Adding chitosan nanoparticles of green tea extract in diluent and thawing temperatures ameliorate the post-thawed quality of Boer buck semen
Suherni Susilowati ,Imam Mustofa✉ ,Tri Wahyu Suparyogi ,Adeyinka Oye Akintunde ,Djoko Agus Purwanto ,Wurlina Wurlina ,Suzanita Utama,Sri Mulyati
1Division of Veterinary Reproduction,Faculty of Veterinary Medicine,Universitas Airlangga,Kampus C Unair,Mulyorejo,Surabaya 60115,Indonesia
2Department of Agriculture and Industrial Technology,Babcock University,Ilishan-Remo,Ogun State 121103,Nigeria
3Department of Pharmaceutical Chemistry,Faculty of Pharmacy,Universitas Airlangga,Kampus C Mulyorejo,Surabaya 601155,East Java,Indonesia
ABSTRACT Objective: To improve the quality of post-thawing Boer buck semen for artificial insemination by adding green tea extract chitosan nanoparticles to skimmed egg yolk diluent,and the proper thawing temperature.Methods: The ejaculate of Boer buck was added to skimmed egg yolk diluent without (the control group) and with adding 1 µg of chitosan nanoparticles of green tea extract per mL of diluent (the treatment group).Then,the diluted semen was filled in French mini straws containing 60×106 live sperm per straw,frozen in a standard protocol,and stored as frozen semen at -196 ℃ for a week.Six replicates from each group were diluted for 30 s at 37 ℃ or 39 ℃sterile water to evaluate the semen quality.Results: Post-thawing (at 37 ℃ or 39 ℃) of live sperm,progressive motility,and plasma membrane integrity were lower compared to those of the pre-freezing stage (P<0.05).Thawing at 37 ℃ resulted in no significant difference in live sperm,progressive motility,and plasma membrane between the control group and the treatment group (P>0.05).The live sperm,progressive motility,and plasma membrane of the treatment group in the pre-freezing stage,and post-thawed at 39 ℃ were higher compared to those of the control group (P<0.05).There was no significant difference in malondialdehyde (MDA) concentration,DNA fragmentation,and catalase concentration of thawing at 37 ℃ compared to those of 39 ℃ in the same group.The MDA concentration and DNA fragmentation in thawing at 37 ℃ and 39 ℃ of the treatment group were significantly lower than those of the control group (P<0.05).However,the catalase concentration in thawing at 37 ℃ and 39 ℃ of the treatment group was not significantly different than the control group (P>0.05).Conclusions: Higher quality post-thawing Boer buck semen is achieved by adding 1 µg/mL of chitosan nanoparticles of green tea extract to the skimmed egg yolk diluent and thawing at 39 ℃.
KEYWORDS: Boer buck semen;Chitosan nanoparticles;Semen diluent;Higher post-thawing semen quality;Prevent undernutrition
1.Introduction
Boer goats,originating from South Africa,have been registered livestock in Indonesia for over 65 years.These meat goats exhibit rapid weight growth and higher productivity than the local Indonesian Kacang goat.Boer goats have an average body weight of (50.73±22.33) kg,whereas Kacang goats average(21.03±5.90) kg[1].Keeping Boer goats can help small farmers reduce poverty and prevent malnutrition.To further increase the goat population,artificial insemination methods using freezethawed semen are employed.However,goat sperm is sensitive to cold shock.Freezing goat semen in skim milk–egg yolk diluent alone results in more than 60% sperm death,which falls below the minimum standard of 40% sperm progressive motility required for artificial insemination[2].This is due to the production of excess reactive oxygen species during the freezing and thawing process,which damages the sperm cell membrane’s double carbon chain fatty acids and increases the concentration of malondialdehyde (MDA).Consequently,this reduces the percentage of live sperm,sperm progressive motility,and increases sperm DNA fragmentation[3].Our previous research demonstrated that green tea ethanol extracts improved post-thawed sperm quality and reduced sperm mitochondrial DNA changes in Kacang buck semen[2].The use of nanoparticle extract is expected to enhance sperm cell penetration due to its increased surface area available for green tea extract particles[4].
Significance
The addition of antioxidants in diluent for frozen buck semen showed higher of post-thawed semen quality.Traditionally,frozen goat semen is thawed in 37 ℃ water for 12–30 s.This study suggested that adding 1 µg/mL of chitosan nanoparticles of green tea extract to the skimmed egg yolk diluent and thawing at 39 ℃°for 30 s can achieve higher quality postthawing Boer buck semen.
The frozen semen was thawed to restore its physiological temperature and reactivate the metabolism of spermatozoa.Previous reports suggest different optimal thawing temperatures and times for frozen goat semen,including 35 ℃–37 ℃ for 30–60 s[5],and 39 ℃ for 120 s[6].Inseminators are advised to thaw frozen semen at 37 ℃ for 30 s[2].It is important to note that the average temperature of a doe is approximately 39 ℃[7],as well as the temperature of the vagina during the estrus in a doe[8].Therefore,this research aims to increase the thawing temperature from 37 ℃ to 39 ℃ for 30 s.
The effect of adding chitosan nanoparticles of green tea extract to the skimmed egg yolk diluent for freezing Boer buck semen,as well as the appropriate thawing temperature to achieve optimum post-thawing semen quality for artificial insemination,has not been previously studied.This research aimed to address this gap by investigating the impact of chitosan nanoparticles of green tea extract on the diluent and determining the suitable thawing temperature for Boer buck semen.
2.Materials and methods
2.1.Chitosan nanoparticles of green tea extraction
Green tea leaves ethanol extract was obtained from previous research[9];0.5 mL of green tea extract and 0.5 mL of chitosan solution (pH 5.0) were mixed and vortexed for 20 s;then 0.03%triphenyl phosphate solution was added and vortex again for 20 s[10].Chitosan nanoparticles of green tea extract were freeze-dried at -20 ℃.The particle size of green tea extract chitosan nanoparticles was measured by a ZEN 3600 Zetasizer Nano ZS produced by Malvern Instruments Ltd.,UK.The measurement used a water dispersant,RI: 1.330,viscosity: 0.887 2,temperature 25.0 ℃;count rate,1 166 kbps;duration,70 s;measurement position,4.65 mm;cell description,disposable sizing cuvette;attenuator: 8.
2.2.Experimental animals
Three male Boer goats aged two to three years weighing 50-60 kg were used.The goats were fed 5 kg of fodder forage and 0.5 kg of pellet concentrate (containing 16%-18% crude protein) daily and had access to drinking water.Boer buck ejaculate was obtained using an artificial vagina on Tuesday and Friday to get six replicates of ejaculate samples for further processing into frozen semen.
2.3.Skim milk–egg yolk diluent
The formulation of skim milk–egg yolk diluent was carried out as in previous studies.A 3.75 g of skim milk powder(Merck 115338) was added with distilled water up to 37.5 mL,heated at 92 ℃-95 ℃ for 10 min,and then cooled down to room temperature (26 ℃).Egg yolk 1.25 mL was added to 23.75 mL of skim milk solution,followed by the addition of penicillin (Meiji Seika Pharma,Tokyo,Japan) 0.5 IU/mL,and streptomycin (Thermo Fisher Scientific,Singapore) 0.5 mg/mL[9].The diluent was divided into two equal parts: the control group (without adding chitosan nanoparticles of green tea extract) and the treatment group (1 µg chitosan nanoparticles of green tea extract/mL diluent).The dose of chitosan nanoparticles of green tea extract added to the skimmed egg yolk diluent was based on previous studies[2].
2.4.Frozen semen
Each diluent was added with glycerol so that the concentration became 8%,and ejaculates reached 60 million live sperm cells per French mini straw.Equilibration of diluted semen was conducted at 5 ℃ in the Cold Handling Cabinet (Minitube) for 1 h and then evaluated for the pre-freezing semen quality.The straws were exposed to liquid nitrogen vapor (-140 ℃) for 10 min and stored immediately in liquid nitrogen (-196 ℃)[11] for three days before quality evaluation.
2.5.Post-thawed sperm quality evaluation
Six straw samples of each group were thawed in aqua dest for 30 s at 37 ℃ and 39 ℃,respectively,to subjective assessment of live sperm percentage,sperm progressive motility,plasma membrane integrity,malondialdehyde,sperm DNA fragmentation,and catalase concentration according to previous studies[12].
2.5.1.Sperm viability evaluation
Dry-smeared semen sample stained with nigrosine drop (Sigma-Aldrich) on an object glass was evaluated under a microscope(Olympus BX-53,Tokyo,Japan) at 400× magnification for 100 spermatozoa.The heads of the viable sperm were brightly transparent,while those of the not-viable spermatozoa were redstained[12].
2.5.2.Sperm motility evaluation
Semen samples of 10 µL and 1 mL of 0.9% NaCl were homogenized and dropped on the object glass and covered.Sperm progressive motility was counted at 400× magnification using a microscope (Olympus BX-53,Tokyo,Japan)[12].
2.5.3.Intactness plasma membrane
The hypo-osmotic solution was made of 0.735 g sodium citrate(Sigma-Aldrich) and 1.352 g fructose (Sigma-Aldrich),diluted in 100 mL of distilled water. A 100 µL of semen sample added with 1 mL of hypo osmotic solution was incubated at 37 ℃ for 30 min and then evaluated for 100 sperm using a 400× Olympus BX-53 light microscope (Tokyo,Japan).Sperm with intact plasma membranes had curved tails,while those with damaged membranes had straight tails[12].
2.5.4.MDA level
The determination of MDA levels was using the thiobarbituric acid(Sigma-Aldrich) method.Color absorption was measured in a Thermo Fisher Scientific spectrophotometer at a wavelength of 533 nm to determine the MDA levels (ng/mL)[12].
2.5.5.Sperm DNA fragmentation
Semen samples were dry-smeared on object glass and stained with acridine orange (Sigma-Aldrich).The DNA fragmentation was examined on 100 sperm at 400× magnification under a light microscope (Olympus BX-53,Shinjuku-ku,Tokyo,Japan).The intact sperm DNA head was yellow-colored,whereas the fragmented sperm DNA head was green-colored[12].
2.5.6.Catalase level
The hydrogen peroxide phosphate-buffered saline (30 mM) 0.5 mL was added to a 1 mL semen sample at room temperature.The UV spectrophotometric absorbance method at 240 nm wavelength was used to measure the catalase level compared to a blank containing the enzyme solution[13].
2.6.Data analysis
The analysis was conducted using Statistical Product and Service Solution version 27,developed by The International Business Machine Corporation,USA at a 95% significance level.Thet-test was using for the two groups comparison (the control groupvs.the treatment group) of pre-freezing stage in the same parameter,and analysis of variance for comparison of post-thawing at 37 ℃vs. 39 ℃and the control groupvs. the treatment group in the same parameter.Data in percent values were arc sin transformed before statistical analysis.The data were expressed as mean±standard deviation(mean±SD).P<0.05 was considered statistically significant.
2.7.Ethics statement
This research protocol was approved by the Airlangga University Animal Research Ethics Committee No.520/HRECC.FODM/VII/2021.
3.Results
The diameters and their percentage of green tea extract chitosan nanoparticles added to the skimmed egg yolk diluent are presented in Figure 1 and Table 1.
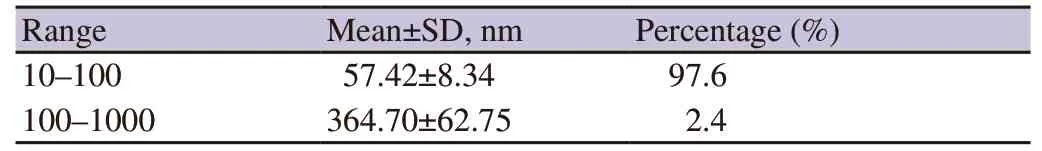
Table 1.Diameter and percentage of chitosan nanoparticles in green tea extract.
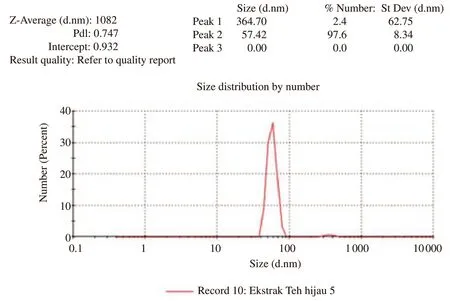
Figure 1.Diameter and percentage curves of chitosan nanoparticles green tea extract (Zetasizer Ver.7.01,MAL1061025,Malvern Instruments Ltd,Worcs.,UK).
3.1.Post-thawed semen quality
The average Boer buck ejaculate parameters met the qualifications for freezing (Table 2).However,there were significant decreases(P<0.05) in post-thawed live sperm percentage,sperm progressivemotility,and plasma membrane integrity compared to the prefreezing values.Adding chitosan nanoparticles of green tea extract in the skimmed egg yolk diluent significantly increased (P<0.05)the live sperm percentage,sperm progressive motility,and plasma membrane integrity before freezing and after thawing at 39 ℃.No significant difference (P>0.05) was observed for these parameters after thawing at 37 ℃ (Table 3).Adding chitosan nanoparticles of green tea extract in the diluent significantly reduced (P<0.05) the post-thawed MDA concentration and DNA fragmentation compared to the control group.However,no significant difference (P>0.05) in catalase concentration between the control group and the treatment group (Table 4).
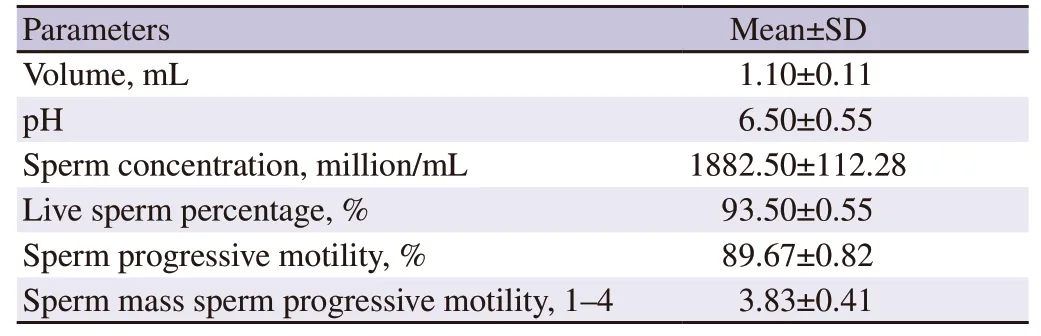
Table 2.The characteristic of Boer buck ejaculate.
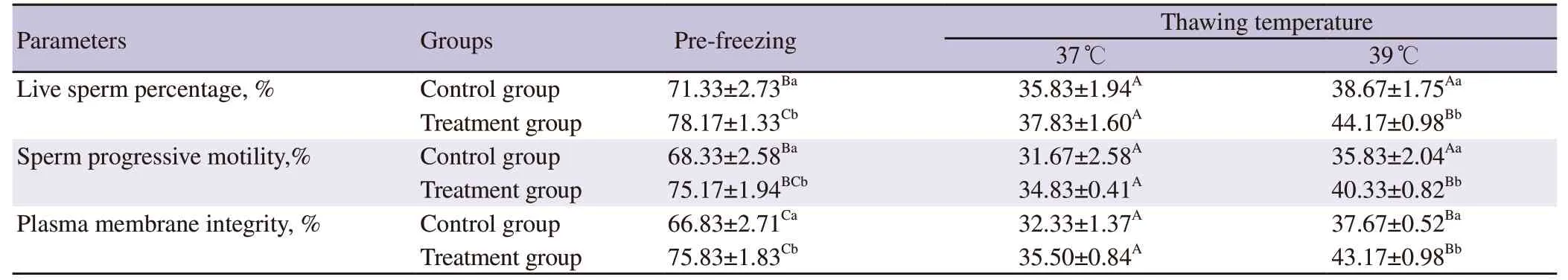
Table 3. Live sperm percentage,sperm progressive motility,and plasma membrane integrity of Boer buck semen.
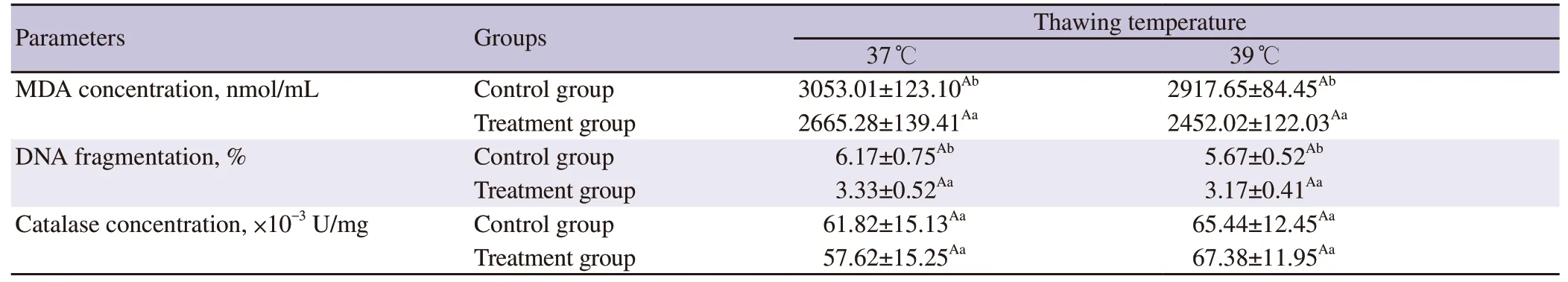
Table 4.Malondialdehyde concentration,DNA fragmentation,and catalase concentration of Boer buck sperm.
3.2.The thawing temperatures
Thawing at 39 ℃ resulted in significantly higher (P<0.05) postthawed live sperm percentage,sperm progressive motility,and plasma membrane integrity of the treatment group than that of the control group (Table 3).There was no significant difference in MDA concentration,DNA fragmentation,and catalase concentration of thawing at 37 ℃ compared to those of 39 ℃ in the same group.The MDA concentration and DNA fragmentation in thawing at 37 ℃ and 39 ℃ of the treatment group were lower(P<0.05) than those of the control group.However,the catalase concentration in thawing at 37 ℃ and 39 ℃ of the treatment group was not significantly different (P>0.05) from that of the control group (Table 4).
4.Discussion
Nanotechnology has enabled the fabrication of particles 0.1–1000 nm in size[14].Nano-sized particles (0.001 µm) exhibit higher chemical reactivity,enhanced cellular penetration,and increased biological activity[15].They also facilitate the dispersion of active compounds in media and enable more precise targeting of cells compared to microparticles[16].Chitosan is a safe material used in nanoparticle technology[17].Green tea extract,which contains epigallocatechin 3-gallate (EGCG) with ortho-dihydroxy or ketohydroxyl groups,which are potent antioxidants[18],was chosen due to its higher effectiveness than vitamins E and C[19].
The sperm progressive motility in the ejaculate of Boer bucks exceeded 70%,meeting the requirements for freezing semen[3].However,buck semen is susceptible to freezing and thawing.Freezing leads to high reactive oxygen species levels,resulting in lipid peroxidation of the sperm cell membrane and increased MDA concentration[20].This oxidative stress leads to a marked decline in sperm progressive motility,live sperm percentage,plasma membrane integrity,and sperm DNA integrity[21].
As for post-thawed semen quality,the treatment group exhibitedhigher live sperm percentage,sperm progressive motility,and plasma membrane integrity than the control group.Additionally,the treatment group had lower MDA concentration and DNA fragmentation.The reduction in post-thawed semen quality is attributed to damage to the spermatozoa’s plasma membrane.The survival and progressive motility of sperm rely on the integrity of the plasma membrane.During the freezing-thawing process,excessive reactive oxygen species production leads to lipid peroxidation in the sperm membrane[22].This lipid peroxidation damages the molecular structure and disrupts the function of the sperm cell membrane,ultimately leading to cell death[21].Reactive oxygen species also damage mitochondrial and axonemal proteins,impairing sperm progressive motility[23].Higher MDA concentration indicates more significant membrane damage caused by lipid peroxidation[24].Furthermore,reactive oxygen species-induced damage to proteins’ disulfide chains results in DNA structural instability and fragmentation[25].Although the ejaculate is equipped with antioxidant enzymes such as glutathione peroxidase,catalase,and superoxide dismutase to defend against reactive oxygen species attack,the limited cytoplasmic space in sperm is mainly occupied by DNA[26].When semen diluents are added,the endogenous antioxidants become insufficient to counteract the excessive reactive oxygen species.Insufficient antioxidants during semen freezing lead to reduced live sperm percentage,sperm progressive motility,and plasma membrane integrity.
Green tea contains epicatechin,epigallocatechin,epicatechin-3-gallate,and epigallocatechin gallate (EGCG).EGCG is an antioxidant that can reduce sperm DNA damage,protein carbonylation,and lipid peroxidation[27].When green tea extract is extracted into nanoparticles,it results in a larger surface area to volume ratio,allowing for more effective entry of the extract into sperm cells[28].The antioxidants in the diluent help decrease lipid peroxidation in the plasma membrane of sperm cells,leading to increased live sperm percentage,sperm progressive motility,and acrosome integrity[29].Adding chitosan nanoparticles to a skimmed egg yolk diluent (1 µg/mL) improved the semen quality compared to the control group.These findings are consistent with previous studies that showed adding green tea extract (0.1 mg/100 mL) in ethanol to a skimmed egg yolk diluent maintained the quality of Simmental bull semen in terms of sperm progressive motility,live sperm percentage,plasma membrane integrity,and DNA integrity[10].
Semen contains antioxidant enzymes,such as glutathione peroxidase,catalase,and superoxide dismutase,to maintain the balance between oxidants and antioxidants[30].These antioxidant enzymes play a role in defending the biological system from free radicals.Catalase converts hydrogen peroxide into water and molecular oxygen,complementing the scavenging function of superoxide dismutase[31].By blocking biochemical reactions that generate reactive oxygen species in cells,catalase reduces lipid peroxidation by breaking down hydrogen peroxide into water and oxygen,leading to improved membrane integrity and fluidity and a lower percentage of acrosomal damage through the reorganization of membrane lipids and proteins and reduced oxidative stress[32].However,when 1 µg/mL of chitosan nanoparticles of green tea extract was added to a skimmed egg yolk diluent,the catalase concentration was similar to the control group.This finding differs from previous reports where adding catalase to a commercially optimized medium enhanced total sperm progressive motility,membrane integrity,and live sperm percentage in post-thawed goats[33] and ram semen[34].The ideal concentration of catalase in the diluent has been shown to mitigate the adverse effects on post-thawed sperm progressive motility,live sperm percentage,plasma membrane,and acrosome integrity[35].Catalase is used as a molecular target for detecting and monitoring human male infertility,and methods to improve sperm parameters are also applied[36].In stallion semen cryopreservation,the concentration of catalase and superoxide dismutase did not significantly differ between ejaculates with good and poor freezing abilities[37].However,ejaculates with good freezing abilities had higher superoxide dismutase activity compared to ejaculates with weak freezing abilities[38].The concentration and activity of catalases in the seminal plasma of stallions are high,but there is no relationship with sperm kinematic parameters[37].
As for the thawing temperatures,thawing frozen Boer buck semen at 39 ℃ for 30 s resulted in higher post-thawing sperm quality in terms of live sperm percentage,sperm motility,plasma membrane intactness,and lower MDA concentration compared to thawing at 37 ℃.Therefore,thawing at 39 ℃ for 30 s is more suitable for frozen Boer buck semen.Different combinations of thawing duration and temperature were studied for the frozen semen of various animals.For example,in frozen yak semen,thawing in water at 35 ℃ for 60 s or 37 ℃ for 30 s yielded better post-thawing semen quality than thawing at 75 ℃ for 9 s[6].In ram-frozen semen,thawing at 39 ℃ for 120 s resulted in higher sperm progressive motility and live sperm percentage,while thawing at 75 ℃ for 5 s improved plasma membrane structural and functional integrity[7].Thawing methods for goat semen can vary.Traditionally,frozen goat semen is thawed in 37 ℃ water for 12–30 s[39,40].Studies also explored thawing at temperatures such as 40 ℃ for 45 s[41] and 40 ℃for 20 s[42],with varying results.Thawing at temperatures suitable for body and vaginal temperatures of does during estrus yields better semen quality compared to the thawing methods used for Boer buck frozen semen.
The limitation of this study is no significant difference between post-thawed frozen semen without or with the addition of green tea extract chitosan nanoparticles in the extender.It needs further exploration of this problem.In addition,this study only included post-thawing semen quality evaluation due to limited research time.A field study is needed by artificial inseminating using post-thawed semen into synchronized female goats[11].
In conclusion,adding 1 µg/mL chitosan nanoparticles of green tea extract skimmed egg yolk diluent and thawing at 39 ℃ for 30 s resulted in higher post-thawed semen quality in Boer buck semen.However,the specific reason why the catalase concentration was not affected by the addition of antioxidants and differences in thawing temperatures in Boer buck semen is unclear and requires further research.
Conflict of interest statement
The authors certify no conflict of interest with any financial,personal,or other relationships with other people or organizations related to the manuscript.
Funding
This study is funded by Universitas Airlangga,Indonesia,contract number: 1405/UN3.1.6/PT/2022.
Acknowledgments
The authors also thank Dikky Eka Mandala Putranto,DMV,M.Sc,the Chairman of the Insemination Center,Airlangga University;Subchan Aziz,and Agus Purwanto for technical support.
Authors’ contributions
Suherni Susilowati and Imam Mustofa compiled ideas and designed this text's main framework for their research work,and conceived the manuscript.Djoko Agus Purwanto and Tri Wahyu Suparyogi prepared green tea extract chitosan nanoparticles.Tri Wahyu Suparyogi and Imam Mustofa collected and assessed the ejaculate's quality.Suherni Susilowati,Suzanita Utama,and Sri Mulyati prepared the extender and freezing of extended semen.Suherni Susilowati and Tri Wahyu Suparyogi evaluated the post-thawed semen quality.Imam Mustofa,Suzanita Utama,and Adeyinka Oye Akintunde interpreted data and statistical analysis.Djoko Agus Purwanto,Adeyinka Oye Akintunde,and Suzanita Utama critically read and revised the manuscript for intellectual content.All authors read and approved the final manuscript.
 Asian Pacific Journal of Reproduction2024年1期
Asian Pacific Journal of Reproduction2024年1期
- Asian Pacific Journal of Reproduction的其它文章
- Engineering of ovarian tissue for ovarian dysfunctions: A review
- Exploring the relationship between ambient sulfur dioxide and semen quality parameters: A systematic review and meta-analysis
- The relationship between DNA fragmentation and the intensity of morphologically abnormal human spermatozoa
- Subsequent pregnancy outcomes and fertility rates in the case series that underwent bilateral hypogastric artery ligation (BHGAL) due to severe postpartum hemorrhage
- Pro-fertility effect of Ficus carica fruit extract in streptozotocin-induced male rats
