Effect of Zeolite Crystal Size on the Catalytic Performance of Zn/ZSM-5 Zeolite for Olefin Conversion
YU Mengnan,PAN Tao,JU Ya′na,ZHANG Ran,WU Pei,WU Zhijie
(1.State Key Laboratory of Heavy Oil Processing and the Key Laboratory of Catalysis of CNPC,China University of Petroleum, Beijing 102249,China;2.Petrochemical Research Institute,PetroChina,Beijing 100195,China)
Abstract:Converting olefins in FCC gasoline into high-octane aromatics or iso-alkanes is an effective approach to produce clean gasoline in line with China Ⅵ vehicle emission standard. Zn-modified ZSM-5 (Zn/ZSM-5)zeolite has been regarded as the best catalyst for olefin aromatization. The structure-activity relationship between the acid properties of ZSM-5 zeolites with different crystal sizes and the selective conversion of olefins was systematically explored by adjusting the crystal size of zeolite. The XRD,SEM,TEM,UV-vis,XPS,FT-IR and NH3-TPD characterization results show that the crystal size of ZSM-5 has an influence on the distribution of acid sites inside and outside the zeolite,and Zn/ZSM-5 zeolite with the smallest crystal size of 100—200 nm has the highest relative content of ZnOH+ active species (57% of all Zn species)and a lower ratio of Brønsted acid to Lewis acid (AB/AL=0.15). At the reaction temperature of 350 ℃,liquid volume space velocity (LHSV)of 1.5 h-1,volume ratio of H2(g)to 1-hexene(1)of 600,and hydrogen pressures of 0.1 and 2.0 MPa,the Zn/ZSM-5 zeolite catalyst exhibits a high aromatics selectivity (17.2%)and a high iso-alkane selectivity (50.0%)in the hydro-conversion of 1-hexene,respectively. The data indicate that the selective conversion of olefins in FCC gasoline into high-octane components can be promoted by reducing the crystal size of ZSM-5 zeolite.
Key words:ZSM-5 zeolite;olefin;aromatization;gasoline;acid catalysis
Reducing olefin mass fraction in gasoline by 15% has been included in China Ⅵ standard gasoline,in which the hydrocarbon and NOxemissions can be reduced by 6%[1-2]. The selective conversion of olefins to aromatics oriso-alkanes with a high octane number has been regarded as one of the best routes to reduce olefin content and recover octane number of FCC gasoline. Zn modified ZSM-5 (Zn/ZSM-5)zeolite based catalysts are frequently reported because of its characteristics of high aromatization activity and selectivity.
The incorporation of Zn into ZSM-5 zeolite micropore can react Brønsted (B)acid site and turn it to Lewis (L)acid (ZnOH+),in which L acid sites enroll in the dehydrogenation reaction and hydrogen transfer reaction for olefin aromatization[3-4]. However,the good capability of hydrogen transfer of Zn/ZSM-5 zeolite also leads to the formation of polycyclic aromatics and then deactivation by coking. Thus,ZSM-5 zeolite with small crystal size has been proposed to overcome the problem,in which the diffusion of reactants and products are improved by reducing diffusion distance within zeolite and the external surface area of zeolite is promoted for coke deposition.
For the Zn modified zeolite obtained by impregnation,a small zeolite crystal size makes more Zn species access to B acid sites,leading to more metal L acid (ZnOH+)sites[5]. Thus,the relation between reactivity and stability of Zn modified zeolite and crystal size is complex and variable by the change of texture properties,acid types and amounts. Comparing with the Zn/ZSM-5 with different crystal size (250—2000 nm),Niu et al.[6]found that the sample with the smallest crystal size possessed most active metal species for aromatization. Li et al.[7]found that ZSM-5 zeolite with intra-crystalline mesopores created by alkali treatment could promote the distribution of Zn species and reacted with B acid sites into L acid (ZnOH+)sites for aromatization. Clearly,the access of B acid sites to Zn species is the key to control aromatization over Zn/ZSM-5. Moreover,our previous work showed that ratio of B/L acid sites,fractions of B acid sites within micropore and strong acid sites were sensitive to the zeolite crystal size,in which the size screening of ZSM-5 zeolites for methanol to propylene was highly required to realize high propylene selectivity and reaction stability[8]. Here,we attempted to clarify the effect of zeolite crystal size on the olefin conversion over Zn/ZSM-5 zeolite for olefin reduction in FCC gasoline via hydro-upgrading treatment. 1-Hexene,the typical olefin component in heavy FCC gasoline from PetroChina,was used as probe to screen suitable zeolite crystal size for Zn/ZSM-5. The relation between zeolite crystal size and olefin aromatization/isomerization performance was focused.
1 Experiment
1.1 Materials
Sodium aluminate (NaAlO2,45% Al2O3),pseudo boehmite (AlOOH·nH2O,65% Al2O3),and zinc nitrate hexahydrate (Zn(NO3)2·6H2O,99%)were purchased from Tianjin Kermel Chemical Reagent Co. Ltd. Silica gel (0.075—0.150 mm, 99% SiO2)was purchased from Qingdao Haiyang Chemical Co. Ltd. Sodium hydroxide (NaOH,99%)and ammonium chloride (NH4Cl,>99% purity)were purchased from Sinopharm Chemical Reagent Co. Ltd. 2,6-di-tert-butylpyridine (C8H18O2,DTBP,98%),pyridine (C5H5N,98%)and cobalt nitrate (Co(NO3)2·6H2O,99%)were purchased from Alfa Aesar. Tetrapropylammonium bromide (C12H28BrN,98%)and 1-hexene (C6H12,98%)were purchased from Aladdin Reagent (Shanghai)Co. Ltd. All samples were used as received.
1.2 Synthesis of zeolite
Three ZSM-5 zeolites with a silicate-alumino gel composition ofn(SiO2)∶n(Al2O3)∶n(H2O)∶n(Na2O)∶n(TPABr)=100∶2∶823∶9∶9 were prepared by a two-step crystallization method,respectively[8]. ZSM-5A was obtained by nucleation (pre-crystallization)at 100 ℃ for 36 h and then crystallization at 170 ℃ for 24 h. ZSM-5B was prepared by nucleation (pre-crystallization)at 100 ℃ for 12 h and then crystallization at 170 ℃ for 24 h. ZSM-5C was obtained by the direct crystallization at 24 h. The commercial ZSM-5 zeolite from PetroChina with a molar ratio of SiO2/Al2O3at 60 was used as reference and recorded as ZSM-5D. Zn/ZSM-5 zeolite was prepared by wetness impregnation with 3.2% theoretical ZnO loading.
1.3 Characterization
The element composition of sample was analyzed by X-ray fluorescence (XRF)of PANalytical AxiosMAX. The structure of zeolite was characterized by Bruker D8 Advance Powder X-ray diffractometer with Cu-Kαradiation (λ=0.15418 nm)and recorded in the 2θrange of 5° to 50° at 5°/min scanning speed. The surface analysis of zeolites was characterized by the X-ray photoelectron spectroscopy (XPS)with a Fluorolog-Tau-3 (ISA-USA),and the binding energies were calibrated referring to C 1s peak at 284.6 eV. Scanning electron micrograph (SEM)images were collected on GeminiSEM 300 at 5—20 kV. The transmission electron micrograph used FEI Tecnai G2 F20 with an acceleration voltage of 200 kV. The UV-Vis spectra were collected on UV-1800-SPC ultraviolet spectrometer of Shanghai Meiji instrument Co. Ltd.,and recorded in the range of 200—800 nm.
Nitrogen adsorption-desorption measurements used on Micromeritics ASAP 2020 multi-function adsorption instrument. The specific surface area and the pore size distribution were calculated by Brunauer-Emmett-Teller (BET)and Barrett-Joyner-Halenda (BJH)method,respectively.
The temperature-programmed desorption of ammonia (NH3-TPD)was performed on a Huasi DAS-7000 chemisorption analyzer. The catalyst of 0.1 g was treated at 600 ℃ with flowing N2gas stream (30 mL/min)for 30 min,then cooled to 100 ℃,and the NH3gas stream (30 mL/min)was switched for 0.5 h. Finally,after switching back to N2(30 mL/min)atmosphere for 30 min,the amount of desorbed NH3was recorded by a thermal conductivity detector (TCD)with the temperature increased from 100 ℃ to 600 ℃ at 10 ℃/min ramping rate.
The Fourier Transform infrared spectra of pyridine (FTIR-Py)was collected on a Bruker Tensor Ⅱ equipped with a liquid-nitrogen cooled mercury-cadmium-telluride (MCT)detector. The wafer samples were pretreated at 10-3Pa and 350 ℃ for 1 h. Then the temperature dropped to 100 ℃ to adsorb pyridine for 30 min,then degassed for 30 min. Finally,the pyridine was desorbed by rising temperature and the spectra were recorded at 200 ℃ and 350 ℃,respectively.
1.4 Acid titration experiment
The 2,6-di-tert-butylpyridine (DTBP)titration experiment was carried out in a fixed-bed reactor of Beijing Kunlun Yongtai Technology Co. Ltd. In the reaction of dehydration of methanol to dimethyl ether (DME),adding DTBP to calculate the content of B acid on the pore mouth and external surface since the bulky DTBP molecule has limited accessibility to B acid sites located in micropores of zeolites[9-10]. The fraction of B acid sites on the pore mouth and external surface (fsum)was calculated by the conversion of methanol before (x1)and after (x2) DTBP titration (formula (1)).
(1)
1.5 Catalytic reaction
The selective conversion of 1-hexene was performed in a fixed-bed reactor,with the stainless-steel reaction tube (9 mm ID,450 mm length),under 2 MPa or 0.1 MPa hydrogen pressure,respectively. 1-Hexene and H2were pumped into a reactor at 350 ℃ and with liquid volume space velocity (LHSV)of 1.5 h-1,and the volume ratio of H2to 1-hexene was set to 600. The products were analyzed by gas chromatography (GC-7820),connected with HP-PONA column (50 m×0.2 mm×0.5 μm). The conversion of the reactants (x,%)and the mass selectivity of the products (s,%)were calculated according to the formula (2)and (3).
(2)
(3)
w0,inandw0,out(%)were the mass fraction of the reactant at the inlet and outlet,respectively,andwi(%)represented the mass fraction of a product.
The hydrogen transfer index (HTI)was defined as formula (4).
(4)

2 Results and discussions
2.1 Structure and texture properties of zeolites
Fig.1 shows the XRD patterns of ZSM-5 and Zn/ZSM-5 zeolites. All samples only show MFI topological structure characteristic diffraction peaks, indicating that ZSM-5 zeolites were successfully synthesized. The disappearance of characteristic diffraction peaks of ZnO at 31.8° and 36.3° may suggest the good dispersion of Zn species[11]. Similar crystallinity,bulk ratio of Si/Al and ZnO loading were obtained among these Zn/ZSM-5 zeolites (Table 1).
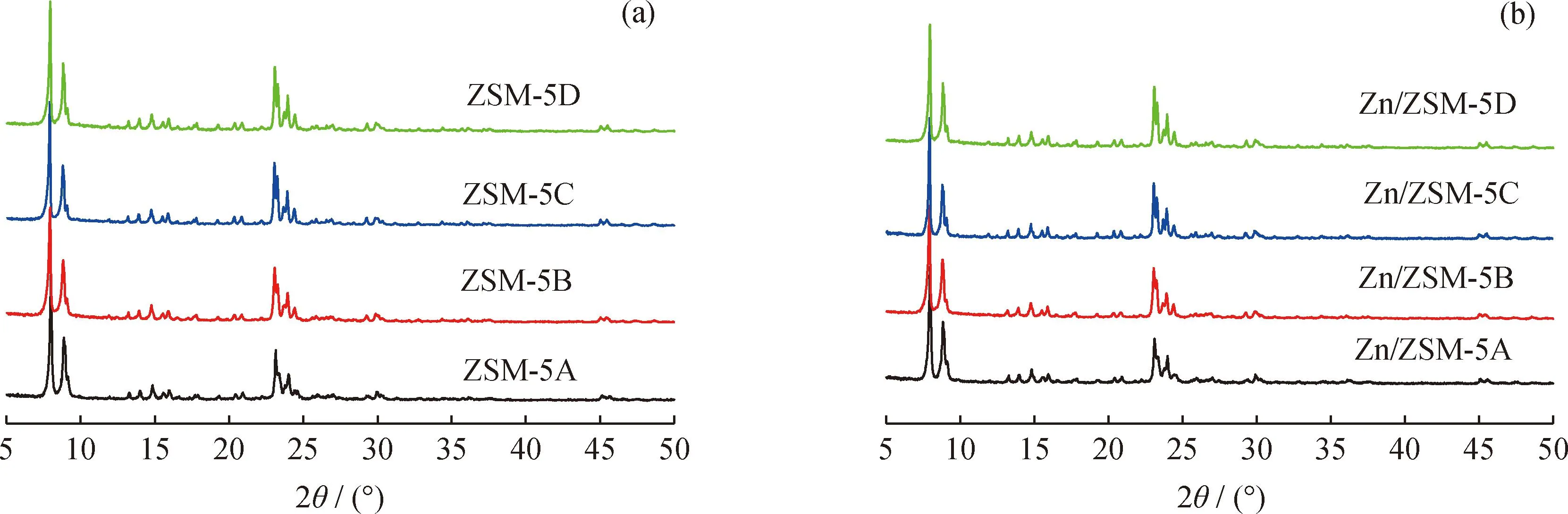
Fig.1 XRD patterns of ZSM-5 and Zn/ZSM-5 zeolites(a)ZSM-5;(b)Zn/ZSM-5
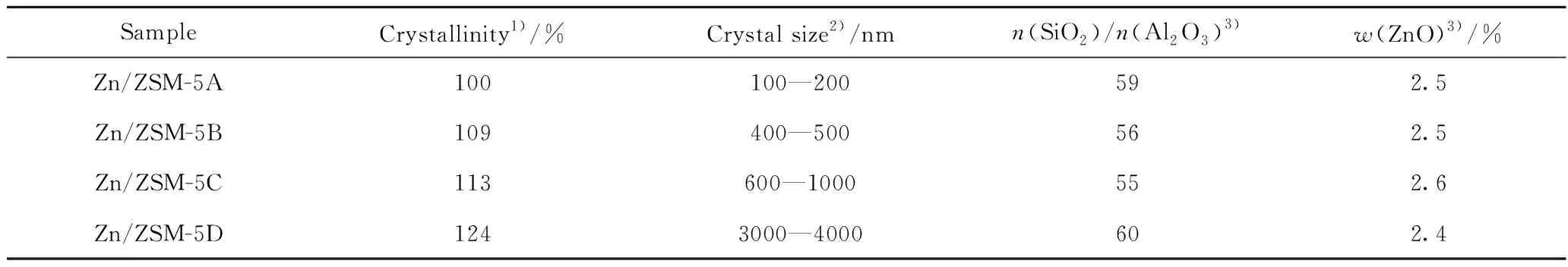
Table 1 Crystallinity,crystal size and composition of Zn/ZSM-5 zeolites
Fig.2 shows the SEM and TEM images of Zn/ZSM-5 zeolite samples. Zn/ZSM-5A and Zn/ZSM-5B are characteristic of crystal assembly morphology with 100—200 nm and 400—500 nm,respectively. Some inter-crystalline mesopores are randomly observed in the TEM images of Zn/ZSM-5A and Zn/ZSM-5B zeolites,agreed with our previous work[8]. Zn/ZSM-5C and Zn/ZSM-5D zeolites show clear crystal boundary and large crystal size at 600—1000 nm and 3000—4000 nm respectively. The increase of crystal size is agreed with the crystallinity shown in Table 1,in which the relative crystallinity of Zn/ZSM-5 zeolite increases from 100% to 124% with the crystal size from 100—200 nm to 3000—4000 nm.

Fig.2 SEM and TEM images of Zn/ZSM-5 zeolites(a),(e)Zn/ZSM-5A;(b),(f)Zn/ZSM-5B;(c),(g)Zn/ZSM-5C;(d),(h)Zn/ZSM-5D
Fig.3 shows the N2sorption curve and pore size distribution of Zn/ZSM-5 zeolite with different crystal sizes. N2adsorption volume of all zeolites increases sharply with the increase of relative adsorption pressure in the range ofp/p0from 0 to 0.2. This,indicates the presence of microporous structures in zeolites. Zn/ZSM-5A and Zn/ZSM-5B zeolites show obvious hysteretic structure withp/p0at 0.45—1.00,characteristic of mesopore structure. All zeolite samples show 4 nm mesopore in their pore size distribution diagrams,which is due to the capillary phenomenon reported by Groen et al.[12-13]. Table 2 shows the texture properties of Zn/ZSM-5 samples. Zn/ZSM-5A zeolite shows the largest mesoporous size range with 20—30 nm,this is agreed with its smallest crystal size. The external surface area and mesoporous volume decreases with increasing zeolite crystal size.
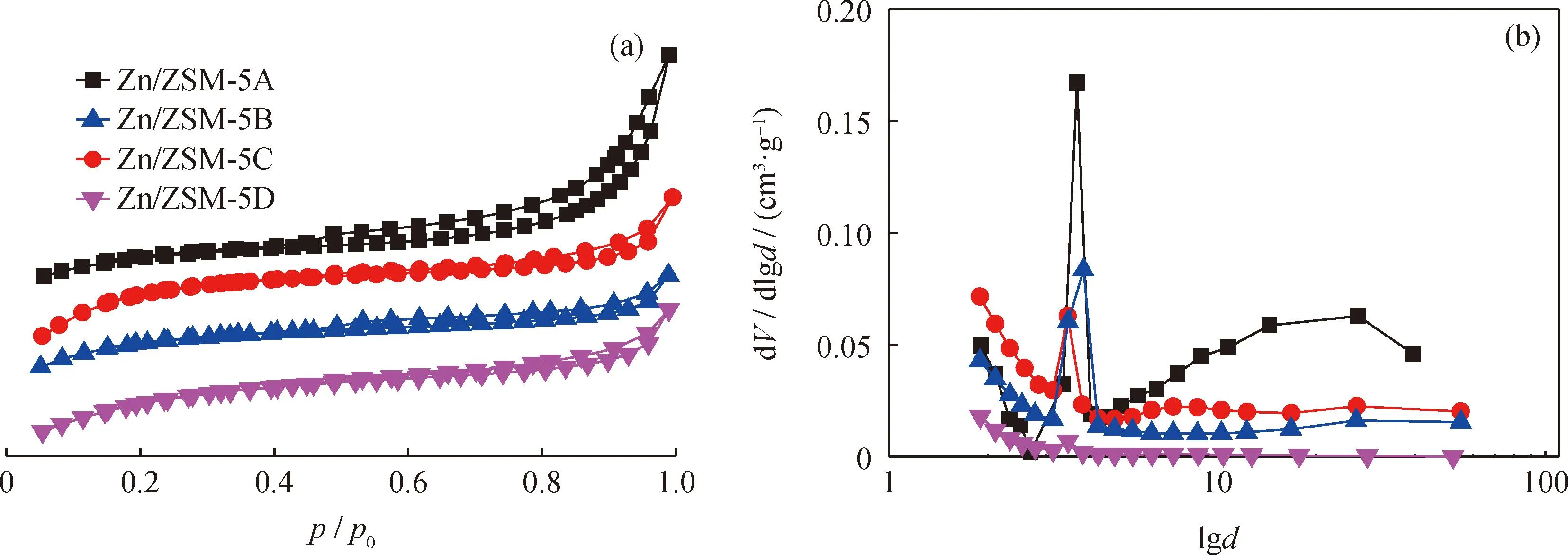
Fig.3 N2 adsorption-desorption isotherms and pore size distribution of Zn/ZSM-5 zeolites(a)N2 adsorption-desorption isotherms;(b)Pore size distribution
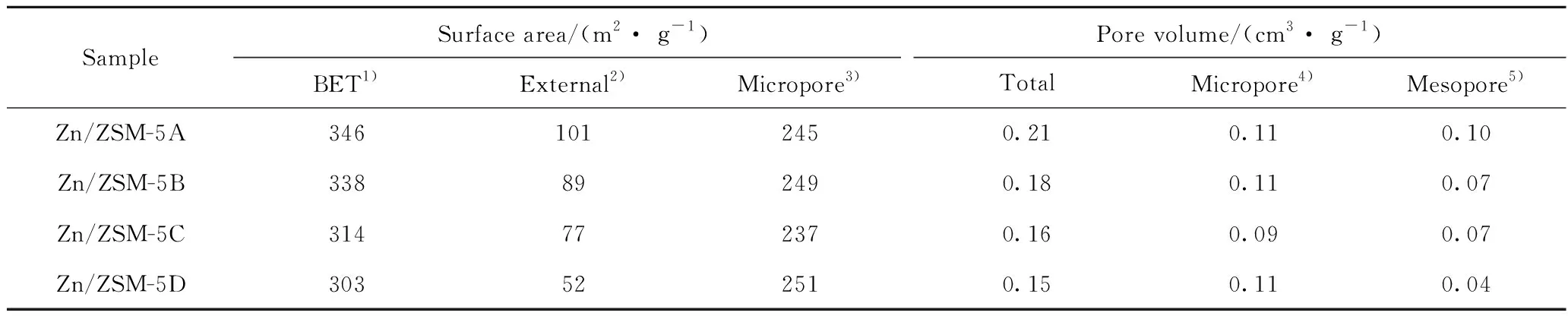
Table 2 Texture properties of Zn/ZSM-5 zeolites
2.2 Al distribution in ZSM-5 zeolite
The distribution and location of framework Al in zeolite is sensitive to catalytic performance of zeolite[14]. Al-O-(Si-O)n-Al (n≤2)denoted as Alpairand Al-O-(Si-O)n-Al (n≥3)denoted as Alsinglehave been proposed to distinguish the Al distribution[15]. Three types of Alpaircan be distinguished in ZSM-5 zeolite:α,βandγtypes due to the location in the straight channel,intersection channel,and sinusoidal channel,respectively[16]. Mlinar et al.[17]proposed that the Alpairin ZSM-5 zeolite is the active sites for propylene dimerization. Sazama et al.[18]found the adjacent Alpairpromotes hydrogen transfer reaction and isolated Alsingleis inclined to cracking reaction. In addition,the distribution of Al on the pore also affects the conversion of hydrocarbons on ZSM-5 zeolites. Janda et al.[19]found that cracking reaction is favorable toαAlpairandγAlpair,while dehydrogenation is more favorable forβAlpair. Liu et al.[20]showed that Alpairwas beneficial to the conversion of olefins to aromatics on ZSM-5. Thus,to compare the aromatization performance of as-prepared Zn/ZSM-5 zeolites,the Al distribution should be firstly distinguished.
For ZSM-5 zeolite,Co2+ion introduced by ion-exchanging treatment can share its balance with A1pairand make the identification of Alpairvia UV-Vis characterization. Fig.4 shows all ZSM-5 zeolites exhibit a wide absorption peak in the range of 14000—24000 cm-1,which corresponds to the d-d transition of Co (Ⅱ)cations at different sites. These sites and their numbers are obtained by deconvolution of the UV-Vis spectra. The peak at 15100 cm-1representsα-type cations (Co2+ions interacting withα-type Alpair),and the peaks at 16000 cm-1corresponding toβ-type cations (Co2+ions interacting withβ-type Alpair). The transition absorption peaks ofγ-type cations (Co2+ions interacting withγ-type Alpair)are at 20100 and 22000 cm-1[20-21]. The contents of these sites are listed in Table 3. Combined with the bulk composition of Al elements from XRF results,the fraction of Alsingleand Alpairin the zeolite framework was calculated. All ZSM-5 zeolite samples show a high fraction of Alsingle(>65%),and similar contents of Alsingleand Alpair. All ZSM-5 zeolites show a high fraction ofβ-type Alpair(>80%),indicating that the Alpairin all ZSM-5 zeolite samples are mostly located in the position of intersection channel. In addition,the similar distribution ofα,βandγtype Alpairalso shows that the distribution of Alpairin ZSM-5 zeolites is basically the same. Thus,the interfere of the Al location on the aromatization performance over these as-prepared Zn/ZSM-5 zeolite can be excluded.

Table 3 Distribution of framework Al in Co-exchanged ZSM-5 zeolites
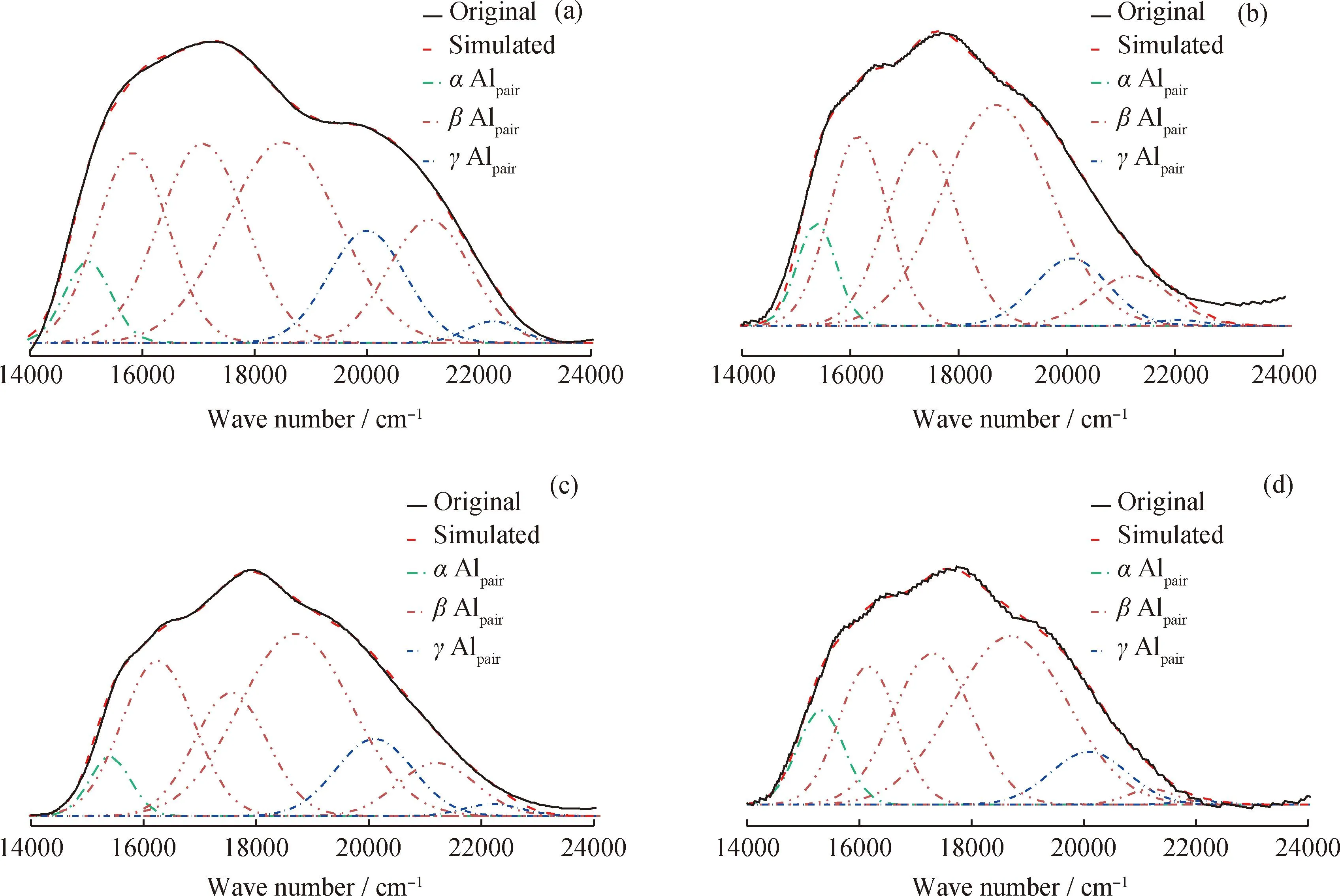
Fig.4 UV-Vis spectra of Co-exchanged ZSM-5 zeolites(a)Co/ZSM-5A;(b)Co/ZSM-5B;(c)Co/ZSM-5C;(d)Co/ZSM-5D
2.3 Zn species in Zn/ZSM-5 zeolite
The surface properties of Zn species were analyzed by XPS spectra. As shown in Fig.5,two Zn species with binding energies at 1021.8 and 1022.8 eV are distinguished to ZnO and ZnOH+species,respectively[22]. A higher binding energy of ZnOH+species is due to the strong interaction between Zn species and zeolite framework Al. Table 4 shows that with the increase of the crystal size of ZSM-5 zeolite from 100—200 nm to 3000—4000 nm the proportion of ZnO gradually increases from 43.0% to 64.1%,while ZnOH+fraction gradually decreases from 57.0% to 30.9%. The results show that smaller crystals are beneficial to the formation of ZnOH+species. This is agreed with the DTBP titration results that more B acid sites at the external surface and open mouth of zeolite are formed in ZSM-5 with small crystal size (Table 6). Then,ZSM-5 zeolite with small crystal size possesses more accessibility of Zn to B acid sites,thus increasing the formation of ZnOH+active species. It can be also found that the small crystal has a relatively higher surface content of Zn species,and the highest value at 5.5% of 100—200 nm Zn/ZSM-5A is observed (Table 4).

Fig.5 XPS spectra of Zn 2p3/2 of Zn/ZSM-5 zeolites

Table 4 Zn species distribution of Zn/ZSM-5 zeolites
Fig.6 shows the FT-IR spectra of hydroxyl over ZSM-5 and Zn/ZSM-5 zeolites. All ZSM-5 zeolites have obvious absorption peaks at 3609 cm-1, corresponding to B acid site from framework Al (Si-O(H)-Al) of ZSM-5 zeolites. The other absorption peak at the higher wave number 3742 cm-1represents the terminal Si-OH at the external surface of zeolite[23-24]. ZSM-5A shows such absorption peak,whose intensity is much higher than other ZSM-5 zeolite with larger crystal size. This is agreed that the Si-OH are generally formed at the external surface of zeolite,the smaller crystal size and the higher content of Si-OH is realized. In addition,internal Si-OH nest formed by the internal defect site of ZSM-5 zeolite is usually observed at peak 3500 cm-1[25]. Here,no internal Si-OH on all ZSM-5 zeolites suggests few defects within zeolite crystals in consistent with the TEM characterization.

Fig.6 FT-IR spectra of hydroxyl groups for ZSM-5 and Zn/ZSM-5 zeolites(a)ZSM-5;(b)Zn/ZSM-5
After the deposition of Zn species onto ZSM-5 zeolite,the intensity of absorption peak due to Si-O(H)-Al peak obviously decreased,and a new absorption peak at 3673 cm-1can be found. This is assigned to the hydroxyl groups originated from L acid sites (ZnOH+species)[26]. The change of absorption peak intensity can be explained by that ZnOH+species is formed via the interaction of Zn cations with Si-O(H)-Al and turn to L acid sites (Si-O[Zn(OH)+]-Al). Among all Zn/ZSM-5 zeolites,a high intensity of absorption peak of Si-O(H)-Al but a low intensity of peak due to ZnOH+sites is observed on the sample with large zeolite crystal. This means that reducing zeolite crystal size is beneficial to the formation of ZnOH+species by the interaction of Zn cations with framework Al,which is consistent with the results obtained by XPS. Again,the absorption peak due to framework Si-O(H)-Al shows a weak shift from 3609 to 3614 cm-1after Zn introduction,this should be due to the fact that Zn cations preferentially react with strong B acid sites,rendering relatively weaker B acid sites. On the other hand,the peak due to terminal Si-OH shows little change after Zn introduction,indicating no obvious interaction between Zn cations and Si-OH.
2.4 Acid property
The acid content and acid strength of zeolite was obtained by NH3-TPD (Fig.7)and the B/L acid amount ratio(AB/AL)was obtained by Py-IR (Fig.8). Three desorption temperatures of NH3-TPD spectra over all ZSM-5 and Zn/ZSM-5 zeolites were observed at the range of 200—220 ℃,300—320 ℃ and 420—440 ℃,assigning to weak,medium and strong acid sites,respectively[27]. All ZSM-5 zeolites have almost the same total acid content (0.40—0.44 mmol/g)owing to close ratio of SiO2/Al2O3. However,the acid strength varied with zeolite crystal size. Table 5 shows that the desorption temperature corresponding to strong acid sites increases from 424 ℃ to 431 ℃ with the growth zeolite crystal size. This should be due to the confinement effect of micropores,in which the strong acid amount also increases gradually from 0.13 to 0.19 mmol/g.
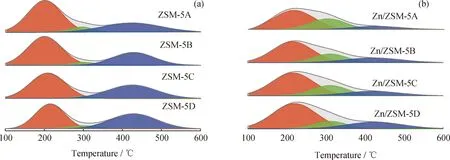
Fig.7 NH3-TPD profiles of ZSM-5 and Zn/ZSM-5 zeolites(a)ZSM-5;(b)Zn/ZSM-5
Fig.8 shows that the intensity of strong B acid absorption peak of ZSM-5 zeolite increases slightly with zeolite crystal size,while decreases for L acid sites,resulting in the growth of the B/L acid amount ratio. NH3-TPD and Py-IR spectra together indicate that a small crystal size of ZSM-5 zeolite is beneficial to weaking acid strength,reduce the ratio of B/L acid sites and increase the fraction of medium strong acid sites. After the deposition of Zn species,the amount of strong acid of Zn/ZSM-5 zeolite decreased,while the amount of medium strong acid increased (Fig.7). Such change should be attributed to that the amount of B acid sites decreases while the amount of L acid site increases (Fig.8). The absorption peak due to L acid sites also shows a red shift from 1450 cm-1to 1455 cm-1,indicating the formation of new L acid sites (ZnOH+). Comparing to NH3-TPD spectra of bulk ZSM-5,the desorption peak temperature of strong acid sites over all Zn/ZSM-5 zeolites decreased,indicating prior interaction between strong B acid sites with Zn cations,agreeing with the results of XPS and FT-IR spectra (Fig.5 and Fig.6).
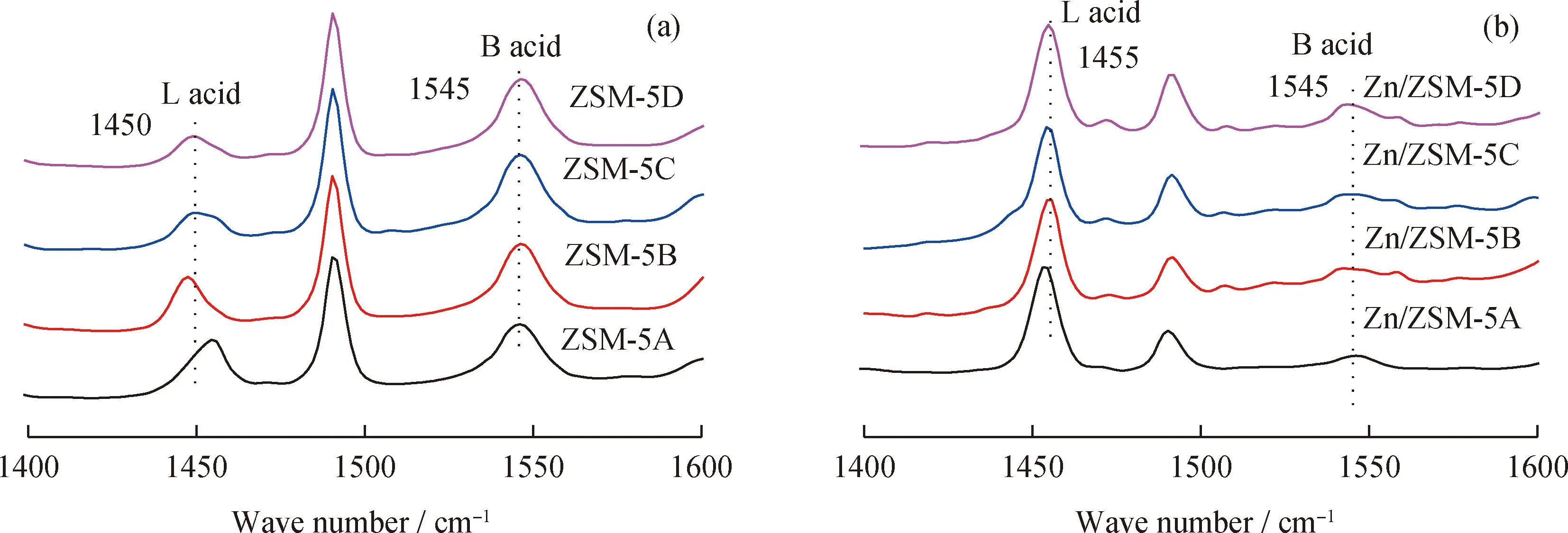
Fig.8 Py-IR spectra of the ZSM-5 and Zn/ZSM-5 zeolites(a)ZSM-5;(b)Zn/ZSM-5
Table 5 shows that the reduction of crystal size can significantly reduce the amount of B acid site from 0.09 to 0.05 mmol/g and the ratio of B/L acid sites from 0.30 to 0.15,and increase the amount and fraction of medium-strong acid from 0.06 to 0.11 mmol/g and 15.1% to 29.6% of Zn/ZSM-5 zeolite,respectively. Zn/ZSM-5A zeolite with the smallest crystal size shows the lowest B/L acid amount ratio at 0.15 and the highest fraction of medium-strong acid at 29.6%.
In addition to the acid amount and strength,the acid site distribution of zeolite is also sensitive to catalytic performance. Table 6 shows the amount and fraction of B acid sites within and outside ZSM-5 and Zn/ZSM-5 zeolite via DTBP titration. With the increase of crystal size,the fraction of B acid sites on the pore mouth and external surface (fsum)of ZSM-5 zeolite decreases gradually from 55.5% to 18.0%,indicating that the small crystal is beneficial for the access of B acid site to reactants. After the impregnation with Zn cations,fsumobviously decreased,suggesting that the consuming surface B acid sites by the interaction between Zn and framework Al. Zn/ZSM-5A zeolite with the smallest crystal size shows the highestfsum,at 44.5%.

Table 5 Acid properties of ZSM-5 and Zn/ZSM-5 zeolites
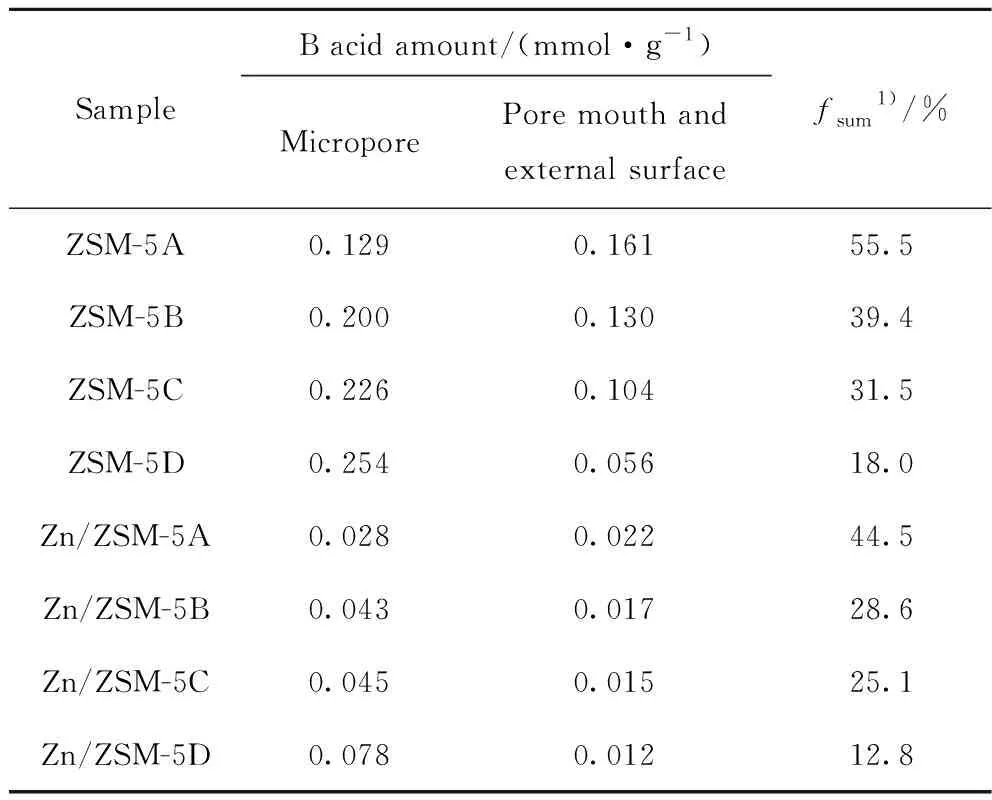
Table 6 Acid sites distribution of ZSM-5 and Zn/ZSM-5 zeolites
2.5 Catalytic performance
Table 7 shows the selective distribution of products for the reaction of 1-hexene catalyzed by ZSM-5 and Zn/ZSM-5 zeolites with different crystal size under 2.0 MPa hydrogen pressure. All samples show a fully complete conversion of 1-hexene and a high selectivity of alkanes (>97%),but a low aromatics selectivity (<3%)using a high H2pressure for olefin conversion. Bulk ZSM-5 zeolite samples show high selectivity of light paraffin (C1—C3selectivity at 48.8%—50.8%)owing to the cracking over excess B acid sites. With the increase of ZSM-5 zeolite crystal size,the selectivity ofiC4—iC4+alkanes decreases whilenC4—nC4+alkane selectivity increases,leading to the decrease of mass ratio ofiso-alkane ton-alkane from 0.43 to 0.37. Clearly,the small ZSM-5 zeolite crystal is good for isomerization.
After the introduction of Zn cations,the selectivity ofnC4—nC4+alkanes and aromatics in Zn/ZSM-5 zeolite changes a bit,but the selectivity of C1—C3alkanes significantly decreases from 48.8%—50.8% to 26.2%—28.4% and the selectivity ofiC4—iC4+alkanes greatly increases from 26.5%—29.3% to 43.6%—50.0%,respectively. These results show that Zn modification promotes the isomerization selectivity and inhibits cracking. The introduction of Zn greatly reduces the amount and strength of B acid sites in ZSM-5 zeolite and produces a large amount of medium strong L acid (ZnOH+).

Table 7 Product distribution of ZSM-5 and Zn/ZSM-5 zeolites at hydrogen pressure of 2.0 MPa
Corado et al.[28]and Corma et al.[29]have shown that both L acid and B acid can carry out isomerization reaction and the acid strength is the important factor determining isomerization performance, in which medium-strong acid site is responsible for isomerization reaction. Fig.9 shows the relationship between the fraction of medium-strong acid sites and the selectivity ofiso-alkanes and the mass ratio ofiso-alkane ton-alkane,respectively. The results show that the fraction of medium-strong acid sites in ZSM-5 and Zn/ZSM-5 zeolites is positively correlated with theiso-alkane selectivity and mass ratio ofiso-alkane ton-alkane,which is consistent with the results of Su et al.[30]. For Zn/ZSM-5 zeolite,the formation of new medium-strong L acid sites (ZnOH+)clearly promotes the isomerization performance. As a result,Zn/ZSM-5A with the smallest crystal size shows the highest fraction of medium-strong acid sites (29.6%),and then the highestiso-alkane selectivity (50.0%)and mass ratio ofiso-alkane ton-alkane (1.08).
In order to clarify the effect of Zn introduction on the aromatization performance of ZSM-5 zeolite,the conversion of 1-hexene at a much lower hydrogen pressure (0.1 MPa)was applied to reduce the hydrogenation rate and equilibrium. The growth of crystal size increases the amount of B acid sites in Zn/ZSM-5 zeolite from 0.05 mmol/g to 0.09 mmol/g (Table 5),which leads to more cracking products (C1—C3alkanes).

ZSM-5; Zn/ZSM-5Fig.9 Relationship of ZSM-5 and Zn/ZSM-5 zeolites between the fraction of medium-strong acid sites and iso-alkanes selectivity (s)and the mass ratio of iso-alkanes to n-alkanes,respectively Conditions:Temperature 350 ℃;H2 pressure 2.0 MPa; molar ratio of H2/1-hexene 600;LHSV 1.5 h-1
For olefin aromatization,the acid amount ratio of B/L is usually screened to balance the roles of B and L acid sites. Fig. 10 shows the relationship between the B/L acid amount ratio and the aromatics selectivity. B/L acid amount ratio shows a negative correlation with aromatics selectivity,in which the lower the B/L acid amount ratio,the higher aromatics selectivity in the products. Therefore,the increase of crystal size increases the B/L acid amount ratio of Zn/ZSM-5 zeolite from 0.15 to 0.30 (Table 5)leading to decreasing aromatics selectivity. In addition,the olefins selectivity in Zn/ZSM-5 zeolite increases from 8.6% to 13.5% (Table 8)with the growth of zeolite crystal size,suggesting that large crystal possesses less dehydrogenation active site (ZnOH+)in Zn/ZSM-5 zeolites,thus making it difficult for dehydrogenation and hydrogen transfer to form aromatics. Here,hydrogen transfer index (HTI,ratio of C4alkanes/C4alkenes)is used to represent the hydrogen transfer ability of zeolites. Table 8 shows that the HTI value decreases with the growth of zeolite crystal size. These data together show that reducing zeolite crystal size is beneficial to form ZnOH+species as dehydrogenation active site,and to reduce the amount of B acid sites and the ratio of B/L acid sites,then improve the olefin aromatization performance.
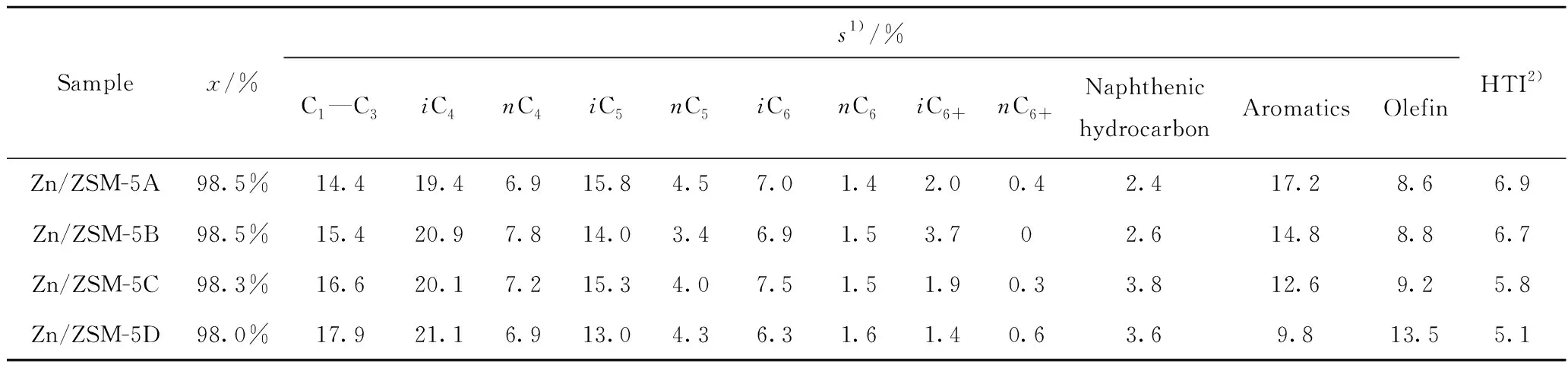
Table 8 Products distribution of Zn/ZSM-5 zeolites at hydrogen pressure of 0.1 MPa
Fig.11 shows the relation of aromatic distribution with thefsumof Zn/ZSM-5 zeolites. C8+aromatics mass fraction increases with thefsum,in agreement with that large aromatics are difficult to form and diffuse throughout ZSM-5 zeolite micropores. Specially,besides the lowfsum,Zn/ZSM-5D possesses nanosheet morphology with about 400 nm (Fig.2). Owing to the reduction in the diffusion path via forming sheet structure as well as the low fraction of B acid sites on the pore mouth and external surface acid sites,Zn/ZSM-5D zeolite possess the lowest selectivity of heavy aromatics (C8+components). In other words,the zeolite with large crystal size (Zn/ZSM-5D)shows good selectivity of light aromatics (C6—C8aromatics). The results show that the change of acid distribution caused by the crystal size of ZSM-5 zeolite has a great influence on the composition of aromatics in the products.
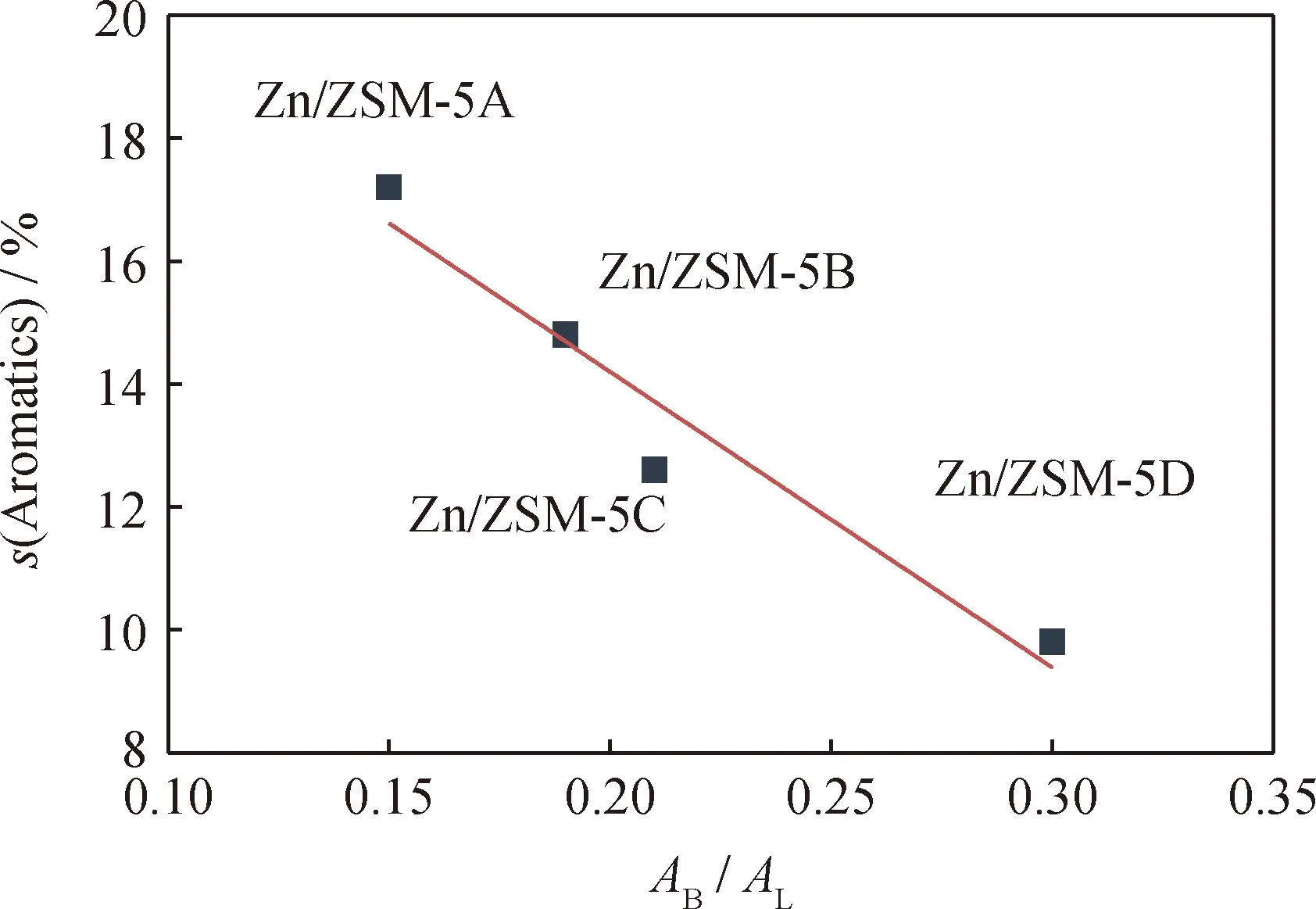
Fig.10 Relationship of Zn/ZSM-5 between aromatics selectivity (s)and B/L acid amount ratio (AB/AL) Reaction conditions:Temperature 350 ℃;H2 pressure 0.1 MPa; molar ratio of H2/1-hexene 600;LHSV 1.5 H-1
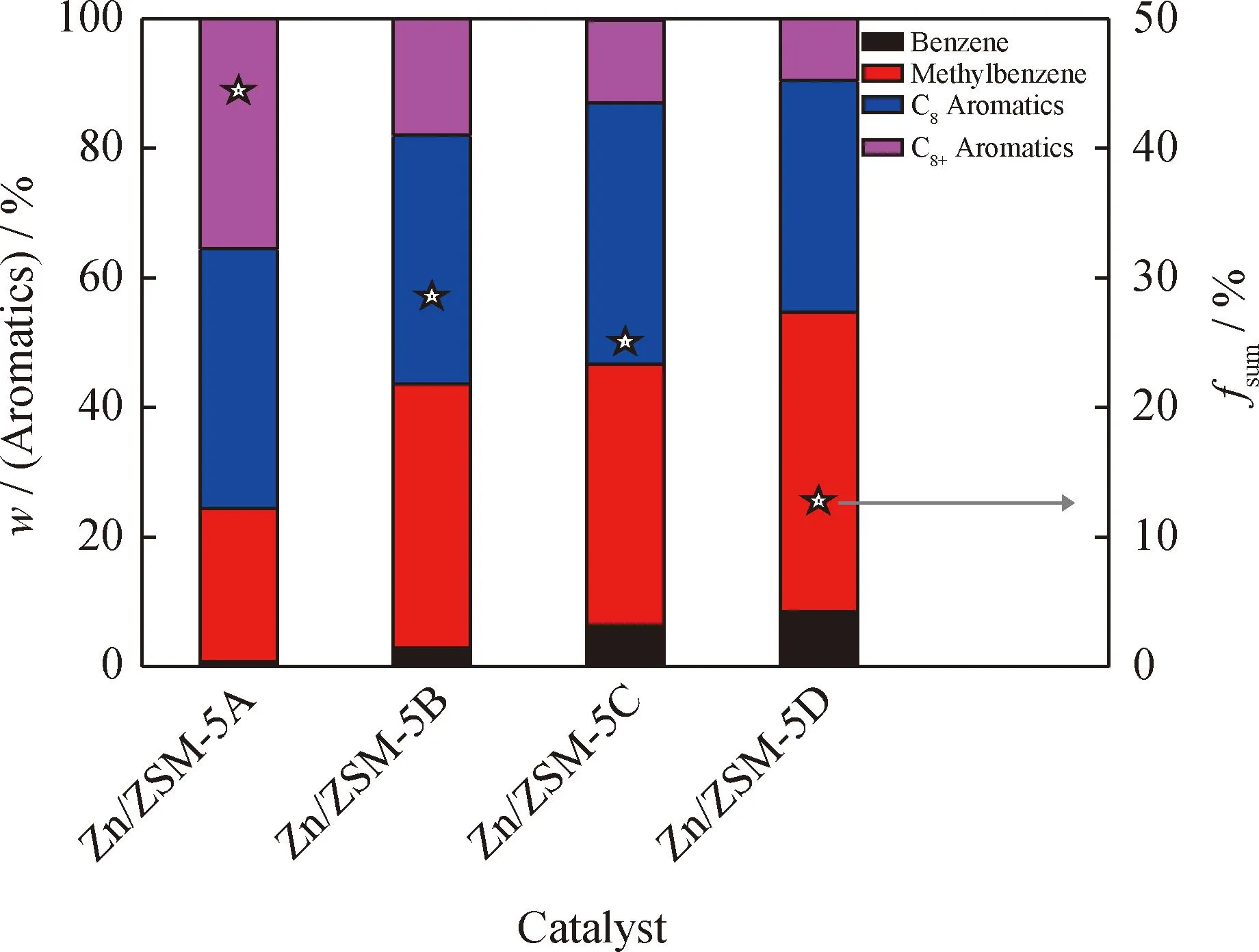
fsum—The fraction of Brønsted acid sites on the pore mouth and external surfaceFig.11 Distribution of aromatics and fsum for Zn/ZSM-5 zeolites Reaction condition:Temperature 350 ℃;H2 pressure 0.1 MPa; molar ratio of H2/1-hexene 600;LHSV 1.5 H-1
In a word,Zn/ZSM-5A with small crystal size (100—200 nm)are more likely to form ZnOH+species as medium-strong L acid sites,and greatly reduce the amount of B acid sites and B/L acid amount ratio. Therefore,it showed the highest isomerization selectivity of 50.0% for 1-hexene conversion at high hydrogen pressure (2.0 MPa),and also the highest aromatization selectivity of 17.2% for 1-hexene conversion at low hydrogen pressure (0.1 MPa). In addition,the acid distribution sensitive to zeolite crystal size has a great influence on aromatics composition of Zn/ZSM-5 zeolite. Reducing crystal size of ZSM-5 zeolite can significantly increase the fraction of B acid sites on the open mouth and external surface of zeolite,leading to a high mass fraction of C8+aromatics.
3 Conclusion
(1)ZSM-5 zeolites with similar SiO2/Al2O3molar ratio (55—60),Al distribution and different crystal sizes (100—4000 nm)were screened for the 1-hexene selective conversion toiso-alkane and aromatics,respectively. The ZSM-5A zeolite with small crystals (100—200 nm)shows hierarchical pore structure formed by nanocrystal aggregation and ZSM-5D zeolite with largest crystal size is the characteristic of sheet morphology. UV-Vis spectra of Co-exchanged ZSM-5 zeolites shows that Alpairis mainly located in the intersection channel (β-type Alpair)for all the ZSM-5 samples.
(2)The modification by Zn impregnation leads to decrease in B acid sites via the interaction between Zn cations and B acid site to form ZnOH+species. Reducing zeolite crystal size can greatly reduce the amount of B acid sites of ZSM-5 zeolite and increase the amount of ZnOH+species as L acid sites,thus further reducing the ratio of B/L acid sites and increasing the fraction of medium-strong acid sites. Zn/ZSM-5A zeolite with the smallest crystal size has the lowest B/L acid amount ratio at 0.15 and the highest fraction of medium-strong acid sites at 29.6%.
(3)For the 1-hexene conversion with 2.0 MPa hydrogen pressure,the isomerization is predominant prior to aromatization,and the selectivity ofiso-alkanes is sensitive to the fraction of medium-strong acid sites,in which Zn/ZSM-5A with the smallest crystal size shows the highest selectivity. Also,Zn/ZSM-5A zeolite with the lowest B/L acid amount ratio is benefited for the formation of aromatics in the conversion of 1-hexene at 0.1 MPa hydrogen pressure.

