一种基于三苯胺-噻吩基团的新型荧光探针在藻细胞中检测CN-的应用
文 丹,喻艳华
(江汉大学 光电化学材料与器件教育部重点实验室,光电材料与技术学院,湖北 武汉 430056)
In recent decades,algae as autotrophic microorganisms found in freshwater,marine,and soil ecosystems,are extensively used in nutraceuticals and as supplements in the diet,due to their abundant plant proteins and biologically active substances including β-carotene,eicosapentaenoic acid(EPA),and docosahexaenoic acid(DHA)[1-2].Moreover,algae have a complete protein genome similar to that of human beings.Eight essential amino acids for human beings,including lysine and threonine,are abundant in algae.The proportion of various amino acids is very close to the recommended standards of the Food and Agriculture Organization of the United Nations(FAO)and the World Health Organization(WHO).They are recognized as "one of the best protein sources" and"the most ideal food for human beings in the world"[3-5].While algae can produce cyanide(CN-)during metabolism or even absorb cyanide from their living environments due to human activities as cyanide compounds are extensively used in industries,such as metallurgy,gold mining,electroplating,and plastic manufacturing[6-9].It is well known that CN-is one of the most toxic anions to human health as it can directly enter the human body through the respiratory system or digestive system,resulting in human metabolic disorder and damage to the central nervous system,blood vessels,and heart[10-12].Therefore,to ensure the safety of algae food and human health,it is meaningful to develop efficient methods for monitoring trace amounts of CN-in algae cells and algae food.
Various methods have been used in CN-detection,such as Raman spectrometry[13],electrochemistry[14],ion chromatography[15],and gas chromatography-mass spectrometry[16].However,the use of these methods is limited due to their complexity,time-consuming,complex operation process,high cost,and dependence on instruments.Among the less traditional CN-detection methods,the chemical method which produces fluorescence and colorimetric reaction has been proved to be the most convenient,because of its simplicity,high sensitivity,and low cost.The most important advantage is that fluorescence probes can be used in living cells.Therefore,the strategy of designing such chemical sensors has received great attention in the past few years[17-23].So far,a large number of fluorescence probes have been reported for CN-detection.The construction of these fluorescence probes is mainly based on two characteristics of CN-:strong affinity with metal ions and unique nucleophilic properties.According to the characteristic of high affinity with metal ions,the fluorescence probes usually contain Co2+,Cu2+,Fe3+,or Zn2+.After binding with CN-,the metal ions in the probe are removed,resulting in fluorescence spectrum changes[24-27].In addition,the structure in the fluorescent probe designed based on nucleophilic property generally has electrondeficient double bonds,such as C=C[28],C=O[29],C=N[30],and C=N+[31].In the nucleophilic addition reaction,the conjugated probe is interrupted,resulting in a dramatic fluorescence response.The method based on nucleophilic addition reaction is most desirable because of its rapid reaction and high sensitivity.However,the fluorescent probes for CN-detection in algae cells and algae food are rarely reported.Furthermore,many algae cells have strong auto-fluorescence emission in the range of 500 to 750 nm.Due to the background fluorescence interference,most reported fluorescent probes are not suitable for CN-detection in algae cells[32-37].Therefore,there is a great need to design and synthesize novel fluorescent probes for sensing CN-detection in algae cells and food.
Herein,we rationally designed a novel probe based on triphenylamine-thiophene bearing indolium salt(TI)for CN-detection in living algae cells and food with high selectivity and sensitivity.Indolium salt was introduced due to its water solubility algae and high reactivity with CN-.Moreover,triphenylamine has strong electron-donating ability.By combining triphenylamine with electron-withdrawing groups,the push-pull electrons in the molecule can be regulated to achieve the purpose of regulating fluorescence.As expected,probe TI exhibits no fluorescence upon excitation at 380 nm because of the intramolecular charge transfer(ICT)process,while it shows highly blue fluorescence with maximum emission wavelength at 473 nm due to the interruption of the ICT process by CN-nucleophilic addition reaction.Meanwhile,the absorption spectra of TI exhibit a significant blue shift(from 570 to 375 nm)with the color of the solution changed from purple to colorless.Importantly,probe TI was successfully applied for CN-detection in living algae cells andChlamydomonas reinhartiilyophilized powder to ensure food safety.
1 Experimental Section
1.1 Materials and instrumentation
1,2,3,3-tetramethyl-3H-indolium iodide was purchased from BidePharm,5-(4-(diphenylamino)phenyl)thiophene-2-carbaldehyde was purchased from Alfa Aesar.Other reagents and solvents used for the synthesis and analytical experiments were purchased from Sigma-Aldrich.All fluorescence spectra were measured by Hitachi F-4600 fluorescence spectrophotometer.1H NMR and13C NMR were recorded by Bruker Advance 400 MHz spectrometer.HRMS data were obtained by SCIEX TripleTOF 5600+high-resolution spectrometer.Perkin Elmer PTI Tribute-UV multilabel reader was used to measure the fluorescence intensity of the sample at 473 nm.All fluorescence images of living cells were collected by Leica TCS SP8 confocal laser scanning microscope.
1.2 Synthesis of probe TI
In a nitrogen atmosphere,5-(4-(diphenylamino)phenyl)thiophene-2-carbaldehyde(0.071 g,0.2 mmol)and 1,2,3,3-Tetramethyl-3H-indolium iodide(0.15 g,0.5 mmol)were dissolved in ethanol(25 mL)with piperidine(0.2 mL),the mixture was stirred and refluxed for 4 h.After completion of the reaction,the solvent was removed by a rotary evaporator under reduced pressure to give a reddish black residue,which was further purified directly by gel column chromatography(CH2Cl2/MeOH=100/1,CH2Cl2/MeOH=50/1,CH2Cl2/MeOH=30/1,CH2Cl2/MeOH=20/1)to give a dark blue solid(0.076 g,0.12 mmol)in 60% yield.1H NMR(400 MHz,DMSO)δ 8.63(d,J=15.8 Hz,1H),8.15(d,J=4.0 Hz,1H),7.84(d,J=7.2 Hz,2H),7.77-7.67(m,3H),7.59(dt,J=14.6,7.3 Hz,2H),7.39(t,J=7.7 Hz,4H),7.24-7.07(m,7H),6.99(d,J=8.6 Hz,2H),4.05(s,3H),1.77(s,6H).13C NMR(101 MHz,DMSO)δ 180.77,154.50,149.41,146.72,145.79,143.68,142.38,140.51,138.76,130.33,129.39,129.20,127.90,125.86,125.74,124.90,123.26,121.83,115.05,110.08,52.09,34.31,26.04.HRMS:calculated:511.220 2,measured:511.220 9.
1.3 General procedure for fluorescence spectra measurement
To measure the fluorescence spectra,the stock solution of probe TI(1 mmol/L)was prepared with EtOH in HPLC grade purity and stored in the refrigerator at 4 ℃.All the tested anions(5 mmol/L)were prepared with distilled water from ammonium(NH4+)salts of AcO-,Br-,Cl-,ClO4-,F-,HSO4-,H2PO4-,I-,NO3-,CN-,respectively.A mixture solution of EtOH and distilled water(V/V=7∶3)was prepared.Before the measurement,the solution of probe TI was diluted to 5 μmol/L by the solution of EtOH and distilled water(V/V=7∶3).The final concentration of the anions was 50 μmol/L.The fluorescence spectra were recorded from 400 to 700 nm using the excitation wavelength of 380 nm at room temperature.
1.4 General procedure for cellular imaging
Cells ofChlamydomonas reinhartiiwere maintained at 25 ℃in cycles of 14 h under light and 10 h in darkness until 106cells/mL,and cells were cultivated photoheterotrophically in Tris-acetatephosphate(TAP)medium.Cells ofChlamydomonas reinhartiiwere obtained by centrifugation at 2 500 r/min for 3 min,washed twice with TAP medium,and resuspended to 107cells/mL.Cells ofChlamydomonas reinhartiiwere incubated with probe TI(20 μmol/L in ethanol)for 30 min and then washed with TAP medium three times.After further incubation with CN-(25 μmol/L in water)for another 30 min,the cells were washed with TAP medium three times again.Afterward,the confocal fluorescence images ofChlamydomonas reinhartiicells were captured by a Leica TCS SP8 confocal laser scanning microscope.The channel(480±50)nm was set to capture the fluorescence emission by excitation upon 380 nm.
1.5 CN-detection in Chlamydomonas reinhartii lyophilized powder sample
Cells ofChlamydomonas reinhartiiwere cultured according to the above method.107cells were added into the solution with 0,0.1,0.5,1,and 10 μmol/L CN-dissolved in TAP medium,these solutions were shaken well and incubated at 25 ℃for 30 min,then the cells were washed with TAP medium three times and lyophilized to powder.Powder ofChlamydomonas reinhartiicells that incubated with different concentrations of CN-were added into probe TI solution(20 μmol/L),and incubated for 30 min as described above.Fluorescence intensity at 473 nm was then recorded by a Perkin Elmer PTI Tribute-UV multilabel reader.
2 Results and Discussion
2.1 Synthesis of probe TI
As shown in Scheme 1,probe TI was synthesized in one simple step through Knoevenagel condensation with 5-(4-(diphenylamino)phenyl)thiophene-2-carbaldehyde and 1,2,3,3-tetramethyl-3H-indolium iodide in 60% yield.Probe TI was analyzed and well characterized by1H NMR,13C NMR,and HRMS data,which were offered in the experimental section.
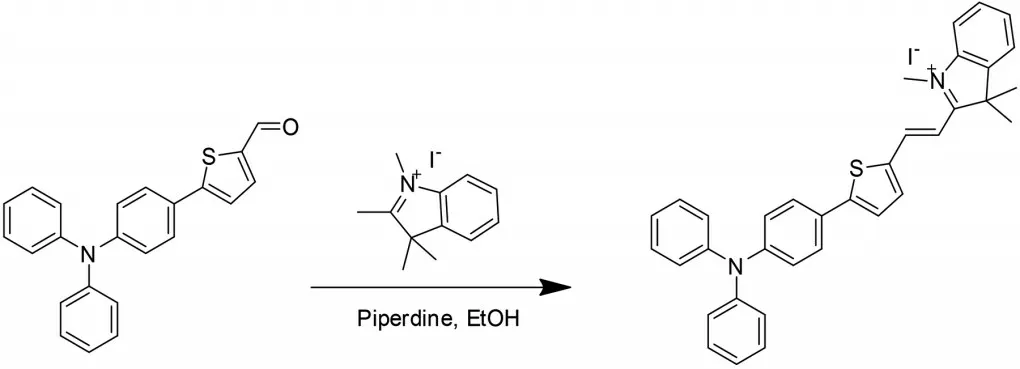
Scheme 1 Synthesis of probe TI
2.2 Sensitivity study of probe TI towards CN-
With probe TI in hand,we first investigated its sensing ability towards CN-through absorption and fluorescence spectra measurements in the mixture solution of EtOH/H2O(V/V=7∶3).As shown in Fig.1(a),probe TI(10 μmol/L)has two absorption bands peaked at 295 and 570 nm,which were respectively attributed to σ-π* and n-π* transition.With the addition of CNincrementally,the absorption band around 570 nm decreased gradually,accompanied by the appearance of a new band peaked at 375 nm.An isosbestic point at 375 nm was observed,which indicated the formation of a new compound.In addition,probe TI(10 μmol/L)in EtOH/H2O(V/V=7∶3)exhibited extremely low fluorescence emission upon excitation at 380 nm,due to intramolecular charge transfer from triphenylamine-thiophene moiety to indolium group through the π-conjugated bridge(Fig.1(b)).When CN-aqueous solution was added into the solution of probe TI bit by bit,the fluorescence emission band peaked at 473 nm appeared,increased gradually,and reached a plateau with the addition of 30 μmol/L CN-.It is well known that CN-processes strong nucleophilicity,it can easily attack the carbon of C=N+bond in the indolium group by nucleophilic addition reaction.Therefore,after the CN-nucleophilic attack on the C=N+bond in the indolium group,the electron acceptance of the indolium group was strongly weakened,and the ICT process was blocked,which led to the activation of the triphenylamine-thiophene moiety fluorescence signal.As shown in Fig.2,the plot of fluorescence intensity at 473 nm of probe TI as a function of CN-concentrations ranging from 0 to 6 μmol/L also gave a good linear regression(R2=0.991).Furthermore,according to the equation 3σ/k,the detection limit of CN-based on fluorescence spectral variation was calculated to be 95.7 nmol/L,whereσwas the standard deviation of the blank measurement andkwas the slope of fluorescence intensity versus the sample concentration plot.The detection limit in the current work is much lower than the maximum permissible level of CN-(1.9 μmol/L)in drinking water recommended by the World Health Organization(WHO).
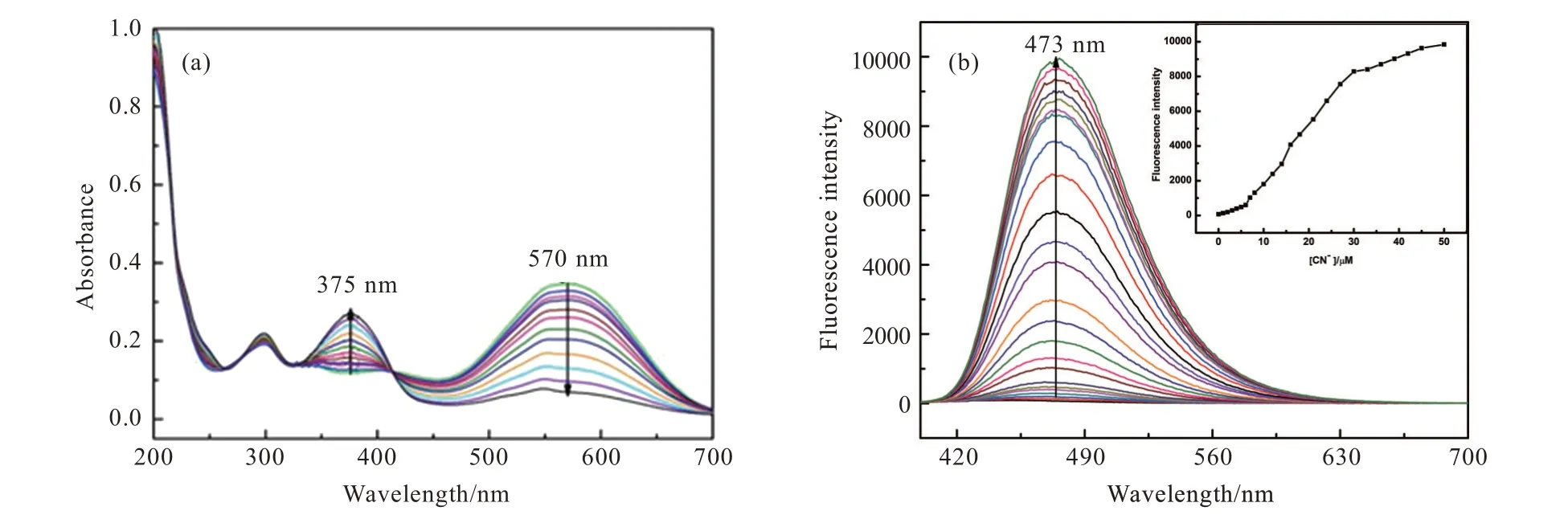
Fig.1 The absorbance spectra(a)and fluorescence spectra(b)changes of probe TI(10 μmol/L)with increasing concentration of CN-(0-50 μmol/L)in EtOH/H2O(V/V=7∶3)
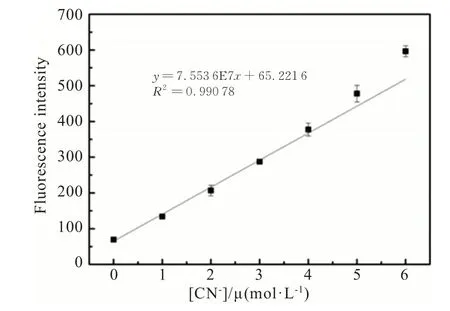
Fig.2 The linear relationship between the fluorescence intensity I473nm of probe TI(10 μmol/L)and concentration of CN- from 0 to 6 μmol/L in EtOH/H2O(V/V=7∶3)
2.3 Selectivity study of probe TI for CN- detection
With probe TI in hand,we firstly investigated its selectivity towards CN-among several anions,such as AcO-,Br-,Cl-,ClO4-,F-,HSO4-,H2PO4-,I-,NO3-,through absorption and fluorescence spectra measurements.As shown in Fig.1(a),probe TI(10 μmol/L)in EtOH/H2O(V/V=7∶3)had two absorption bands peaked at 295 and 570 nm,which were respectively attributed to σ-π*and n-π* transition.With the addition of CN-,the absorption band around 570 nm disappeared along with a new band peaked at 375 nm.An isosbestic point at 375 nm was observed,which indicated the formation of a new species.
To our delight,probe TI showed high selectivity towards CN-among the tested anions.As shown in Fig.3,probe TI displayed no obvious fluorescence emission band,but CN-addition could induce the appearance of a new emission band centered at 473 nm.Moreover,the color of probe TI in sunlight changed immediately from purple to transparent after the addition of CN-(Fig.4(a)),and the color of probe TI under 365 nm UV lamp changed immediately from blank to green after the addition of CN-(Fig.4(b)).The other anions addition showed an obvious fluorescence spectrum change of probe TI.In order to further confirm the high selectivity of probe TI for CN-detection,we tested the fluorescence resonance of probe TI towards CN-in multiple anions environments.As shown in Fig.5,I473nmof probe TI in the absence and presence of different anions almost remained basically unchanged.WhileI473nmwas significantly increased with the addition of CN-,indicating a great anti-interference ability of probe TI for CN-detection,which further validated the excellent selectivity of the probe TI for CN-detection.
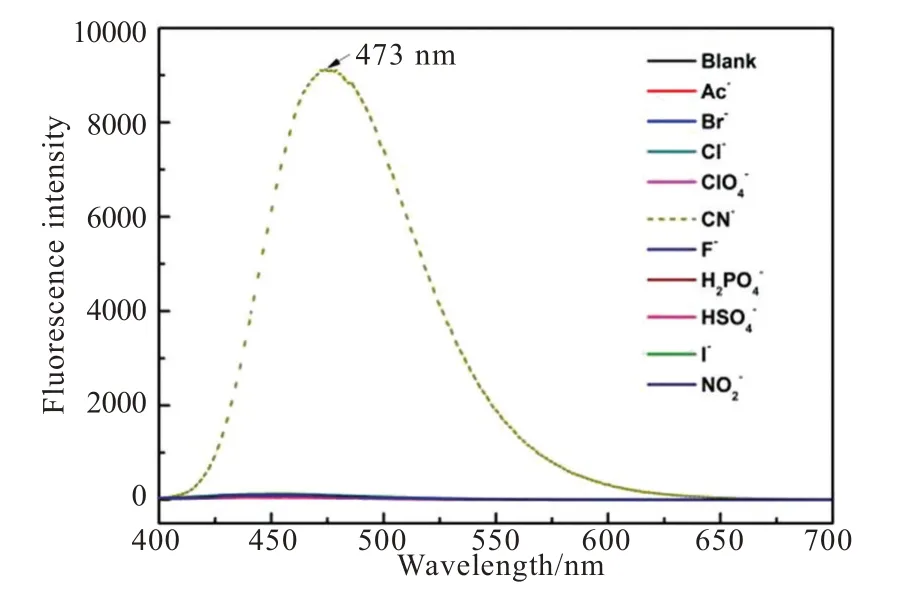
Fig.3 The fluorescence emission spectra of probe TI(10 μmol/L)with 10 different anions(50 μmol/L)in EtOH/H2O(V/V=7∶3)
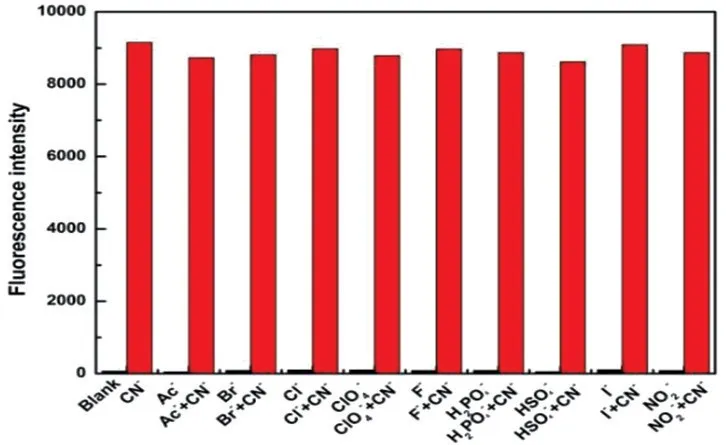
Fig.5 I473nm of probe TI(10 μmol/L)with and without different anions(50 μmol/L)in EtOH/H2O(V/V=7∶3),and upon addition of CN-(50 μmol/L)in the presence of background anions
2.4 Kinetic study
The time dependence of probe TI toward CN-in different concentrations was studied and shown in Fig.6.The value ofI473nmwas augmented with the increasing concentration of CN-.It was noted thatI473nmwas significantly increased after 30 s and almost reached equilibrium within 5 min,indicating that the probe TI could detect CN-in real-time.In addition,there were no significant changes ofI473nmof probe TI over 10 min,which meant that probe TI is stable at room temperature in EtOH/H2O(V/V=7∶3).
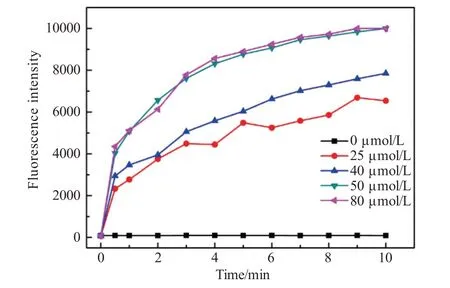
Fig.6 Time-dependent fluorescence intensity I473nm of probe TI(10 μmol/L)with different concentration of CN-(0-80 μmol/L)in EtOH/H2O(V/V=7∶3)
2.5 pH influence
The influence of pH for probe TI to detect CN-was investigated.I473nmresponses of probe TI for CN-detection at different pH values were measured to study a suitable operating pH range.As shown in Fig.7,in the absence of CN-,I473nmof probe TI was stable in the pH range of 4-8,but when the pH was above 9,I473nmof probe TI begins to change and increase,which meant that probe TI was unstable in basic conditions.In the presence of CN-,I473nmof probe TI showed no obvious changes at pH below 4 or above 10,which meanst that CN-could not promote the nucleophilic reaction under strongly acidic or basic conditions.In the range of pH 6-10,the presence of CN-significantly improvedI473nmof probe TI,indicating that probe TI was suitable for CN-detection under physiological conditions.

Fig.7 I473nm of probe TI(10 μmol/L)without and with CN-(50 μmol/L)as a function of pH
2.6 Mechanism study
Based on the data achieved above,the proposed sensing mechanism of probe TI toward CN-was the nucleophilic reaction between probe TI and CN-.With the addition of CN-to the probe,the ICT process was blocked from the triphenylamine part to the hemicyanine moiety,and the fluorescence emission was switched on upon excitation at 380 nm.To verify this hypothesis,we did1H NMR titration and HRMS experiments.The1H NMR titration experiment was carried out in(CD3)2SO solvent,due to its high solubility for probe TI.Moreover,the response of probe TI to CN-in(CD3)2SO was the same as that in EtOH/H2O(V/V=7∶3).As shown in Fig.8,the vinyl protons(Haand Hb)were shifted from 8.63 ppm,8.15 ppm to 6.75 ppm,6.11 ppm,and the N-methyl protons(He)was shifted from 4.05 ppm to 2.73 ppm by the addition of CN-to probe TI,due to the increased electronic density of the indolium group through CN-nucleophilic addition reaction.Furthermore,the singlet peak of C-methyl protons(Hc,Hd)was shifted from 1.77 ppm(s,6H)to 1.47 ppm(s,3H)and 1.11 ppm(s,3H),which meant it was adjacent to a chiral center.Furthermore,the HRMS spectrum of probe TI with CN-was recorded.The observed peak at m/z 537.221 9 was corresponded to the calculated m/z at 537.223 9 for[probe TI-CN](SI).1H NMR and HRMS data supported the mechanism that was depicted in Scheme 2.
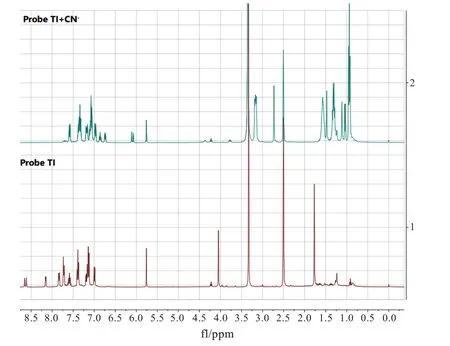
Fig.8 The 1H NMR spectra of probe TI and probe TI with the addition of CN-in DMSO
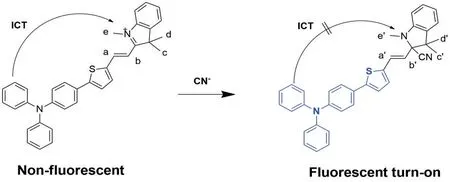
Scheme 2 The proposed mechanism of probe TI for CN-detection
2.7 Living cellular imaging
The Leica TCS SP8 confocal laser scanning microscope was used to obtain fluorescence images ofChlamydomonas reinhartiicells.Set the channel(480±50)nm to capture fluorescence emission images by excitation at 380 nm.As displayed in Fig.9,after incubating theChlamydomonas reinhartiicells in the TAP medium,almost no fluorescence was observed.In addition,almost no fluorescence was observed neither in pure CN-(25 μmol/L)nor probe solution(20 μmol/L).This meant probe TI was stable and there was no interference for CN-detection inChlamydomonas reinhartiicells.Incubated theChlamydomonas reinhartiicells stained with probe TI with CN-in TAP for 30 min,the blue fluorescence emission in the channel was increased significantly,which meant CN-could promote nucleophilic reaction in living algae cells.

Fig.9 The confocal fluorescence and DIC images of Chlamydomonas reinhartii cells incubated with buffer(a),CN-(b),probe PI(20 μmol/L)(c),probe TI with CN-(25 μmol/L)over 30 min (d)
2.8 Probe application in detection of CN- in Chlamydomonas reinhartii cells
Chlamydomonas reinhartiicells as food are in the form of lyophilized powder.Chlamydomonas reinhartiicells that incubated with different concentrations of CN-were added into probe TI solution(20 μmol/L)respectively.The fluorescence intensity increased dramatically with the increasing amount of CN-and reached an equilibrium with the addition of 1 μmol/L CN-(Fig.10).According to this result,the concentration of CN-in the powdery algae food could be quantitatively determined.The above results showed that the probe TI could be used as a fluorescent probe for CN-detection in living algae cells.
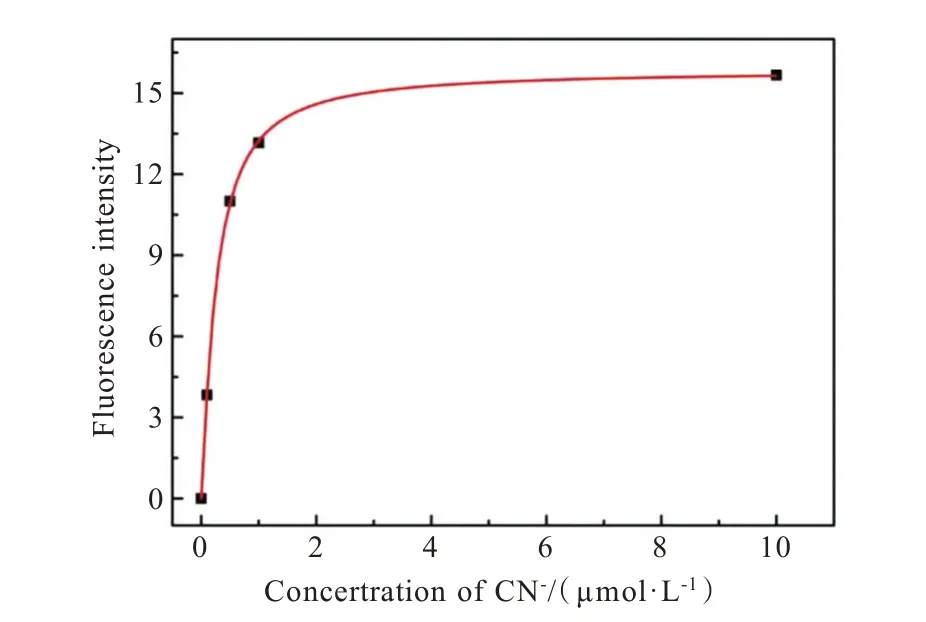
Fig.10 Nonlinear relationship of fluorescence intensity I473nm of probe TI toward CN- in Chlamydomonas reinhartii powder sample solution
3 Conclusion
In summary,we have designed and synthesized a triphenylamine-thiophene dyad fluorescent probe TI with blue fluorescence emission,which shows high sensitivity and selectivity towards CN-.This probe could detect CN-in EtOH/H2O(V/V=7∶3)with LOD 95.7 nmol/L in the range of pH 6-10.The addition of CN-induced an increasing fluorescence emission band at 473 nm through ITC mechanisms.Under daylight and UV lamps at 365 nm,noticeable color changes can be observed with the naked eye.Most importantly,probe TI has been successfully applied in the imaging and detection of intracellular CN-inChlamydomonas reinhartiipowder.

