Core level excitation spectra of La and Mn ions in LaMnO3
Fujian Li(李福建), Xinlu Cheng(程新路), and Hong Zhang(张红)
Institute of Atomic and Molecular Physics,Sichuan University,Chengdu 610065,China
Keywords: lanthanum manganate, the core level excitation spectra, free-ion multiplet calculation, oxidation state
1.Introduction
The manganese-based perovskite LaMnO3has garnered significant attention due to its numerous applications in various fields, including solid-oxide fuel cells, electrocatalysis,supercapacitors, and colorimetric sensing.[1-6]As a type of A-type antiferromagnetic material, the N´eel temperature of LaMnO3is~141 K,[7]and various interesting phenomena have been discovered and reported, such as colossal magnetoresistance effects,[8]exchange bias in superlattices[9]and orbital ordering,[10,11]indicating its great potential for use in the field of materials science.The spectral analysis is essential for understanding the electronic structure of LaMnO3.X-ray photoelectron spectroscopy(XPS)and x-ray absorption spectroscopy (XAS) are typical spectral analysis methods.They can provide information on the reducibility,oxygen vacancies,and oxidation state of metal elements of perovskite oxides,which are important properties arising from structural features.
Accurate spectral simulations can be used not only to identify electronic structures but also to distinguish clearly between electronic and geometric effects on the spectral shape and edge position.[12]In recent decades,many theoretical approaches have been used to simulate LaMnO3transition spectra.A study by Elfimovet al.revealed that the polarization dependence ofK-edge scattering is mainly caused by 4p orbital effects and energy band structure effects.[11]Ravindranet al.examined the ground and excited state properties of LaMnO3through full-potential calculations,successfully simulating the XANES spectra and determining the respective contribution of different elemental components to the spectral intensity within distinct energy ranges near the Fermi surface.[13]Based on the dipole approximation, Takahashiet al.calculated theK-edge absorption spectrum of Mn and observed that the spectral intensity increased with local lattice distortion and is not sensitive to the magnetic order.[14]Taguchiet al.calculated the MnL2,3-edge scattering and absorption spectra in MnO6using a cluster model that considered the Mn intra-atomic multiplet state coupling.[15]According to their findings,the intensity of theL3-edge is strongly associated with the Jahn-Teller distortion, while the intensity of theL2-edge is closely correlated with the orbital ordering.Moreover,numerous theoretical approaches have been employed to calculate the near-edge structure of transition metals.For instance,Pinjariet al.simulated the XAS of ironL-edges using the multiconfigurational wavefunction theory.[16]TheL-edge spectra of transition metals were calculated with the CTM4XAS program package by Stavitskiet al.[17]de Grootet al.reviewed a series of principles and methods for calculating the 2p x-ray absorption spectra of transition metals, considering atomic multiplet effects,charge transfer effects,crystal field effects,and x-ray magnetic circular dichroism.[18]
The investigation of the valence state of Mn in manganese-based perovskites is significant.The Mn 3d orbitals undergo energetic degeneracy with O 2p orbitals,resulting in strong covalent interactions,which determine the formation of electronic structures and ferromagnetism.[5]Zhanget al.conducted experiments to explore the lanthanum-oxygen and manganese-oxygen interaction in LaMnO3.Their findings confirmed that lanthanum is present in a+3 valence state,while manganese exists in both+3 and+4 valence states,excluding the+2 valence state.[19-21]Valmoret al.investigated the local structure of the Mn site in the LaMnO3sample using MnK-edge x-ray absorption spectroscopy and showed that the Mn ions are at the intermediate oxidation state with an average valency of +3.4.[22]Depending on the valence state of manganese atoms, temperature and pressure, they exhibit different electrical and magnetic phases, including antiferromagnetic insulators or ferromagnetic-metallic, and also exhibit charge-orbit ordered phases.[5,23,24]LaMnO3usually has an Mn3+-O-Mn4+double exchange caused by the cation deficiency, which increases the average valence of Mn ions.[25]Under an oxygen-excess environment, the content of Mn4+increases as the oxygen vacancy decreases,resulting in an elevation of the average valence of Mn ions in LaMnO3,thereby facilitating the formation of mixed valence states.The strong tilting of the MnO6octahedra with respect to the ideal cubic perovskite structure and the remarkable distortion of the lattice by the Jahn-Teller (JT)effect leads to a change in the oxidation state of the Mn.In addition, many studies have demonstrated that proper doping of metal cations (e.g., Sr, Ba, and Ca) in LaMnO3can regulate the valence of Mn ions and enhance the electronic,magnetic,and transport properties of the material.[22,26,27]
The spectra show different features with different valence states of La and Mn in LaMnO3.The 3d spectrum of La shows well spin-orbit splitting, and the binding energy of La2+is shifted 3.1 eV lower than that of La3+.For Mn ions, the spectrum exhibits exchange splitting on theM-edge, and the magnitude of this splitting energy shows a certain linear relationship with the valence of the Mn ion.[28]In theL2,3-edges andK-edges, the spectrum shifts toward higher energy as the valence of the Mn ion increases.[27,29]The values of the whiteline intensity ratio(L3/L2)and spin-orbit splitting on theL2,3-edges depend on the valence of the Mn ion.[30,31]This article adopts a calculation method based on configuration interaction, which successfully avoids the interference of other ions on the x-ray absorption spectrum of the central ion in the experiment spectrum.As a result, more accurate ion spectral data was obtained.The origin of spectral peaks in LaMnO3crystals was identified through x-ray spectroscopy,and the oxidation states of La and Mn in LaMnO3were analyzed in detail.This helps us understand the electronic and geometric structure information of the material, and may also provide a new perspective for explaining some phenomena caused by the structural characteristics inside LaMnO3.In addition, identifying the redox states of Mn and La ions in LaMnO3can provide valuable insights into the electrochemical properties,which can be used to design and optimize material catalytic performance.
We organize the article as follows.A description of the computational methods is given in Section 2, which contains both atomic multiplet calculations and crystal field multiplet calculations.TheM-edge spectra of La ion,M-edge,L-edge andK-edge spectra of Mn ion in LaMnO3are discussed in Section 3 respectively.A conclusion is given in Section 4.The experimental spectra compared with this article were obtained by XAS and XPS.[27,28,32-35]
2.Methods
2.1.Free-ion multiplet calculation
A series of calculations were performed using the flexible atomic code(FAC)program for the transition spectra of the LaM-edge,the MnM-edge,L2,3-edge,andK-edge.These calculations primarily involve excitations: La 3d104f0→3d94f1;Mn 3s23d4→3s13d5, 2p63d4→2p53d5, 1s23d4→1s13d5,1s24s0→1s14s1and 1s24p0→1s14p1.The calculation of the multiple structure of the ionic core level is based on the configuration interaction of independent particle ground state wavefunctions.To account for the electronic screening effect resulting from the self-consistent field of the nuclear potential, the ground state wavefunction includes both the core potential and electron-electron interactions.The standard Dirac-Coulomb Hamiltonian is employed in the FAC, which thoroughly considers significant relativistic effects such as spin-orbit interactions and mass effects.[36]The standardj j-coupling mechanism is employed to simulate the transition spectra of La and Mn ions,and the Gaussian functions are used to fit the spectra in the MATLAB program.
The FAC program primarily investigates excitation transitions of free ions.However, in crystals, these transitions are additionally influenced by crystal field effects and interatomic interactions.In LaMnO3crystals, the Jahn-Teller effect changes the bond length of Mn-O in the MnO6octahedra,resulting in energy level shifts.[37,38]We considered the experimental spectrum of lanthanum oxide and manganese oxides to explain the deviations of experimental and calculated spectra of LaMnO3.[19,20,34,39,40]Our calculations reveal that the energy deviation in the La ion spectrum is less than 0.5 eV,while in the Mn ion spectrum it ranges from 2.5 eV to 5 eV.This can be attributed to the comparatively weaker interaction between La ions and O ions in the crystal, in contrast to the interaction between Mn ions and O ions.Consequently, the spectrum of La ion in the crystal closely resembles that of free ions.
2.2.Crystal field multiplet calculation
In crystal field multiplet theory,the transition metal ion is treated as an independent particle surrounded by point charges,which effectively captures the symmetry of the intermediate ion.[41]The 3d orbital energy of the transition metal(Mn)ion is influenced by the octahedral arrangement of the six oxygen atoms.The cubic crystal field plays a crucial role in splitting the five 3d orbitals by symmetry into a two-fold degenerate Eg(dx2-y2,d3z2-r2)and a three-fold degenerate T2g(dxy,dyz,and dxz).The splitting between the Egand T2gorbitals is defined by the parameter 10 Dq,where Egaccounts for 6 Dq and T2gfor 4 Dq.The Egorbital, oriented towards the oxygen ion in the octahedral environment, exhibits stronger antibonding interactions in comparison to the T2gorbital situated between the oxygen ions,thereby leading to its elevated energy state.The crystal field within the perovskite structure originates from covalent interactions with neighboring oxygen atoms, with the Egorbitals exhibiting stronger hybridization characteristics than the T2gorbitals.The existence of a large crystal field splitting indicates a high degree of hybridization,with typical splitting distances ranging from 1 eV to 2 eV.[18,42]
3.Results and discussion
3.1.Spectral study for M-edge of Mn in LaMnO3
In terms of shallow-core spectroscopy, Sen Guptaet al.propose a theory about the valence excitation of shallow-core between orbitals of the same principal quantum.[43]Their theory implies that the energy of the multipole transition corresponding to the bound state is lower than that of the dipole transition.This transition can be effectively simulated by the local single-ion model.[44]The spectral line with energy between 78 eV and 94 eV corresponds to the excitation of Mn3+:3s23d4→3s13d5, which is a quadrupole transition.The calculated results are shown in Fig.1.
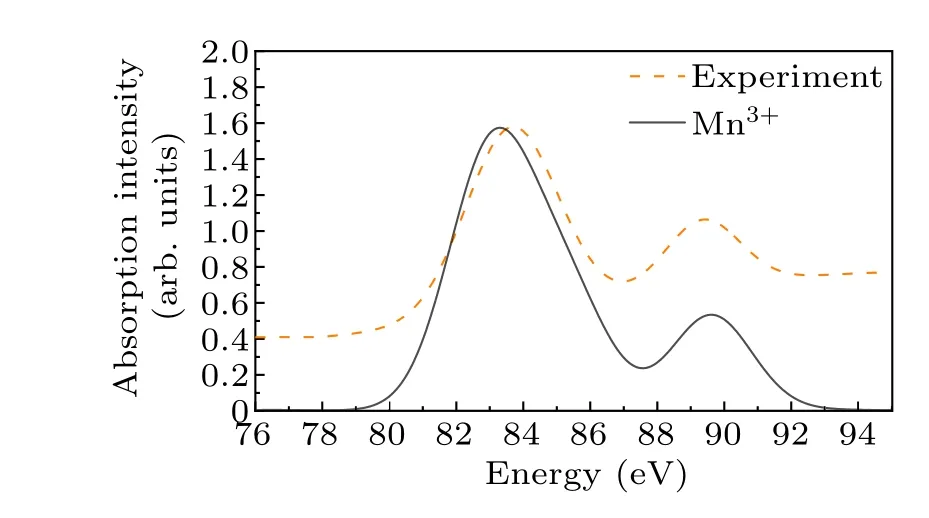
Fig.1.The calculated and experimental spectra at the M-edge of Mn3+in LaMnO3.
In the 3s core-level spectral of manganese ions,an exchange splitting is detected with a size of (2S+1)G2(3s,3d)/5, whereSis the local spin of the ground state 3d electron,andG2(3s,3d)is the Slater exchange integral between 3s and 3d electrons.[45,46]The exchange splitting of Mn3+is about 5.5 eV,but this value decreases as the Mn oxidation state increases.[28]Meanwhile, the presence of Mn2+and Mn4+during the experimental procedure has an impact on the spectra.In conclusion, free-ion multiplet calculations serve as a valuable tool in identifying Mn ion spectra, and the calculated spectrum matches the experimental spectrum closely.[47]
3.2.Spectral study for M-edge of La in LaMnO3
The oxidation state of La in LaMnO3was determined by analyzing theM-edge spectra of La ions.Figure 2 compares the calculated spectra of La2+and La3+ions and the experimental spectra of La in LaMnO3.The spectra with energies between 825 eV and 860 eV are produced by electron transition from La 3d to La 4f orbitals.The transition spectra show that the relative intensity of La2+is much greater than that of La3+,and the bond energy of La2+is 3.1 eV lower than that of La3+.The peaks observed at 833.92 eV and 836.70 eV in the La3+spectrum correspond to the splitting peaks of La 3d5/2,and the peaks at 850.73 eV and 853.09 eV correspond to La 3d3/2.The calculated spectrum of La2+exhibits a spin-orbit splitting energy of 17.22 eV, whereas La3+displays a spinorbit splitting energy of 16.81 eV.The experimental spectrum of La2O3exhibits comparable features to that of La3+, further confirming the reliability of the accuracy of the calculation results.[19,20,40]We compare the experimental spectra of La in LaMnO3with the calculated spectra of La2+and La3+.The analysis showed that the experiment spectra agree with the calculated spectra of La3+in terms of energy and shape.Consequently, it can be concluded that La in LaMnO3exists at a valence state of+3,which agrees with the results obtained by Flores-Lasluisaet al.[19,21,35,48]The experimental spectrum displays a discrepancy in multiple splitting values, which is 0.9 eV higher than the calculated value.This disparity can be attributed to the presence of lanthanide oxides in the experiment and the transfer of electrons from the oxygen center to the La 4f shell layer.[35,49]
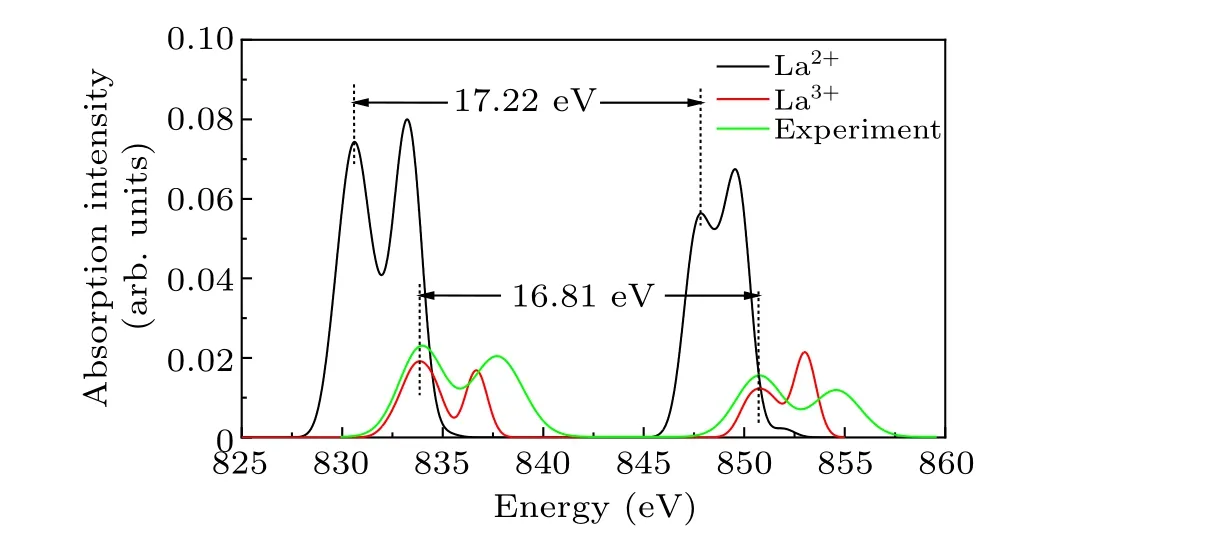
Fig.2.The calculated spectra in M-edge of La2+and La3+ and experimental spectra from XAS of LaMnO3.
3.3.Spectral study for L2,3-edge of Mn in LaMnO3
Figure 3 shows the calculated spectrum of Mn3+L2,3-edge alongside the experimental spectrum of LaMnO3.The spectral lines within the energy range of 635 eV to 660 eV are generated by the electron transition from Mn 2p orbitals to 3d orbitals.The Mn 2p core hole exhibits two broad multiplet states,namely,the high spin 2p1/2state(L2-edge)and low spin 2p3/2state (L3-edge), as a result of spin-orbit splitting.In addition,we incorporate crystal field corrections to account for the multiplet calculation of free ions, with a crystal field splitting energy of 10 Dq=2.0 eV for Mn.There are four possible transitions: 2p1/2→T2g, 2p1/2→Eg, 2p3/2→T2g,and 2p3/2→Eg.The interaction between the ligand and Egorbitals is significantly stronger than that of T2gorbitals in octahedral symmetry.[42]Therefore, the absorption peaks of Egare higher than T2gin theL2,3-edge spectra.This is illustrated in Fig.3,which showcases the enhanced resemblance between the ionic and crystal spectra resulting from the incorporation of the crystal field correction.

Fig.3.The experimental spectra from XAS of LaMnO3 and the calculated spectra in L2,3-edge of Mn3+: j-j coupling and j-j coupling+CF.
The influence of Mn2+, Mn3+, and Mn4+on theL2,3-edge spectrum varies, as depicted in Fig.4.To facilitate a more accurate comparison with the experimental spectra, all calculated spectra have been adjusted by 2.5 eV towards the lower energy direction.The obtained spectra are consistent with those experimentally derived by Moraleset al.[50,51]Two main factors are considered in this study.Firstly,the shape of theL2-edge peak indicates a stronger agreement between the theoretical and experimental spectra for Mn3+than for Mn4+,while no agreement is observed for Mn2+.Secondly, an increase in the valence state of the manganese ion leads to a shift in peak energy towards higher values.These descriptions suggest that the majority of the Mn ions in LaMnO3exist in the Mn3+state,with a minor fraction in the Mn4+state,while Mn2+is insignificant.
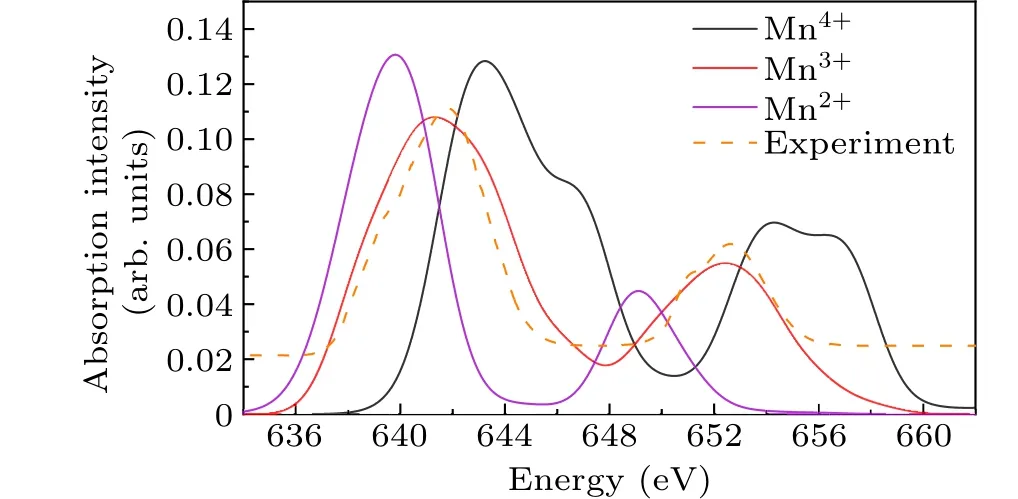
Fig.4.The calculated spectrum in L2,3-edge of Mn2+,Mn3+,and Mn4+and experimental spectrum from XAS of LaMnO3.
TheL2,3-edge spectrum of Mn is commonly employed in spectroscopy research for determining the valence state.Previous studies have documented various techniques for determining the oxidation state of Mn,with a focus on the shift of the peak toward higher energies as the Mn valence increases and the observed valence-dependent behavior of theL3/L2whiteline intensity ratio.[29-31]In our research, we calculated the spectrum of Mn3+and found that the white line intensity ratio was 1.972:1,which is near the single-particle theoretical value of 2:1.This result further supports the reliability of the computational method.Additionally, we synthesized the calculated spectra of Mn in various proportions and compared them with the experimental spectra.Figure 5 shows that a high degree of agreement between the theoretical and experimental spectra was observed when Mn3+is 90%and Mn4+is 10%.
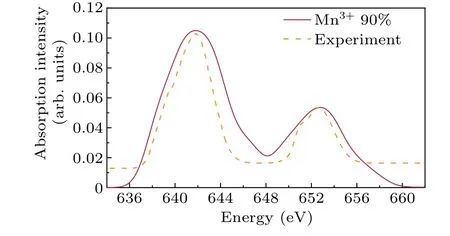
Fig.5.The calculated spectrum in L2,3-edge of Mn3+ and Mn4+ at different ratios and the experimental data for LaMnO3.
3.4.Spectral study for K-edge of Mn in LaMnO3
Figure 6 presents a comparative analysis of theK-edge spectrum of Mn2+, Mn3+, and Mn4+.The spectrum shifts toward higher energies as the valence of Mn ions increases,making theK-edge spectrum a valuable tool for determining the valence of manganese in compounds.[27,52]Notably, theK-edge spectrum of Mn3+is closest to the experimental spectrum in both shape and energy, while Mn4+displays a lesser degree of overlap.Conversely,theK-edge spectrum of Mn2+exhibits substantial deviation from the experimental spectrum,which echoes our conclusion inL2,3-edge.Our analyses indicate that Mn3+and Mn4+are the primary oxidation states of Mn ions in LaMnO3,while Mn2+is not detected.
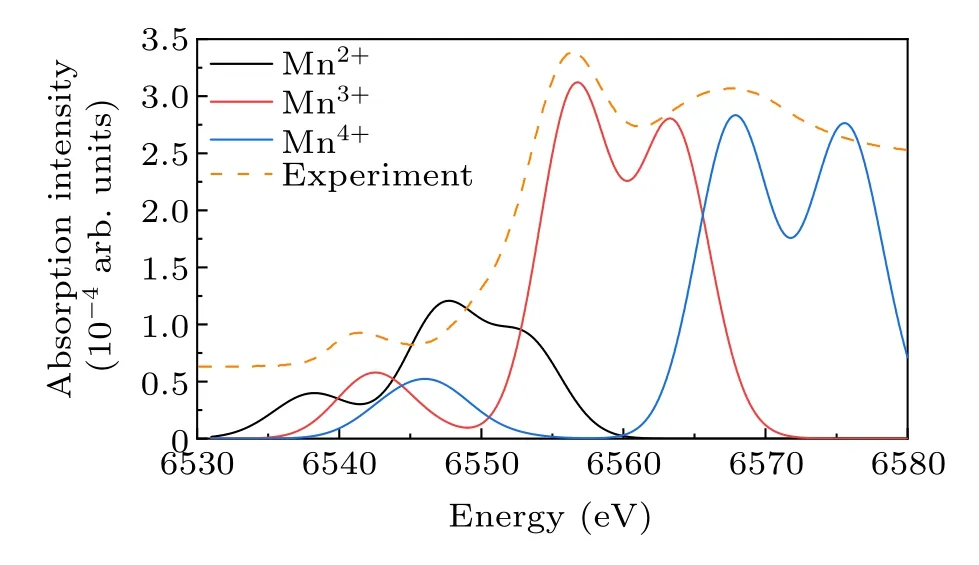
Fig.6.Calculated spectrum in K-edge of Mn2+, Mn3+, and Mn4+ and experimental spectrum from XAS of LaMnO3.
The ionic multiplet model allows us to identify spectral transitions that assist in analyzing the origin of the peaks.Specifically, in theK-edge spectrum of Mn3+, the prepeak structure at 6542 eV is located about 14 eV below the mainK-edge peak of manganese.This structure corresponds to a quadrupole transition occurring from the 1s core level to the empty 3d level,which represents a dipole-forbidden transition and consequently exhibits limited intensity.Elfimovet al.has proposed the existence of a dipole contribution to the prepeak in LaMnO3, which arises from the strong hybridization between the Mn 4p orbitals and the adjacent Mn atom’s 3d orbitals facilitated by oxygen 2p orbitals.[11]The peak observed at 6556 eV corresponds to the excitation from 1s orbital to 4s orbital of Mn3+, while the peak observed at 6563 eV corresponds to a dipole transition from 1s orbital to 4p orbital.Correspondingly, the peaks observed at energies of 6546 eV,6567 eV, and 6575 eV can be attributed to the excitations of Mn4+from the 1s orbital to the 3d,4s,and 4p orbitals,respectively.
The influence of different ratios of Mn3+and Mn4+in LaMnO3on theK-edge spectra is simulated in Fig.7.The results suggest that with an increasing proportion of Mn3+, the prepeak A shifts to lower energy.Moreover, there is a discernible trend of intensity transfer from a relatively weaker peak B (compared to peak C) to a relatively stronger peak B(compared to peak C).The synthetic spectra show a significant degree of concordance with the experimental spectra when the proportion of Mn3+falls within the range of 88%-90%.However,a discrepancy arises between the synthetic spectrum and the experimental spectrum at peak C.This discrepancy is attributed to the strong hybridization of the Egorbitals in the 3d of Mn with the 2p orbitals of the adjacent O ions,which causes a broadening of the energy band within the solid.
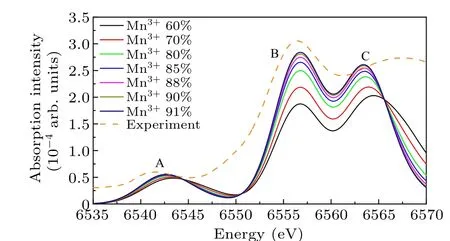
Fig.7.The calculated spectrum of Mn3+ and Mn4+ at different ratios and the experimental spectrum for LaMnO3.
There are several reasons for the appearance of mixed valence states of Mn ions in the LaMnO3: (i) The material exhibits the presence of cationic defects,such as vacancies in La or Mn ions, which lead to the valence state of some Mn ions changing from +3 to +4 in order to maintain overall electrical neutrality.(ii) An excess of oxygen ions in the material,which presents in metal ion vacancies or lattice gaps.This excess of oxygen ions causes an increase in the valence of Mn ions.(iii) The crystal structure can be affected by deviations from standard stoichiometric ratios,as well as the temperature and duration of the annealing process during its preparation,which further changes the valence state of Mn ions.
4.Conclusion
The core electron excitation spectrums for La and Mn are calculated,and a theoretical estimation for determining the effective oxidation states of La and Mn in LaMnO3is provided.From the point of free ions, we analyzed the contribution of La and Mn ions in various valence states to the spectrum by considering that the spectral shapes and mainline positions are mainly influenced by the oxidation state of the central atom.TheM-edge spectra of La demonstrate a concurrence between the experimental spectra and the calculated spectra of La3+in terms of the spin-orbit splitting values and peak positions,indicating that the La ion is positively trivalent in LaMnO3.However,theL2,3-edge andK-edge spectra of Mn,clearly indicate the presence of a mixed valence state in LaMnO3,predominantly composed of Mn3+with a minor fraction of Mn4+,while the absence of Mn2+is evident.The synthetic spectrum at theL2,3-edge aligns with the experimental spectrum when the Mn3+content is 90% and Mn4+is 10%, which is consistent with the Mn3+proportions ranging from 88% to 90%obtained at theK-edge.The results also indicate that a weak interaction between lanthanum and manganese in LaMnO3,while the interaction between manganese and oxygen is robust.This investigation enhances our ability to analyze crystal spectrum and deepens our understanding of the electronic structure information in perovskite materials.
Acknowledgment
Project supported by the National Natural Science Foundation of China(Grant No.11974253).
- Chinese Physics B的其它文章
- Does the Hartman effect exist in triangular barriers
- Quantum geometric tensor and the topological characterization of the extended Su–Schrieffer–Heeger model
- A lightweight symmetric image encryption cryptosystem in wavelet domain based on an improved sine map
- Effects of drive imbalance on the particle emission from a Bose–Einstein condensate in a one-dimensional lattice
- A new quantum key distribution resource allocation and routing optimization scheme
- Coexistence behavior of asymmetric attractors in hyperbolic-type memristive Hopfield neural network and its application in image encryption

