Defined hydrogels for spinal cord organoids: challenges and potential applications
Wai Hon Chooi,Yuewen Wu,Shi-Yan Ng
Organoids of the central nervous system,primarily derived from pluripotent stem cells or neural stem cells,are three-dimensional tissue cultures with self-organizing properties.When exposed to the right combinations of signals,they differentiate into a 3D tissue consisting of complex cytoarchitecture and native cell types,including various neuron subtypes and glial cells.These features closely mimic native tissues,making them invaluable for developmental studies and disease modeling.In recent years,spinal cord organoids (SCOs) have been developed to investigate spinal cord development,injuries,and various neurological disorders.As an integral part of the central nervous system,SCOs play a vital role and serve as a site for studying both neurodevelopment and neurodegenerative diseases.
During the generation of organoids,including brain organoids and SCOs,the use of Matrigel in encapsulation or supplementation appears to be a critical step (Hor et al.,2018;Lee et al.,2022).Matrigel,also marketed under other brand names like Cultrex and Geltrex,is a basement membrane extract derived from the Engelbreth-Holm-Swarm mouse sarcoma.It provides essential extracellular matrix (ECM) components such as laminin,collagen IV,and heparan sulfate,along with various growth factors.Its widespread use highlights its significance in organoid culture.However,Matrigel’s complexity and undefined nature limit precise culture conditions and introduce batch-tobatch variability.Furthermore,Matrigel’s deviation from tissue-specific ECM restricts its universal suitability for diverse organoid types and research directions.Consequently,numerous ongoing studies are eagerly exploring Matrigel substitutes to overcome the limitations of this animal-derived ECM.Αn intermediate solution to mouse-derived Matrigel,such as human cell-derived MaxGel,is available but remains underutilized.It is important to note that opting for a human-derived source only partially mitigates these problems,rather than offering a comprehensive solution.Recently,we demonstrated that alginate hydrogel can be a viable alternative for culturing SCOs,supporting neurogenesis and gliogenesis with similar efficiency to Matrigel (Chooi et al.2023).
Can hydrogel completely replace Matrigel for SCO culture? Currently,the majority of SCO cultures rely on Matrigel as a substrate (Hor et al.,2018;Lee et al.,2022).When following directed protocols for spheroid formation,in which small molecules and morphogens efficiently specify cell fates,the supplementation of Matrigel may not be essential (Andersen et al.,2020).SCOs generated using these directed protocols typically exhibit a more spherical shape,but they manage to recapitulate certain organoid signatures,such as neural rosettes and a diverse array of neuron subtypes and glial cells (Hor et al.,2018,Chooi et al.2023).Conversely,it appears that if the protocol starts at an early developmental stage,the inclusion of Matrigel might be necessary to replicate the required biomimetic conditions,thus inducing the basal-apical orientation (Gribaudo et al.,2023).
Even if Matrigel is considered necessary,a significant challenge arises from the fact that we lack precise knowledge of which components among its more than 1000 proteins are responsible for its crucial properties (Hughes et al.,2010).While early indications suggest that laminin,the major component of Matrigel,plays a role,it is not the sole contributor.The necessity of Matrigel might extend beyond the ECM since laminin alone cannot sufficiently support the formation of brain organoids (Lancaster et al.,2017).Consequently,exercising caution is essential when utilizing Matrigel,given the somewhat elusive nature of its precise role.Nevertheless,the impact of other Matrigel components on culture outcomes remains unclear.In our recently published study,we found that Matrigel might harbor growth factors or unknown factors contributing to the emergence of non-specific cells within SCOs,highlighting a substantial drawback associated with Matrigel usage (Chooi et al.,2023).In our experiments employing a directed protocol,we observed an increase in mid-and hind-brain markers when using Matrigel.The undefined components of Matrigel become a double-edged sword;while these factors could aid in organoid self-organization,they also pose risks when one seeks precise control over the fate of organoids.This becomes particularly critical if we aim to develop region-specific organoids for applications such as drug screening or transplantation,where non-specific cell types could mask meaningful results.Caution is imperative not only to ensure the acquisition of desired cell types but also to scrutinize unwanted cell populations.To address this,single-cell RNA sequencing emerges as a valuable tool for identifying and characterizing these cells,making it an almost essential component in contemporary organoid studies.
In the absence of Matrigel,SCOs generated using the directed protocol appear to develop satisfactorily,displaying complex structures with diverse cell types.This observation is supported by both the literature and our own experiences(Αnderson et al.,2020;Chooi et al.,2023).However,a notable challenge arises in the form of their tendency to fuse,a problem also encountered with cortical organoids (Narazaki et al.,2022).To address this issue,one promising approach involves alginate encapsulation or the addition of non-adhesive biopolymers to the growth medium to mitigate clumping (Narazaki et al.,2022;Chooi et al.,2023).Our observations indicate that this strategy significantly contributes to reducing intra-batch variation in organoid size(Figure 1).Organoid size serves as a valuable indicator of the necrotic core and the types of cells obtained,providing critical insights into their developmental progress.Alginate encapsulation protects the organoids from aggregation and shields them from shear stress caused by the movement of the medium in the spinner flask or on a shaker.
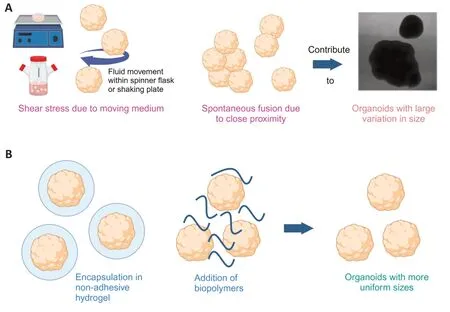
Figure 1|Strategies to reduce intra-batch variation in organoid size.
Beyond alginate,numerous hydrogel options have been explored and tested for organoid development,although relatively less attention has been paid to neural organoids,particularly SCOs.A particular challenge in choosing a hydrogel for SCO culture lies in the sensitivity of neural cells.Natural hydrogels,such as alginate and collagen,usually offer good biocompatibility but have a limited degree of tunability compared to synthetic hydrogels,like poly(ethylene glycol).Conversely,synthetic hydrogels exhibit limitations in terms of biocompatibility and bioactivity when compared to natural hydrogels.Furthermore,while many polymers are biocompatible,the gelation process demands careful consideration,as improper gelation mechanics can lead to cell death.On the other hand,when studying organoid/cell-ECM interactions,non-adhesive bioinert materials such as alginate and poly(ethylene glycol) are preferred.Materials like hyaluronic acid,collagen,or other cell-adhesive substances can provide a scaffolding for adhesion but may also obscure the outcomes.To ensure biocompatibility and increase the success rate,researchers can draw inspiration from recently developed hydrogels used in other organoid models.Similarly,many of the biomaterials developed for spinal cord injuries may also prove suitable for this purpose.It is also important to consider the compatibility of the hydrogel with efficient encapsulation methods,such as the high-throughput formation of multiple gel droplets in a short period and ease of adoption.
The shift towards utilizing defined materials in organoid production holds immense promise,particularly in the context of large-scale drug screening.The adoption of defined hydrogels should alleviate some challenges that current organoid models face associated with the use of Matrigel,such as reproducibility concerns,the need for low temperatures to prevent Matrigel gelation,and high cost.Furthermore,many of these defined materials are amenable to customization for compatibility with manufacturing or good manufacturing practice standards.For instance,the combination of alginate and organoids can facilitate high-throughput production through microfluidics,paving the way for automation and translational applications(Cohen et al.2023).This approach could prove particularly beneficial for SCOs,where there is a pressing need to develop novel drugs targeting conditions like amyotrophic lateral sclerosis,infectious diseases,and developmental disorders.
Customizable hydrogels also provide an intriguing opportunity to engineer regionalized and polarized SCOs (Figure 2).The idea involves conjugating different parts of the hydrogel with morphogens in a gradient that surrounds the organoids.A more advanced method involves adding tunability to the hydrogel properties,allowing post-encapsulation changes in components.This approach enables precise spatiotemporal control of organoid development.While these concepts have been explored in the field of organoid engineering,their application to the spinal cord,with its distinct regional characteristics—basal-apical,rostralcaudal,dorsal-ventral—remains largely uncharted territory.Recent advancements suggest that early developmental stages can capture these axes in organoids using Matrigel (Gribaudo et al.,2023),but it will be fascinating to ascertain whether engineered hydrogels can replicate them.Customizable hydrogels offer precise control over organoid development and ideally reduce variability when scaled up for broader usage.This approach has the potential to elucidate the varying manifestations of spinal cord diseases across different regions and neuronal networks,with organoid engineering using hydrogels serving as a valuable tool in this endeavor.
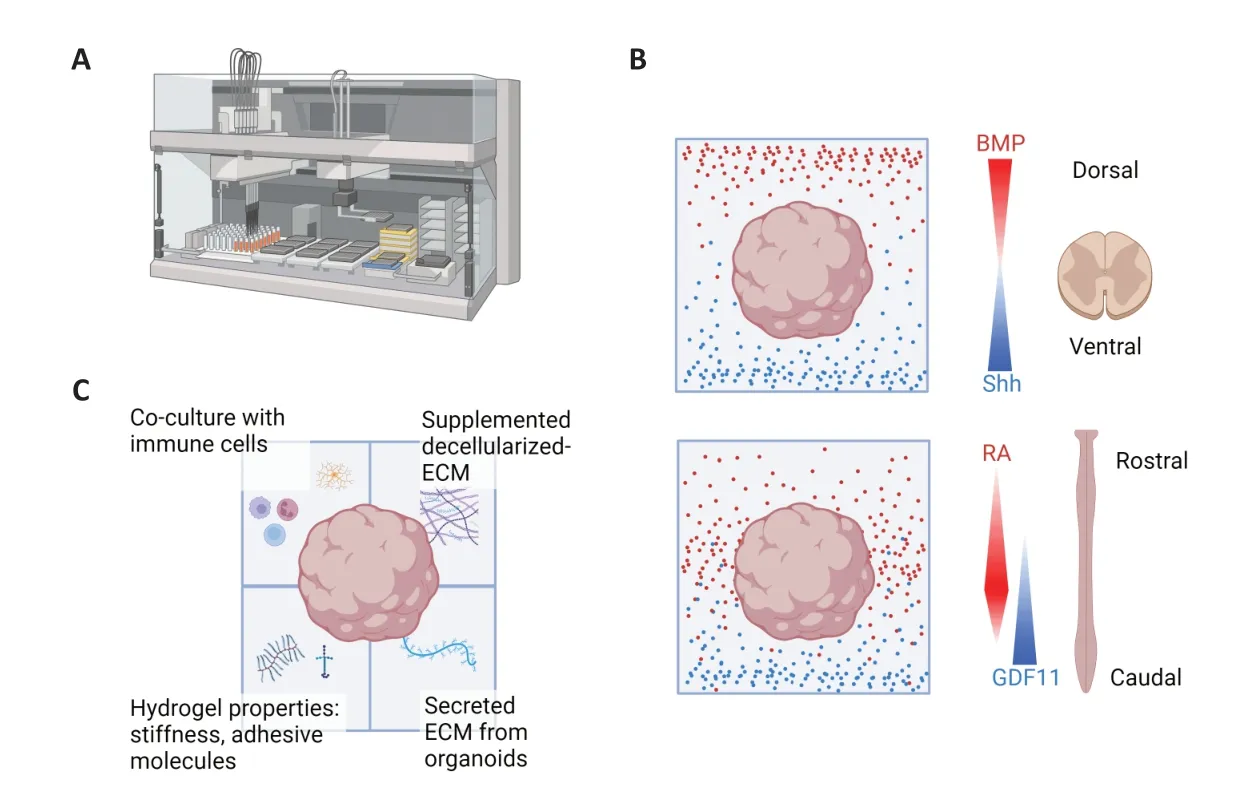
Figure 2|Potential applications for spinal cord organoids in hydrogels.
In addition to controlling organoid development,manipulating the microenvironment of organoids using hydrogels offers another avenue for investigation.In the field of gut organoids,hydrogels have been employed to encapsulate organoids,enabling the study of interactions between organoids and immune cells (Jowett et al.,2021).This approach allows for the exploration of secreted factors or ECM components trapped within the gel—an aspect that the use of Matrigel might obscure.Notably,Matrigel may contain matrix metalloproteinase inhibitors that hinder the study of matrix remodeling (Jowett et al.,2021).In the context of spinal cord research,where injury-induced ECM changes and inflammation are known to occur,this could serve as an invaluablein vitromodel.A similar approach can facilitate the examination of interactions between the SCO and other tissues or cells over time in precisely controlled co-culture systems.Such a method is also possible if one desires to study the effect of decellularized matrices that do not instantaneously form a solid ECM gel on spinal cord aging,diseases,or regeneration.
In conclusion,the field of SCO research is evolving rapidly,offering tremendous potential for applications in disease modeling,drug screening,and regenerative medicine.The transition from traditional Matrigel-based cultures to defined hydrogels presents both challenges and exciting opportunities.Defined hydrogels,with their tunable properties and customizable nature,hold the promise of enhancing the reproducibility and scalability of spinal cord organoid cultures.These materials can be tailored to facilitate large-scale drug screening endeavors,engineer regionalized SCOs,and enable the study of the dynamic interactions between the organoids and the microenvironment.The urgent need for drug development in conditions like amyotrophic lateral sclerosis and developmental disorders makes this transition all the more crucial.The shift towards defined hydrogels in spinal cord organoid research signifies a significant step towards overcoming the limitations associated with Matrigel.While challenges remain,the potential applications and insights gained from this transition are promising.As we continue to unravel the mysteries of spinal cord development and diseases,defined hydrogels will play a crucial role in advancing our understanding and therapeutic strategies.
This work was supported by A*STAR Career Development Fund(C210112011)and National Medical Research Council(MOH-001248-00)(to WHC);Singapore International Graduate Award(to YW);National Research Foundation(NRFNRFF-2018-003)and Biomedical Research Council,A*STAR Research Entities(to SYN).
Wai Hon Chooi*,Yuewen Wu,Shi-Yan Ng
Institute of Molecular and Cell Biology (IMCB),Agency for Science,Technology and Research(A*STAR),Singapore,Singapore (Chooi WH,Wu Y,Ng SY)
Department of Physiology,Yong Loo Lin School of Medicine,National University of Singapore (NUS),Singapore,Singapore (Wu Y)
*Correspondence to:Wai Hon Chooi,PhD,chooi_wai_hon@imcb.a-star.edu.sg.
https://orcid.org/0000-0003-0268-0938(Wai Hon Chooi)
https://orcid.org/0000-0003-3418-2757(Shi-Yan Ng)
Date of submission:October 7,2023
Date of decision:December 7,2023
Date of acceptance:December 26,2023
Date of web publication:January 31,2024
https://doi.org/10.4103/NRR.NRR-D-23-01665
How to cite this article:Chooi WH,Wu Y,Ng SY(2024)Defined hydrogels for spinal cord organoids:challenges and potential applications.Neural Regen Res 19(11):2329-2330.
Open access statement:This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- A sphingolipid message promotes neuronal health across generations
- Krüppel-like factor 2 (KLF2),a potential target for neuroregeneration
- Neuronal trafficking as a key to functional recovery in immunemediated neuropathies
- Advancements in personalized stem cell models for aging-related neurodegenerative disorders
- New insights into astrocyte diversity from the lens of transcriptional regulation and their implications for neurodegenerative disease treatments
- Harnessing endothelial cells and vascularization strategies for nerve regeneration

