Advancements in personalized stem cell models for aging-related neurodegenerative disorders
Mingxi Weng,Ralf Jauch
Neurodegenerative diseases (NDDs) are a class of disorders characterized by the gradual loss or malfunction of specific cell populations in the nervous system,which can be triggered by genetic or environmental factors.As a result,patients often experience a decline in mobility,sensation,memory,and cognition,which can ultimately lead to a fatal outcome.The global incidence of NDDs,including Alzheimer’s disease,Parkinson’s disease,Huntington’s disease,amyotrophic lateral sclerosis (ALS),and multiple sclerosis,is increasing.Many NDDs are associated with aging,and they pose significant challenges to affected families,healthcare systems,and societies,which will only be exacerbated by the current demographic megatrend.Clinical management of NDDs is primarily focused on mitigating physical or mental impairment,slowing disease progression,and providing palliative care.Despite extensive research efforts spanning decades,there are currently no effective cures for NDDs.This is largely due to a limited understanding of disease mechanisms,the heterogeneity of the pathophysiology,and the failure to identify specific pathways and molecular targets that cause the diseases.For example,most ALS cases are sporadic without clear-cut genetic cause and a unique patient-specific etiology.Disease-associated cell types,such as neurons in the patient’s brain,are not easily accessible for clinical evaluations.The lack of patient biopsies and the scarcity of representative animal models hinder mechanistic studies and the discovery of potential treatments.
Probing the pathophysiology of NDD with human cell models:To uncover the fundamental mechanisms of NDDs and evaluate therapeutic approaches,various animal models of neurodegenerative disorders have been generated across different species and diseases.However,while these models have proven useful,they fall short of accurately representing the pathophysiology of human diseases and the complexity of an intact human nervous system.Consequently,attempts to translate findings from research solely relying on animal models to clinical and therapeutic applications have largely failed.Therefore,it is essential to establish authentic human cell or organoid models that can reflect normal neural biology and pathogenesis to develop NDD therapeutics.Direct access to cells from the human central nervous system is limited,which hinders studies on primary human cells.However,advances in stem cell research and cell fate engineering technologies have enabled the study of the root causes of diseases using cells directly from patients.Through cell fate engineering,the identity of cells can be manipulated using genetic or pharmacological means,despite profound epigenetic barriers.This way,cells from human skin,blood,or urine can be turned into a neuronal cell type that dies in a given NDD.These tailored approaches using ‘biopsy-like’tissues could lead to the discovery of reversible pathological processes,and empower early diagnosis and personalized therapeutic strategies.Cell fate engineering by cellular reprogramming and stem cell differentiation has led to cell models to study human disease in a dish and to evaluate therapies using cultured cells.Typically,human cell models can be derived from pluripotent stem cells (PSCs),including embryonic stem cells and induced pluripotent stem cells (iPSCs).More recently,alternative cell models have begun to emerge.
Limitations of pluripotent stem cell models to study age-linked NDDs:To study NDDs,primary human cells can be turned into iPSCs with the ectopic expression of defined transcription factors including OCT4,SOX2,KLF4,and C-MYC.iPSCs resemble embryonic stem cells naturally found in the blastocyst of pre-implantation embryos.These cells can be kept infinitely,are amenable to gene editing,and can be produced on a large scale.PSCs can be differentiated into all cell types of the human body such as neurons and glial cells.This way,cellular defects and disease features can be examined in a patient-specific genetic background or following the intentional introduction of disease-linked mutations.It is also possible to make more complex models by combining multiple iPSC-derived cell types and or by generating self-organizing brain organoids (Cederquist et al.,2019).iPSC-derived motor neurons of ALS patients die faster,their neurite growth is impaired and they show abnormal protein aggregation compared to healthy controls (Fujimori et al.,2018).Such cell models have successfully been used to understand disease mechanisms and to identify potential therapeutic interventions with pathology-reversing effects (Fujimori et al.,2018;Αbo-Rady et al.,2020;Hung et al.,2023).The majority of NDD cases arise sporadically with unknown causes.Making individual cell models istime-consuming and costly.It usually takes around 2–3 months to generate iPSCs and a further 6–15 weeks to differentiate them into mature neurons.This makes it difficult to quickly and efficiently generate personalized iPSC-based models of sporadic NDD patients.Furthermore,iPSCs exhibit rejuvenation to an embryonic state,evident through various markers such as DNΑ methylation,global gene expression profiles,telomere length,and cellular senescence.The reset of aging-related features in iPSCs represents a major challenge to utilize stem cell technologies to study and revert age-linked diseases.One recent study showed that iPSC-derived neurons of ΑLS patients show similar transcriptomes as healthy controls,regardless of age and disease status suggesting that iPSC generation masks critical biomarkers (Workman et al.,2023).iPSC-based cell models for NDDs usually require long culture or external stressors,maturations via co-cultures with gliogenic cells,or forced overexpression of maturation genes (Hung et al.,2023) to evoke disease phenotypes.Hence,alternative cell fate engineering methods have been applied to obtain cell models that better capture the pathogenesis of NDDs.
Directly reprogrammed neurons exhibit aging features:Αfter the discovery of iPSCs,it was found that direct interconversion of somatic cell types is also possible.For example,skin fibroblasts can be directly transdifferentiated into induced neurons (iNs) without transitioning through a stem cell state.Unlike the rejuvenation seen in iPSC generation,this direct lineage reprogramming maintains the epigenetic and cellular aging characteristics of the donor cells.To study agingassociated NDDs,iNs can be generated using specific transcription factors and microRNAs.For example,iNs derived from fibroblasts of Alzheimer’s disease patients show agingdependent transcriptional and cellular defects,and increased cellular senescence (Herdy et al.,2022).Similarly,induced striatal neurons from Huntington’s disease patient fibroblasts exhibit age-related disease phenotypes,including protein aggregates,DNA damage,and spontaneous neurodegeneration (Oh et al.,2022).In contrast,iPSC-derived neurons retain a fetal-like state and mask disease phenotypes.iNs generated from patient cells are unique cell sources to understand the link between aging and NDDs.However,iN-based models are fraught with two major challenges.Firstly,iNs are post-mitotic and cannot divide,making it difficult to scale up their production.Secondly,they are unable to differentiate into other cell types such as glial cells.These limitations restrict the utility of iNs in complex NDD modeling,organoid generation,and high-throughput screening.
Towards alternative stem cell models to capture and revert neuronal aging and disease:An ideal cell model for NDDs would combine the scalability,editability,and developmental plasticity of iPSCs with the maturity and accessibility of iNs to accurately evoke age-related disease phenotypes.Induced neural stem cells (iNSCs) have emerged as a potential alternative cell model that fulfills these requirements.
Previous studies have demonstrated the direct reprogramming of human somatic cells such as fibroblasts and blood cells into iNSCs with defined transcription factors (Sheng et al.,2018;Xiao et al.,2018;Thier et al.,2019).However,the limited reprogramming efficiency,lengthy procedures,and unclear cell conversion trajectories during iNSC generation have hindered their widespread application.Α recent study took advantage of an engineered SOX17 (eSOX17) factor that is distinct from wild-type SOX17 and acquired biochemical features of a super SOX2 (Hu et al.,2023).eSOX17 could enhance and expedite the generation of iNSCs from both mouse fibroblasts and human blood cells (Weng et al.,2023).In mice,the reprogramming of iNSCs driven by eSOX17 was direct and did not involve transitioning through an intermediate pluripotent state,thus avoiding the complete epigenetic reset seen in iPSCs.In the case of human iNSC reprogramming,a combination of eSOX17 and c-MYC successfully transformed erythroid progenitor cells into neural lineages that exhibited self-renewal capabilities and could be further differentiated into neurons,astrocytes,and oligodendrocytes.
From a conceptual standpoint,iNSCs offer several advantages over cells derived from iPSCs and iNs.Firstly,direct reprogramming bypasses the pluripotency state,resulting in iNSCs that lack the oncogenic features of iPSCs,thereby substantially reducing the risk of tumorigenesis.This is of critical importance forin vivoreprogramming and if cells are considered for replacement therapies.Secondly,generating neurons from iPSC pipelines can take several months,whereas the direct reprogramming of somatic cells into iNSCs and further differentiation into mature neurons is markedly faster,requiring fewer steps.This would accelerate the generation of specific models of NDDs.Thirdly,iNSCs closely resemble primary NSCs in terms of morphology,global gene expression profile,and tripotencyin vitro.They exhibit self-renewal in long-term culture,which bestows them with clonogenicity and potential for genome editing.In lineage-favoring conditions,iNSCs can efficiently differentiate into neurons,astrocytes,and oligodendrocytes.While postmitotic iNs also exhibit some advantages over cells derived from iPSCs,their inability to divide limits their application in research,drug screening,and complex organoid cultures on a large scale (Figure 1).If iNSCs can lead to the generation of mature neurons that capture age-linked pathologies like iNs,their proliferation,and multipotency could represent a significant advancement for personalized stem cell models of age-associated NDDs.
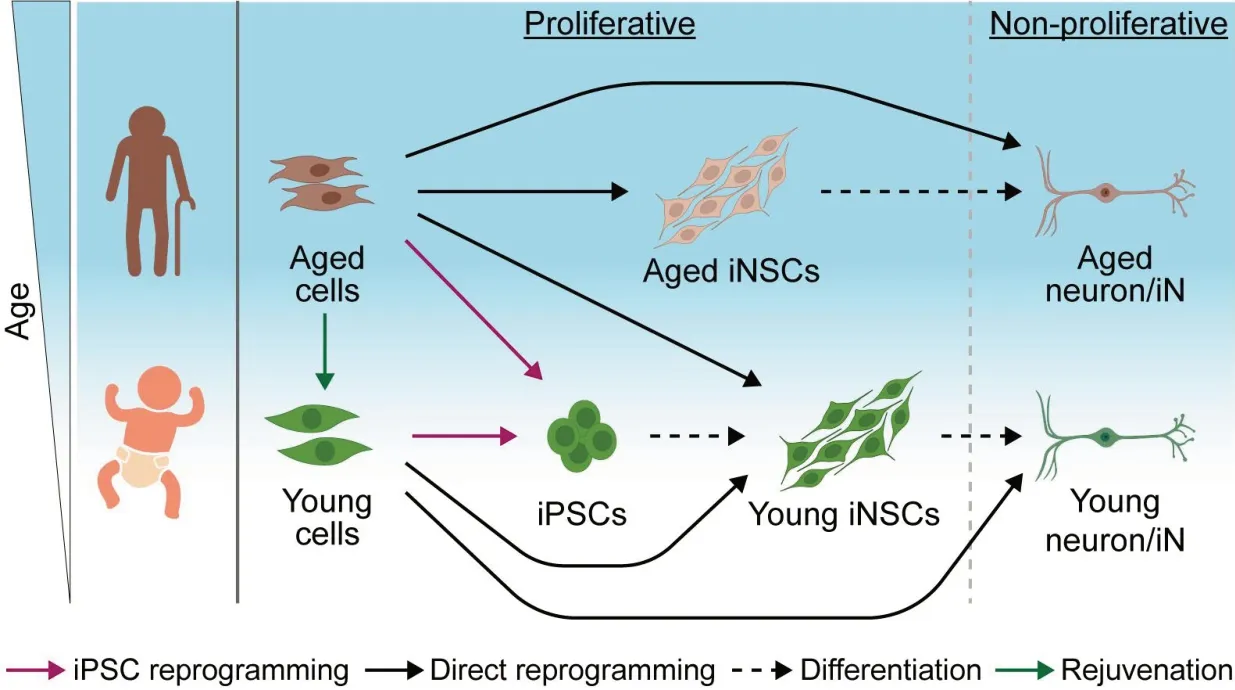
Figure 1|Cell models for neurodegenerative diseases.
In conclusion,the progress made in stem cell research and cell fate conversion technologies holds promise for the development of new therapies for NDDs.Nonetheless,there are still challenging studies to be conducted to establish scalable and authentic cell and organoid models of NDDs that can faithfully represent the disease phenotypes.iNSCs could overcome the limitations of current iPSC or iN-based models.A milestone would be the demonstration that iNSCs provide a mature or even aged stem cell model.This could then provide a platform to discover hitherto unknown causative pathways and genes and eventually lead to breakthroughs in therapeutic interventions.
Mingxi Weng,Ralf Jauch*
School of Biomedical Sciences,Li Ka Shing Faculty of Medicine,The University of Hong Kong,Hong Kong Special Αdministrative Region,China(Weng M,Jauch R)
Center for Translational Stem Cell Biology,Hong Kong Special Αdministrative Region,China(Weng M,Jauch R)
*Correspondence to:Ralf Jauch,PhD,ralf@hku.hk.
https://orcid.org/0000-0002-6590-9579(Ralf Jauch)
Date of submission:November 1,2023
Date of decision:December 1,2023
Date of acceptance:December 27,2023
Date of web publication:January 31,2024
https://doi.org/10.4103/NRR.NRR-D-23-01793
How to cite this article:Weng M,Jauch R(2024)Advancements in personalized stem cell models for aging-related neurodegenerative disorders.Neural Regen Res 19(11):2333-2334.
Open access statement:This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- A sphingolipid message promotes neuronal health across generations
- Krüppel-like factor 2 (KLF2),a potential target for neuroregeneration
- Defined hydrogels for spinal cord organoids: challenges and potential applications
- Neuronal trafficking as a key to functional recovery in immunemediated neuropathies
- New insights into astrocyte diversity from the lens of transcriptional regulation and their implications for neurodegenerative disease treatments
- Harnessing endothelial cells and vascularization strategies for nerve regeneration

