevertheless concerned that it might spark a COVID-19 outbreak or become a plausible cause of clusters with existing outbreaks by transmitting the virus to practitioners and the environment [9].It is important to note that viral RNA can remain relatively stable in chicken, pork, and salmon for three weeks at cold-chain temperatures of 4, -20, and -80 °C for 3 weeks, which increases the danger of direct or indirect exposure to SARS-CoV-2 during food storage, processing, and transportation [10]. In addition, it has been demonstrated that SARS-CoV-2 was more stable in frozen salmon(-20 °C) than in fresh salmon stored at 4 °C [11]. SARS-CoV-2 is also more stable in polyethylene packaging materials at a lower temperature [12]. Therefore, polyethylene-packaged salmon at long-term low temperatures is more conducive to preserving SARS-CoV-2, posing high safety risks that threaten human life and significantly impede industrial development. Prior to the salmon’s distribution on the market, it is crucial to ensure its safety.
There are a few potential methods for reducing the risks of SARS-CoV-2 in the food industry, including avoiding underheating in food processing, utilizing the appropriate chemical sanitizers, employing the
different light spectrums, and producing foods fortified with vitamin,
amino acid, nicotinamide adenine dinucleotide and tannins [3,4].Utilizing disinfectants such as ethanol, propanol, povidone-iodine,and sodium hypochlorite and ultraviolet (UV) light are two of the most employed ways for eliminating SARS-CoV-2 in the frozen seafood industry. However, the lack of standardization has made it difficult to assess the effective concentration and duration of used disinfectants in different situations; consequently, the problem of chemical residues and insufficient salmon sterilization is highly probable [13]. UV light is less applicable in the food chain due to its higher cost and lower SARS-CoV-2 inactivation rate [14].Therefore, it is more than necessary to identify a method that can effectively inactivate SARS-CoV-2 without negatively affecting the quality of salmon. E-beam irradiation has become one of the most promising technologies for preventing food spoilage, as it uses ionizing radiation to kill microorganisms and is characterized by strong penetration and rapid inactivation without heat generation and residual toxicity. Compared to disinfectants and UV light, it is inexpensive, environmentally friendly, and time-saving, and it is frequently used in sealed, fully packed items [15]. The Food and Agriculture Organization of the United Nations (FAO), WHO, and the International Atomic Energy Agency (IAEA) have all concurred that irradiation doses of 10 kGy or less do not harm food safety and pose only minor effects on food nutrition [16]. Yang et al. [17]demonstrated that 0.5 kGy E-beam irradiation effectively maintained salmon’s biochemical properties and gel-forming capacity. In October 2020, it was discovered that the inactivation rate of SARS-CoV-2 can reach 99.9% on the surface of food packaging, food surface, and food gap at the lowest effective dose of 2 kGy E-beam irradiation [18],laying the groundwork for the application of E-beam irradiation technology to salmon to prevent SARS-CoV-2 contamination.
Before promoting the application of E-beam technology to the inactivation of SARS-CoV-2 in salmon, it is necessary to determine if the extinguishing doses will alter the nutritional, textural, and
sensory qualities of the fish. Although some researchers have reported changes in the quality of salmon subjected to E-beam irradiation,there is currently no study focusing on the nutrition, physical and chemical properties, and sensory characteristics of fresh salmon exposed to irradiation doses capable of inactivating SARS-CoV-2.To comprehensively assess the effects of irradiation and clarify the application of E-beam irradiation to imported salmon, we determined several nutritional, textural, and sensory parameters of irradiated salmon.
2. Material and methods
2.1 Salmon sample preparation and irradiation
Chilled divided salmon was purchased from the market (Wholesale Market of Aquatic Products, Hangzhou, China) and was transported to the Electronic Accelerator Platform in Zhejiang University with the cold chain. Salmon muscle from the back area was cut into the following dimensions: 20 cm in length, 10 cm in width, and 4 cm in thickness. Each piece of salmon used in the study weighed approximately 500 g and was vacuum-packed in polyethylene.To monitor the temperature changes, temperature recorder probes(ZDR-20Pro, Zeda Instruments Co., Ltd., Hangzhou, China)were inserted into the center of each sample perpendicular to the irradiated surface.
During irradiation, salmon samples were placed on open iron boxes on the linear accelerator belt (ESS-010-03, Japan) and subjected to high-energy electron beam irradiation at doses of 0, 2, 4, 7, and 10 kGy, respectively. The irradiation doses were determined based on the effectiveness of 2, 4, 7, and 10 kGy in killing SARS-CoV-2 found in a previous study [18]. After irradiation, the salmon samples were stored at -20 °C in the refrigerator and transported to the laboratory using a freezing incubator for further analysis. All sampling was performed in triplicate.
2.2 Changes in nutritional composition
2.2.1 Determination of fat and fatty acid
The fat content of salmon samples was determined using Soxhlet extraction, as described previously [19]. For the determination of saturated fatty acid (SFA), monounsaturated fatty acid (MUFA), and polyunsaturated fatty acid (PUFA), the internal standard method of Chinese standard GB 5009.168-2016 was used.
2.2.2 Determination of protein and amino acid
The protein content of irradiated salmon samples was determined using the Kjeldahl Method in accordance with Mæhre et al. [20].Amino acid determination was performed by the amino acid automatic analyzer (Biochorm, the UK) based on Chinese standard of GB 5009.124-2016.
2.2.3 Determination of vitamin A and vitamin E
The vitamin A and vitamin E content of salmon samples were determined using reversed-phase high-performance liquid chromatography according to the Chinese standard GB 5009.82-2016.
2.2.4 Determination of moisture content
We determined the water content of salmon samples by dehydrating them to a constant weight in accordance with the Chinese standard GB 5009.3-2016.
2.2.5 Determination of ash
Based on the Chinese standard GB 5009.4-2016, salmon samples were burned, and the residual inorganic matter was weighed to calculate the ash content.
2.2.6 Determination of reducing sugar
The content of reducing sugar in salmon was determined by the direct titration method according to the Chinese standard GB 5009.7-2016.
2.3 Changes in biochemical properties
2.3.1 Determination of the total volatile basic nitrogen (TVB-N)
Semi micro steam distillation method was used for the determination of TVB-N [21]. The content of TVB-N (mg) in the sample (per 100 g) was determined by multiplying the consumed volume of hydrochloric acid standard titration by its concentration,and the corresponding formula is provided below:

whereVis the difference of the consumed volume of the hydrochloric acid standard titration between the test solution and the reagent blank;cis the concentration of hydrochloric acid standard titration solution.
2.3.2 Determination of 2-thiobarbituric acid (TBA) value
TBA value was determined using previously described protocols [22]. The oxidation degrees of salmon oil were determined by the amount of malondialdehyde that could be detected by reacting with TBA to produce red-colored compounds, which was measured at 530 nm.
2.4 Changes in physical properties
2.4.1 Texture determination
The texture profile was analyzed referring to Zzaman et al. [23],with modifications. Salmon was defrosted in a refrigerator at 4 °C and cut into cubes with dimensions of 8 cm × 8 cm × 1 cm in order to determine its texture further. For texture profile analysis, a TA-XT2i Texture Analyzer (Stable Micro System, London, UK) equipped with P/5 flat-bottom cylinder probe was used. The sample was placed on the loading cell and compressed at a cross-head speed of 1 mm/s with a compression rate of 50% by the probe. The trigger force was 5 g, and each sample was measured for 5 s. The software of the TA-XT2i Texture Analyzer would calculate the hardness, elasticity,cohesiveness, and chewiness. For each group, at least 6 samples were detected.
2.4.2 Color evaluation
Based on the method of Zonoubi et al. [24], the color of the salmon samples was evaluated using theL*a*b* system. There were three samples detected for each group. Salmon samples were cut into the following sizes: length is 8 cm, width is 8 cm, and depth is 1 cm.After calibration, a colorimeter (Konicamonilta, Japan) was used to determine the lightness (L*), the red chromaticity (a*), and the yellow chromaticity (b*) of the salmon. Browning index (BI), representing the purity of brown color and color changes relative to sample irradiation, was calculated for each treatment using the following equations [25,26].

2.5 Sensory assessment
The sensory characteristics of salmon samples were evaluated in accordance with Pedrós-Garrido et al. [27] with modifications.Twenty trained sensory assessors for cooked salmon were recruited.Approximately 20 g of salmon fillets from each group were boiled for 5 min in water and then blind-coded with numbers. The panelists were not informed of the number of samples and were required to drink water after tasting each sample to cleanse their palates. The properties of odor, flavor, and texture were evaluated using a 1-10 nonstructural scale (1 = extremely bad odor/flavor/low intensity, and 10 = excellent odor/flavor/high intensity). The judges evaluated at their own pace and scored each salmon sample immediately after tasting it.
2.6 Statistical analysis
All experiments were conducted in triplicate for each group. All measurements were presented as mean ± SEM. GraphPad Prism 8.3(GraphPad Software Inc., New York, USA) was used to conduct a one-way analysis of variance (ANOVA) followed by Dunnett’s post hoc comparison between groups. Different letters indicated significant differences between groups. Differences atP< 0.05 were considered significant.
3. Results
3.1 Changes of the salmon fat
The effects of E-beam irradiation at different doses on salmon fat were shown in Fig. 1. There was no significant difference in fat and fatty acid content between the control and irradiated groups (Fig. 1A, C,P> 0.05). As irradiation doses increased, TBA values increased significantly from (0.33 ± 0.00) mg/kg to (0.45 ± 0.00), (0.60 ±0.00), (0.66 ± 0.01), and (0.68 ± 0.01) mg/kg, respectively (Fig. 1B,P< 0.05).
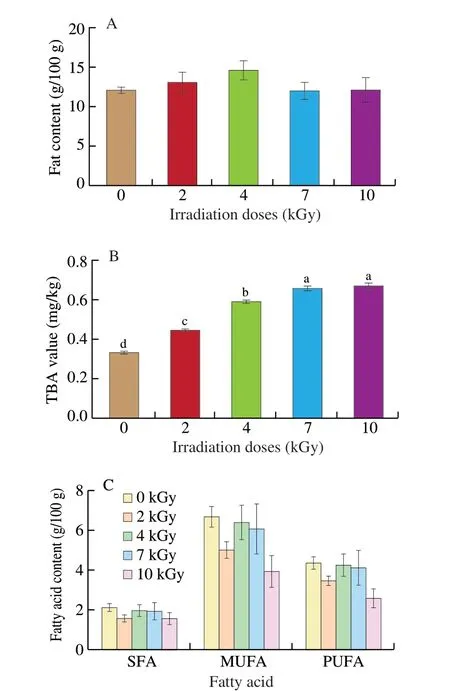
Fig. 1 Changes in (A) fat content, (B) TBA value, and (C) fatty acid content,including SFA, MUFA PUFA in salmon exposed to varying E-beam irradiation doses (0, 2, 4, 7, 10 kGy). Different letters differ significantly (P < 0.05).
3.2 Measurement and changes of amino acid content and TVB-N content
As shown in Fig. 2A, E-beam irradiation had no effect on the salmon protein content (P> 0.05). Thirteen amino acids,excluding proline, glycine and histidine, increased with 10 kGy irradiation, whereas valine increased from (0.87 ± 0.03) g/100 g to(1.00 ± 0.03) g/100 g with 7 kGy irradiation (P< 0.05). The overall amino acid content of irradiated salmon increased (Table 1).In addition, TVB-N content decreased slightly to (8.41 ± 0.62),(8.24 ± 0.91), (7.75 ± 0.89), and (7.40 ± 0.43) mg/100 g following 2-10 kGy E-beam irradiation compared to (10.33 ± 0.18) mg/100 g in the control group in Fig. 2B (P> 0.05).
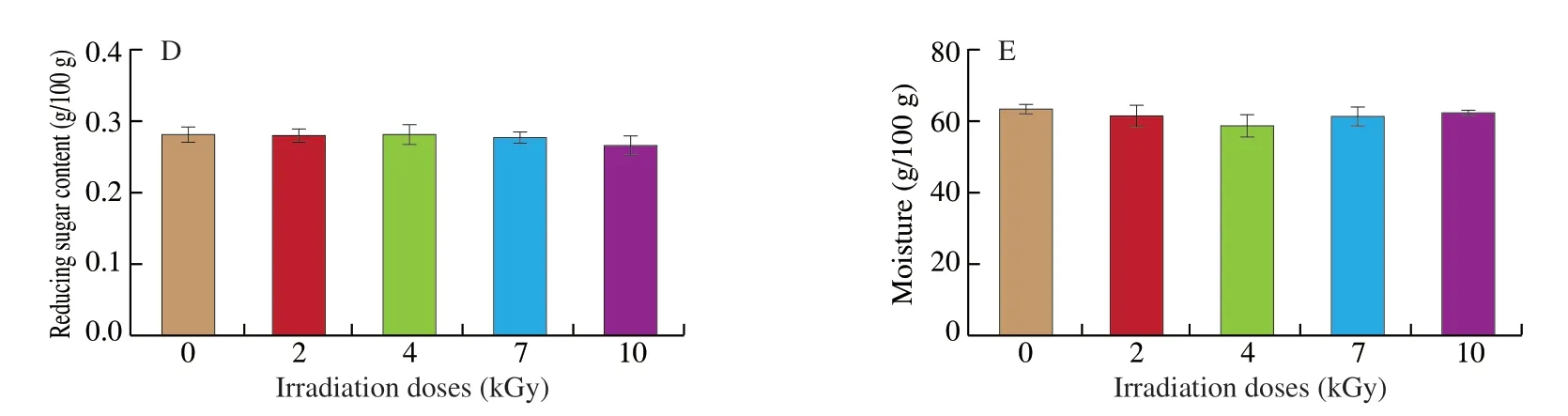
Fig. 3 (Continued)
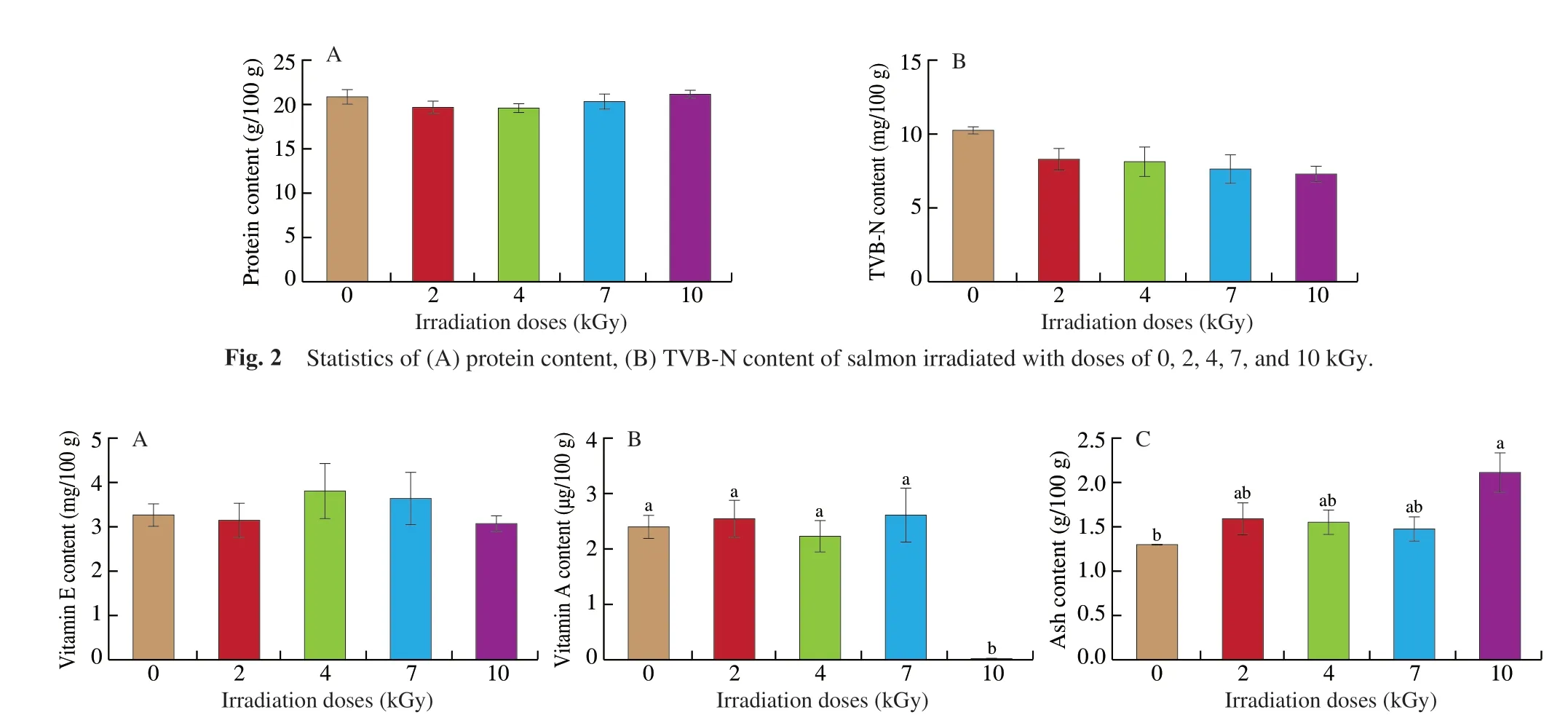
Fig. 3 Content of (A) vitamin E, (B) vitamin A, (C) ash, (D) reducing sugar, and (E) moisture in salmon irradiated with doses of 0, 2, 4, 7, and 10 kGy. Differentletters differ significantly (P < 0.05).
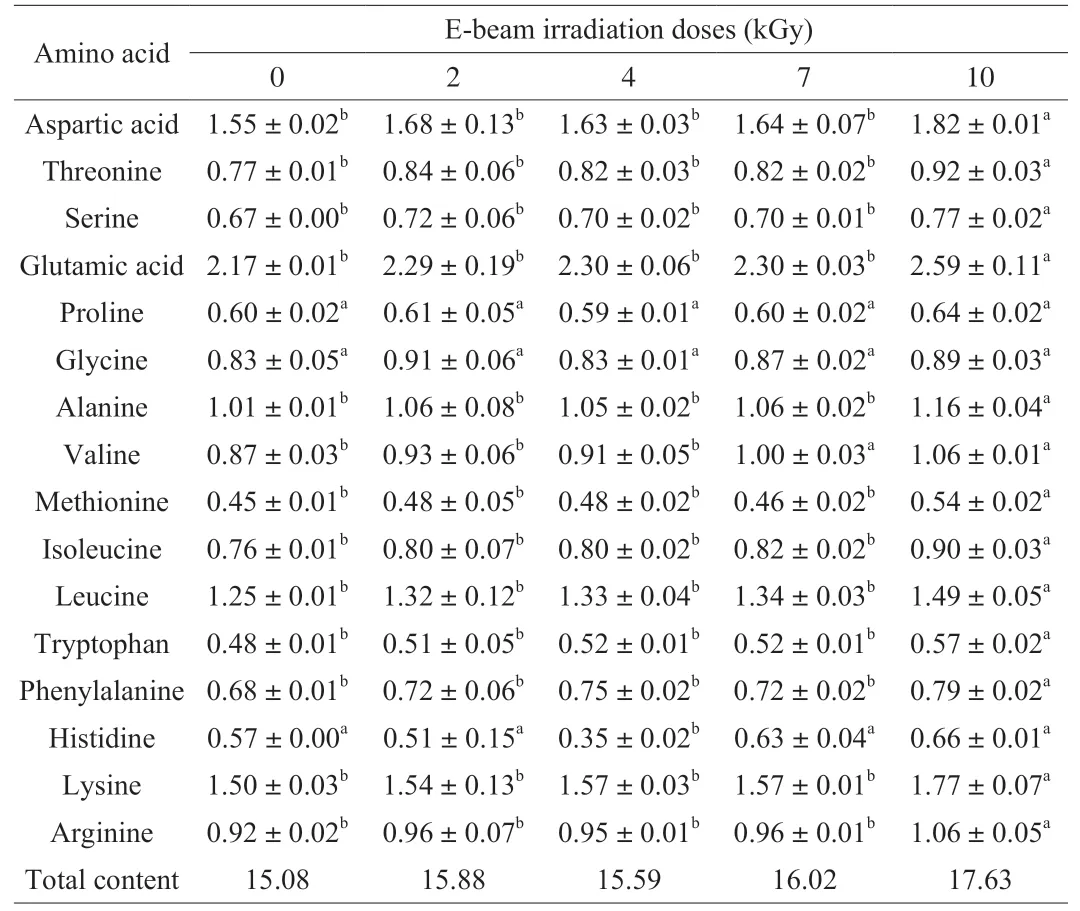
Table 1Amino acid (g/100 g) of salmon under different irradiation doses.
3.3 Determination and content changes of vitamin A,reducing sugars, ash, and moisture
Although vitamin E content remained stable under 4 doses of irradiation (P> 0.05), 10 kGy E-beam irradiation eliminated vitamin A from salmon samples in our experiment (P< 0.05) (Figs. 3A-B).Ash content increased significantly at a dose of 10 kGy, from(1.30 ± 0.00) g/100 g to (2.13 ± 0.20) g/100 g (P< 0.05) (Fig. 3C). After E-beam irradiation, the reducing sugar content and moisture content of the salmon samples remain unchanged (P> 0.05) (Figs. 3D-E).
3.4 Assessment of texture properties and color of salmon
In the experiment, we determined the hardness, elasticity,cohesiveness, and chewiness of E-beam-irradiated salmon to evaluate its textural properties (Table 2). At 7 and 9 kGy irradiation, the hardness of salmon increased significantly to 217.96 ± 28.66 and 279.68 ± 28.11, respectively (P< 0.05), while remaining stable at other doses (P> 0.05). Based on our findings, the change in elasticity was irregular, with the highest value reaching 0.70 ± 0.16 after 7 kGy of irradiation. In the 2-7 kGy irradiated groups, cohesiveness values decreased to 0.40 ± 0.01, 0.36 ± 0.01 and 0.30 ± 0.02, before increasing to 0.45 ± 0.04 in the 10 kGy group. Under 4 irradiated doses, E-beam irradiation significantly increased the chewiness from 21.71 ± 0.59 to 32.73 ± 2.15, 25.97 ±0.33, 45.56 ± 7.01 and 45.50 ± 0.55, respectively (P< 0.05). E-beam irradiation induced decolorization of salmon fillets, which appeared pale throughout,as indicated by the values ofL*,a*, andb* (Table 3).L* value,representing brightness and lightness, has increased significantly from 47.65 ± 8.95 in control to 56.89 ± 5.78, 55.27 ± 2.92, and 64.03 ± 4.57 at 4, 7, and 10 kGy (P< 0.05). Increasing irradiated doses from 2, 4, 7,10 kGy resulted in a decrease ina* value representing the redness of salmon by 40.1%, 67.1%, 76.9%, and 80.7%, respectively (P< 0.05).b* value reflected the yellowness of the salmon muscle and decreased significantly to 23.12 ± 2.27, 21.30 ± 2.25, 22.34 ± 2.25, and 23.66 ± 5.74 under 2, 4, 7, and 10 kGy E-beam irradiation,respectively (P< 0.05). E-beam irradiation significantly decreased the BI of salmon from 173.90 ± 73.14 to 58.81 ± 15.43, 59.10 ± 10.26,52.41 ± 17.66 at 4, 7, and 10 kGy, respectively (P< 0.05). It is evident that irradiation significantly increased the ΔEvalue (P< 0.05).

Table 2Texture properties of salmon under different irradiation doses.

Table 3L*, a*, b* values of salmon under different irradiation doses.
3.5 Sensory evaluation of salmon
Results of sensory evaluation was shown in Fig. 4. The total score for each characteristic (odor, flavor, and texture) was 15.73, 17, 16.09,14.82, and 15.18, respectively. There was no significant difference between the control and irradiated groups in terms of odor, flavor, and texture (P> 0.05), indicating that E-beam irradiation would not result in detrimental effects on sensory qualities.

Fig. 4 Effect of E-beam irradiation on the odor, flavor, and texture of salmon irradiated at doses of 0, 2, 4, 7, and 10 kGy. No significant difference was observed between the control group and the irradiation-treated groups.
4. Discussion
In this study, we investigated the effects of 4 doses of E-beam irradiation, which inactivates SARS-CoV-2, on the nutritional value,texture, color, and sensory qualities of salmon. We found that E-beam irradiation increased TBA value while leaving the fat and fatty acid(SFA, MUFA, PUFA) content unchanged. TBA value reflected the degree of oxidative rancidity, which was determined by the relative malondialdehyde in the second stage [28,29]. Several studies confirmed that TBA content of irradiated foods was always greater than that of unirradiated foods [30,31]. Yang et al. [17] reported that after E-beam irradiation, the TBA value of vacuum-packaged Atlantic salmon increased, which is consistent with our findings. Although vacuum packaging plays a crucial role in maintaining TBA value by isolating oxygen, we speculated that the increase in TBA value after irradiation in salmon was due to residual oxygen after vacuuming [17].In addition, the degree of fat oxidation after irradiation is closely related to the type of irradiated food in terms of their varying oxidative stability. Gecgel [32] found that the increase in TBA value of meatballs was accompanied by a dose-dependent decrease in unsaturated fatty acid and an increase in SFA. In comparison, Javanmard et al. [33] proved that neither 0.75 kGy nor 5.0 kGy irradiation increased the peroxide values of chicken meat. Al-Kahtani et al. [34]discovered that oleic acid and linoleic acid increased after irradiation treatment in tilapia fish, with TBA value increasing dose-dependently.The TBA reaction in food is comprised of two distinct processes,which make for a rather complex course. In our study, even though TBA value of salmon increased significantly, the fat, SFA, MUFA and PUFA content did not change significantly (P > 0.05), indicating that the fat and fatty acids of salmon were highly stable.
Irradiation with a 10 kGy E-beam could also increase the ash and amino acid content while keeping the crude protein and TVB-N content constant. Moreover, 10 kGy of irradiation resulted in a significant decrease in vitamin A, whereas reducing sugar and moisture content were unaffected by E-beam irradiation. Salmon proteins can be broadly categorized as sarcoplasmic, myofibrillar, and stromal, which correspond to the texture characteristics. Similar to our findings, Al-Kahtani et al. [35] discovered that irradiation caused minimal changes in protein and an increase in amino acids in tilapia and Spanish mackerel. Even though irradiation in the experiment did not alter the crude protein content, it was still necessary to confirm the stability of protein properties in further studies for quality assurance. Several studies have also reported increases in amino acids following high dose E-beam irradiation [35-37]. Increased proteolytic enzyme activity, cleavage of peptide bonds, destruction of salmon tissue structure, and possible transformation from other amino acids likely facilitated the extraction of more free amino acids from salmon samples in the experiment [38,39]. The TVB-N content was an indicator of the freshness of salmon, which originated from protein degradation. Tyr, Arg, and Lys, which could produce biogenic amines through decarboxylation, became the key indices for salmon spoilage [36]. At 10 kGy of irradiation, the high concentration of these relevant amino acids might be a possible reason for the slight decrease in TVB-N content. Vitamin E is regarded as both a radiation-sensitive fat-soluble compound and an antioxidant. Some researchers have proposed that vitamin E played a crucial role in protecting vitamin A from irradiation-induced degradation. However, this correlation was not observed in our experiment with irradiated salmon, and vitamin E showed high stability throughout the test. Antonio et al. [40] noted that the high vitamin E content in food samples might be one of the primary reasons for its irradiation stability. In addition, oxygen, water activity, the tested system, and radiation conditions may influence the sensitivity of vitamin E to irradiation [40-42]. Vitamin A was traditionally regarded as one of the most sensitive fat-soluble vitamins to irradiation, as it was especially susceptible to oxidation by air, especially in light and high humidity [42]. Under irradiation doses ≤ 7 kGy, vitamin A in salmon samples remained stable, whereas irradiation at 10 kGy caused vitamin A to disappear completely.The results indicated that vitamin A was less stable when irradiated at higher doses than lower ones. Mameesh et al. [43] studied the changes in vitamin A and α-tocopherol content of dogfish (Squalus acanthias) fillet under 3.0 and 0.3 Mrad irradiation and found that vitamin A was unaffected by 0.3 Mrad but was halved by 3.0 Mrad,whereas α-tocopherol was unaffected by both doses of irradiation,similar to our findings. Hata et al. [44] observed that vitamin A was severely degraded at the highest irradiation doses of 1.88 Mrad. In future research, a greater emphasis should be placed on vitamin A stability following E-beam irradiation, particularly at high doses. The presence of minerals in salmon tissue was reflected in the ash content.According to Huque et al. [45], the ash content of dried Chepa (Puntius stigma), Loitta, and Chingri did not change significantly after 3 and 5 kGy irradiation. The high stability of ash under irradiated treatment ≤ 7 kGy was confirmed by our experiment. Moreover,we first reported a significant increase in ash content when salmon was irradiated with 10 kGy E-beam. The significant increase in ash content at the highest irradiation dose may be due to an increase in the oxidized matter. Moisture content has been considered one of the most important parameters for food storage, preservation, processing,and quality. Similar to our findings, Yang et al. [17] also discovered no significant difference in the moisture content of salmon samples between the control and 2-7 kGy irradiated groups.
Following irradiation, the hardness and chewiness of salmon were significantly increased. Under irradiation, salmon samples became brighter and whiter, accompanied by a decrease in BI and an increase in ΔE value. Montiel et al. [46] and Lv et al. [47] also reported an increase in the hardness of salmon following irradiation.Muscle compaction resulted from the tighter packing of amino acids during the pressure denaturation process, and an actin-myosin closer interaction induced by E-beam irradiation may account for the change [46]. Ca2+ions from the sarcoplasmic reticulum were less released by irradiation, resulting in a decrease in the activity of calcium-activated proteolytic enzymes, which may cause the salmon samples to become harder [48]. Lv et al. [47] and Hultmann et al. [48]reached similar conclusions regarding the resilience and chewiness of salmon subjected to various irradiation doses. In contrast to the previous study, the cohesiveness of salmon in the present work was not affected by irradiation [47]. The difference may result from the two studies’ different sampling locations. Changes in L*, a* and b*values of irradiated salmon in the test were consistent with several previously published studies. It has been revealed that irradiating salmon steaks with 2, 3, or 10 kGy causes a distinct loss of color [49].McKenna et al. [50] compared the least squares means for L*, a*,and b* values of salmon fillets irradiated by 0, 3, 6 kGy E-beam and found that L* value increased while a* and b* values decreased.The decrease in a* value after E-beam irradiation accounted for the majority of the observed color change in the present study, which also reflected the observed color damage from typical cherry-red to beige-white. Salmon fillets have a pink hue due to carotenoids and haem pigments. Astaxanthin was the predominant carotenoid found in salmon, providing the characteristic orange hue. Yagiz et al. [51]concluded that the increasing irradiated dose level resulted in the linear decrease ofa* value and astaxanthin in salmon under the same conditions. Desmonts et al. [52] reported that the reduction ina*andb*values was due to the degradation of carotenoid, as astaxanthin lost 64% andβ-carotene lost 73% following irradiation. Snauwaert [53]also found that 3 kGy irradiation caused a significant carotenoid loss in fresh salmon. Accordingly, it is speculated that the decrease ina*value in salmon samples is due to the loss of carotenoids,particularly astaxanthin, which is predominantly deposited in the flesh of salmon in a form that is sensitive to photo-oxidation. Furthermore,astaxanthin is known for its exceptional antioxidant capacity, which can neutralize irradiation-generated free radicals. The decrease of astaxanthin in salmon likely contributed to the oxidation of lipids,which corresponded to the rise in TBA levels in our study.
No significant differences in the sensory evaluation were observed between irradiated and unirradiated salmon, indicating that E-beam irradiation has a lesser impact on sensory quality than on nutrition and texture. Annamalai et al. [54] proposed similar results to ours,namely that irradiated cookedLitopenaeus vannameiat the dose of 2.5-10 kGy did not exhibit any sensory changes. Badr [55] also observed that all cold-smoked salmon were acceptable, and there were no significant changes in sensory properties such as odor, flavor and texture of salmon after 0-4 kGy gamma irradiation. However, the overall acceptability of irradiated salmon may change during storage due to lipid oxidation, which may lead to an unpleasant foreign odor.
5. Conclusion
The present study showed that E-beam irradiation altered the nutritional and physicochemical characteristics of salmon. Irradiation at doses of 2-10 kGy has stimulated lipid oxidation, resulting in an increase in TBA value. In addition, 10 kGy of irradiation significantly increases the content of free amino acids. Vitamin A content was markedly decreased by 10 kGy irradiation. Besides,the hardness and chewiness of salmon were greater in irradiated groups than in unirradiated group, while resilience and cohesiveness varied irregularly across irradiation doses. Salmon color changed
dramatically after irradiation, with a significant increase inL* value and a significant decrease ina* andb* value, which was most likely due to astaxanthin degradation. Despite several changes caused by irradiation, sensory qualities such as odor, flavor, and texture in salmon remained stable in our study. In conclusion, we recommended 2 kGy of irradiation for SARS-CoV-2 inactivation in salmon while maintaining quality for future industrial applications.
Conflicts of interest
The authors declare no conflict of interest.
Acknowledgement
The researchers gratefully acknowledge the grants from Hangzhou Global Scientific and Technological Innovation Center of Zhejiang University (KC2021ZY0B0003).
- 食品科学与人类健康(英文)的其它文章
- Emerging natural hemp seed proteins and their functions for nutraceutical applications
- A narrative review on inhibitory effects of edible mushrooms against malaria and tuberculosis-the world’s deadliest diseases
- Modulatory effects of Lactiplantibacillus plantarum on chronic metabolic diseases
- The role of f lavonoids in mitigating food originated heterocyclic aromatic amines that concerns human wellness
- The hypoglycemic potential of phenolics from functional foods and their mechanisms
- Insights on the molecular mechanism of neuroprotection exerted by edible bird’s nest and its bioactive constituents

