转录组和代谢组联合分析桑葚发育过程中可溶性糖和有机酸代谢的变化
张若彤 李蒙 齐一鸣 王晓萍 孙志超


摘 要:【目的】探究桑葚發育过程中可溶性糖和有机酸代谢及转录表达水平,揭示桑葚品质形成的分子机制。【方法】以青果期(W1)、转色期(W2)、成熟期(W3)白色桑葚为试验材料,分别测定3个阶段可溶性糖和有机酸含量及转录组变化,并基于转录组与代谢组联合分析揭示调控可溶性糖和有机酸代谢的分子机制。【结果】共检测到64种代谢物,其中有机酸52种、可溶性糖12种。分析发现,蔗糖、葡萄糖和D-果糖为桑葚中主要可溶性糖类物质,苹果酸、柠檬酸和琥珀酸为桑葚中主要有机酸类物质。转录组测序共获得58.65 Gb Clean Data,差异基因分析发现W3 vs W1组获得的差异基因数量最多为9098个。而KEGG富集分析表明,W2 vs W1和W3 vs W2组中差异基因富集到与糖酸代谢相关的通路,主要为淀粉和蔗糖代谢及三羧酸循环通路,在W2 vs W1组中有52个上调的差异基因富集到淀粉和蔗糖代谢,27个上调的差异基因富集到柠檬酸循环,在W3 vs W2组中有27个上调的差异基因富集到淀粉和蔗糖代谢。代谢组和转录组关联分析表明,NINV、HK、CS、ACO、MDH和ICDH是桑葚糖酸积累的关键调控基因。荧光定量分析(qRT-PCR)表明,关键调控基因在不同发育时期表达上调,与转录组中表达趋势一致。【结论】基因NINV、HK、CS、ACO、MDH和ICDH在桑葚成熟中可溶性糖和有机酸的合成与代谢中具有重要调控作用,初步揭示了桑葚口感变化的生物学基础。
关键词:桑葚;代谢组;转录组;可溶性糖;有机酸
中图分类号:S663.2 文献标志码:A 文章编号:1009-9980(2024)04-0690-13
Transcriptome and metabolome combined analysis metabolism change of soluble sugars and organic acids in mulberry fruit during development stages
ZHANG Ruotong, LI Meng, QI Yiming, WANG Xiaoping, SUN Zhichao*
(Institute of Sericulture, Chengde Medical University, Chengde 067000, Hebei, China)
Abstract: 【Objective】 Through the systematic study of the metabolism and molecular mechanism of sugar and organic acids, the mechanism of fruit taste formation was well revealed. In this study, we investigated the metabolism of soluble sugar and organic acid and transcriptome expression levels during the development of mulberry (Morus alba) in order to reveal the molecular mechanism of fruit quality formation of mulberry. 【Methods】 White mulberry fruits were used as experimental materials at greening stage (W1), transforming stage (W2) and ripening stage (W3) . The content and transcriptome of the soluble sugar and organic acid at three stages were determined separately, and the molecular mechanism of regulation of the soluble sugar and organic acid metabolism were analyzed based on the combination of transcriptome and metabolome. By exploring the key differential genes regulating the synthesis and metabolism of the soluble sugar and organic acid during mulberry ripening, the metabolic network was proposed to elucidate the influence of fruit development on the metabolism of the soluble sugar and organic acid. The UPLC-MS/ MS targeted metabolomics method was used to detect the changes of metabolites at 3 developmental stages, and the Cluster analysis was performed on the obtained different substances. The expression of the differential genes was analyzed by qRT-PCR and the obtained differential genes were further analyzed through KEGG (Kyoto encyclopedia of genes and genomes) pathway enrichment analysis. 【Results】 A total of 64 metabolites were detected, including 52 organic acids and 12 soluble sugars. According to the cluster analysis of different substances, the results showed that there were obvious changes in sugar and acid metabolism during the mulberry fruit development. Through the data analysis, it was found that the sucrose, glucose and D-fructose were the main soluble sugars in mulberry fruits, and their contents continued to increase during the development of mulberry fruits, and reached a peak at W3. The malic acid, citric acid and succinic acid were the main organic acids in mulberry fruits. According to the assembly analysis of the transcriptome sequencing data of the mulberry samples at different developmental stages, a total of 58.65 Gb was obtained. The differential gene analysis of gene expression at different developmental stages showed that W3 vs W1 group had the largest number of differential genes, reaching 9098. The Venn map was drawn for the 3 different genes in comparison combinations, among them 762 genes were expressed in common. The W3 vs W1 group contained the largest number of the unique differential genes, with 2836 differential genes. The second group was W2 vs W1 with 499 unique differential genes, and the least group was W3 vs W2 with 195 unique differential genes. The results showed that transcription and translation of a large number of genes were activated at the beginning of fruit development, while transcription and translation of some genes were inhibited at maturity. The KEGG enrichment analysis showed that the differential genes in W2 vs W1 and W3 vs W2 groups were enriched into carbohydrate-related metabolic pathways, which were mainly starch and sucrose metabolism and tricarboxylic acid cycle pathways. In the W2 vs W1 group, 52 upregulated differential genes were enriched in the starch and sucrose metabolism, and 27 upregulated differential genes were enriched in the citric acid cycle. In the W3 vs W2 group, 27 upregulated differential genes were enriched for the starch and sucrose metabolism. Combined with the differential gene identification, correlation analysis and common KEGG pathway analysis of the differential genes and differential metabolites related to soluble sugar and organic acid metabolism were carried out, there were significant differences in the expression of some candidate genes related to the soluble sugar and organic acid metabolism in mulberry. In this study, four differentially expressed SUSY genes were detected, and their expression levels were high in the early stage of fruit development, but significantly decreased with fruit development; three differentially expressed NINV genes were detected, and their expression increased with the development of fruit. Two differentially expressed FRK genes were identified, which were highly expressed at the early stage of fruit development; one differentially expressed HK gene was identified, and its expression gradually increased with the fruit development. In addition, this study also found that the expression of the two MDH genes increased during fruit ripening, and the expression of the MDH was significantly correlated with malic acid content. These results indicated that these genes play a significant role in the regulation of mulberry maturation. The metabolome and transcriptome association analysis showed that the NINV, HK, CS, ACO, MDH and ICDH were the key regulatory genes of saccharic acid accumulation in mulberry. The qRT-PCR analysis showed that the expression of key regulatory genes was up-regulated at different developmental stages, which was consistent with the expression trend in the transcriptome. The TCA cycle was promoted in the ripening process of mulberry fruits, and then affected the change of the organic acid content, and the change of the organic acid content ultimately affected the taste difference of the fruits. 【Conclusion】 The NINV, HK, CS, ACO, MDH and ICDH would play important regulatory roles in the synthesis and metabolism of the soluble sugars and organic acids during mulberry maturation, which initially revealed the biological basis of mulberry taste change. The rich metabolites and differential genes identified will not only provide a lot of information for high-quality genetic improvement of mulberry, but also provide valuable reference for other mulberry crops.
Key words: Mulberry; Metabolome; Transcriptome; Soluble sugars; Organic acids
桑樹是桑科(Moraceae)桑属(Morus)多年生木本植物,广泛分布在亚洲亚热带区域(包括韩国、日本、中国和印度)、北美和非洲,中国是世界桑树种类最多的国家[1-2]。桑葚为桑树的果实,其具有较高的营养价值,部分桑葚品种被用作传统的中草药。桑葚中富含黄酮、有机酸、酚酸、糖醇、氨基酸和多羟基生物碱等多种生物活性化合物,与沙棘、悬钩子一起被誉为“第三代水果”[3-4]。近年来国内外广泛关注基于桑葚代谢组学的相关研究,桑葚中含有大量的营养物质,包括可溶性糖、氨基酸、有机酸含量等理化指标,且这些理化指标对桑葚的代谢途径产生重要影响,进而影响桑葚的生长发育全过程[5]。而目前关于桑葚可溶性糖和有机酸代谢分子机制的研究却少有报道。
甜度是水果感官质量评估中的一个重要特征,由果实的代谢物组成决定,例如糖和有机酸[6]。在大多数水果中,蔗糖是决定果实品质的主要成分[7-8]。在甜瓜果实研究中发现,蔗糖积累是甜瓜果实中一个受发育调控的过程,经历了果实生长早期到蔗糖积累阶段的代谢转变,其中涉及十几种酶促反应[9]。此外,糖与有机酸的比例对果实品质有显著影响[10]。一般来说,果实中有机酸的代谢是一个复杂的生理过程,有机酸的含量是由酸合成与降解的平衡决定的[11]。迄今为止,利用转录组测序、基因组和功能分析对水果中蔗糖和有机酸积累进行了大量研究,其中大多数研究只关注少数酶的活性[12-14]。因此,对糖和有机酸的代谢和分子机制的系统研究将很好地揭示果实口感形成的机制。
近年来,基于功能“组学”方法的综合分析为识别生命系统中的基因网络及其调控机制提供了一种有效手段[15-16]。特别是转录组和代谢组的结合分析已被广泛用于确定植物果实中糖和有机酸积累的信号通路和机制。如利用转录组分析结合靶向代谢组学研究了两个杧果品种的差异糖积累机制,发现蔗糖和D-葡萄糖的合成伴随着淀粉的降解,直接导致了果实的高糖积累[17]。然而,对桑葚果实中糖和有机酸调控的关键基因网络的全面研究还很缺乏。因此,为深入研究桑葚果实中糖和有机酸关键调控基因网络,笔者在本研究中以白色桑葚为研究对象,通过整合转录组学和代谢组学分析,挖掘桑葚成熟过程中调控可溶性糖和有机酸合成与代谢的关键差异基因,进而探明代谢网络,阐明果实发育对可溶性糖和有机酸代谢的影响。
1 材料和方法
1.1 试验材料
选择河北省承德市承德医学院蚕业研究所桑园为试验区,选取大小、生长势基本一致,气候条件和栽培管理基本相同的7年生稳定结果的白色果实的珍珠白品种为试验材料,依据果实发育的颜色进行取样,对不同果实分别在授粉后(DAP)10 d(青果期)、30 d(转色期)、50 d(成熟期)3个时期进行取样,取样均在桑树外围进行,选5株树进行取样,每株树每时期各取10个整果,3次重复,样品名分别为W1(W11,W12,W13);W2(W21,W22,W23);W3(W31,W32,W33),用液氮冷冻后放入?80 ℃超低温冰箱备用。
1.2 可溶性糖及有机酸含量检测及分析
将样品真空冷冻干燥后,利用研磨仪研磨(30 Hz,1.5 min)至粉末状;称取20 mg的样品粉末,加入500 μL提取液(V甲醇∶V异丙醇∶V水=3∶3∶2),涡旋3 min,冰水中超声30 min。4 ℃,14 000 r·min-1 离心3 min,吸取50 μL上清液,加入20 μL质量浓度为100 μg·mL-1的核糖醇内标溶液,氮吹并冻干机冻干。加入100 μL甲氧铵盐吡啶(15 mg·mL-1),37 ℃孵育2 h,随后加入BSTFA 100 μL,37 ℃孵育30 min,得到衍生化溶液。取50 μL的衍生化溶液,用正己烷稀释至1 mL,保存于棕色进样瓶中,用于气相色谱串联质谱(GC-MS)分析[18-19]。VIP>1且p<0.05的代谢物被认为是差异代谢物。
1.3 RNA提取及转录组测序
使用TRIzol(Invitrogen,CA,USA)法对样品的总RNA进行分离和纯化。使用Bioanalyzer 2100(Agilent,CA,USA)对RNA的完整性进行检测,选择RNA完整性数(RIN)≥7的样品进行后续分析。使用oligo(dT)磁珠[Dynabeads Oligo(dT),货号25-61005,Thermo Fisher,USA]通过两轮的纯化对其中带有PolyA(多聚腺苷酸)的mRNA进行特异性捕获。将捕获到的mRNA在高温条件下利用镁离子打断试剂盒(NEBNext? Magnesium RNA Fragmentation Module,货号E6150S,USA)进行片段化,94 ℃ 5~7 min。将片段化的RNA在逆转录酶(Invitrogen SuperScript? Ⅱ Reverse Transcriptase,货号1896649,CA,USA)的作用下合成cDNA。然后使用E. coli DNA polymeraseⅠ(NEB,货号m0209,USA)与RNase H(NEB,货号m0297,USA)进行二链合成,将这些DNA与RNA的复合双链转化成DNA双链,同时在二链中掺入dUTP Solution(Thermo Fisher,货号R0133,CA,USA),将双链DNA的末端补齐为平末端。再在其两端各加上一个A碱基,使其能够与末端带有T碱基的接头进行连接,再利用磁珠对其片段大小进行筛选和纯化。以UDG酶(NEB,货号m0280,MA,US)消化二链,再通过PCR预变性95 ℃保持3 min,98 ℃变性总计8个循环每次15 s,退火到60 ℃保持15 s,72 ℃下延伸30 s,延伸72 ℃保留5 min,使其形成片段大小为(300 ± 50) bp的文库。最后,使用Illumina Novaseq? 6000(LC Bio Technology CO.,Ltd. Hangzhou,China),按照标准操作对其进行双端测序,测序模式为PE150。原始读取首先使用Trimmomatic进行质量控制处理,以获得干净的读取。使用HISAT2将干净的reads比对到桑树基因组[20]。基因表达水平由每千碱基每转录本每百万映射读数(FPKM)的片段数反映。使用Cufflinks计算每个基因的FPKM值,使用HTSeqcount计算每个基因的读取计数。使用R包DESeq2[21]对样本之间进行差异显著性分析,采用p<0.05、|log2FC|≥1的阈值确定差异表达基因,并对其进行GO和KEGG(Kyoto encyclopedia of genes and genomes)富集分析。
1.4 糖合成相关基因qRT-PCR分析
使用大连宝生物工程有限公司生产的TaKaRa MiniBEST Universal RNA Extraction Kit试剂盒提取桑树10、30、50 DAP果实总RNA,反转录使用大连宝生物工程有限公司生产的PrimeScript? RT reagent Kit试剂盒合成cDNA,qRT-PCR使用大连宝生物工程有限公司生产的SYBR Premix Ex TaqTM Ⅱ。以桑树Ribosomal protein L15为内参基因(表1)。qRT-PCR反应体系组成:SYBR Premix Ex TaqTM Ⅱ 5 μL,cDNA 0.5 μL,正向引物0.4 μL,反向引物0.4 μL,加水至10 μL。反应程序:95 ℃预变性30 s;95 ℃变性5 s,60 ℃退火20 s,72 ℃延伸40 s,共40个循环。PCR扩增反应在CF×96 TM Real-Time PCR Detection System(Applied Biosystems,Forter City,CA,美国)仪器上进行,每样品3次生物学重复,3次技術重复,反应结束后应用2-△△Ct算法进行分析。
1.5 数据分析
使用SPSS 27.0软件进行统计分析,使用单因素方差分析计算样品之间的差异显著性,在0.05水平进行Duncans检验(p≤0.05),数据表示为平均值± SD(标准差),每个样本3个独立重复。相关性分析采用皮尔逊方法,用SPSS 27.0软件进行。
2 结果与分析
2.1 代谢物分析与代谢物差异积累
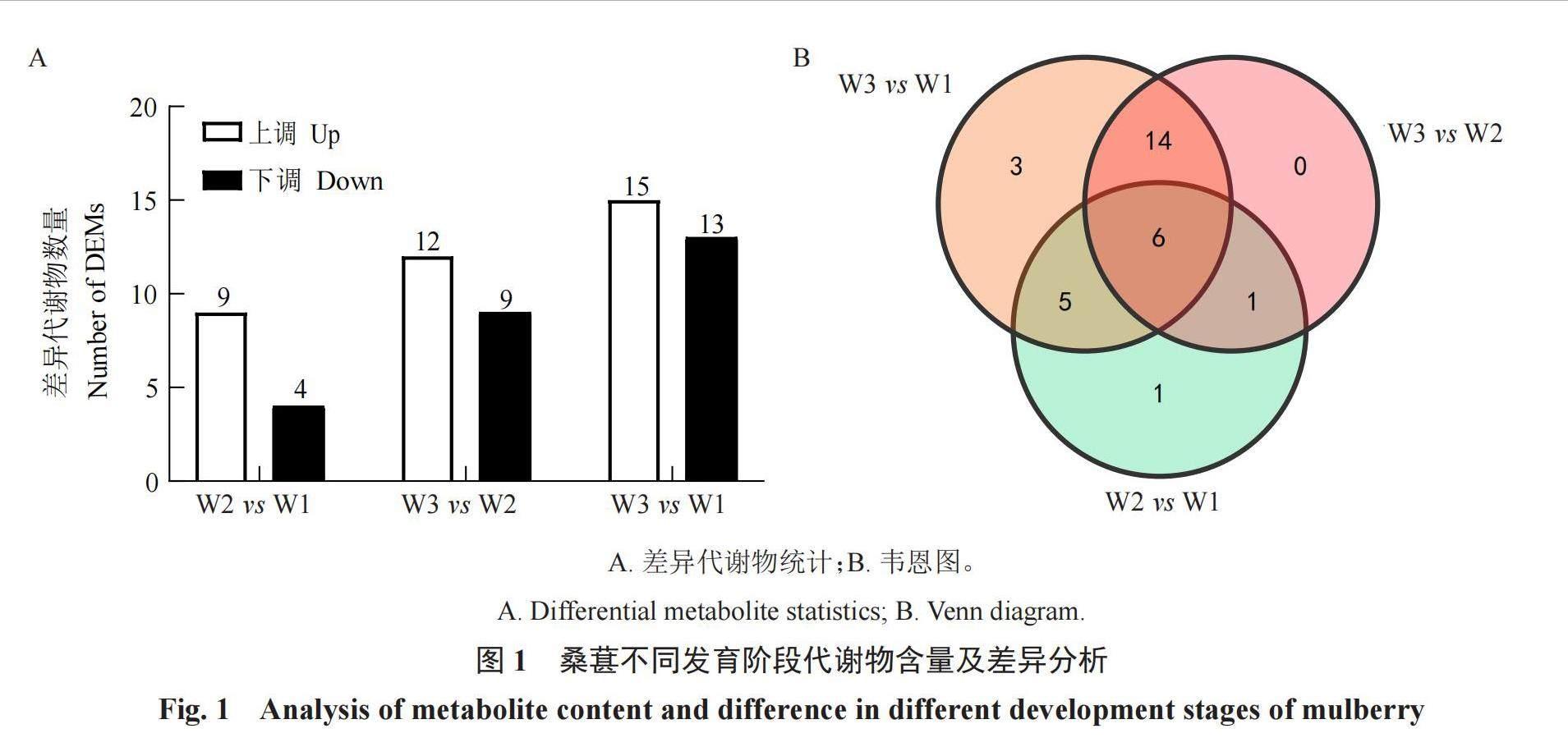
为了解桑葚发育过程中糖和有机酸成分含量的变化,采用GC-MS方法检测3个发育期代谢物成分含量的变化。研究共检测到64种代谢物,其中有机酸52种、可溶性糖12种(表2)。通过差异代谢物质分析发现,在W2 vs W1、W3 vs W2和W3 vs W1中,差异代谢物上调和下调的数量分别为9和4个、12和9个、15和13个(图1-A)。Venn图显示,所有组有6种相同的差异代谢物,W2 vs W1仅有1个特有的差异代谢物,为肉桂酸(图1-B),W3 vs W2没有发现特有的差异代谢物,W3 vs W1中检测到莽草酸、3,4-二羟基苯乙酸、5-羟基吲哚-3-乙酸。通过数据分析发现,蔗糖、葡萄糖和D-果糖为桑葚中主要可溶性糖类物质,其含量在桑葚发育过程中持续增加,并在W3达到峰值。苹果酸、柠檬酸和琥珀酸为桑葚中主要有机酸类物质,苹果酸和琥珀酸的含量在桑葚果实成熟过程中呈先上升后下降的趋势,而柠檬酸的积累呈现持续上升趋势,说明这3种可溶性糖和有机酸为影响桑葚口感的主要糖和酸类物质(表2)。
2.2 转录组测序
不同处理发育时期桑葚样品转录组测序数据的组装分析见表3,共获得58.65 Gb有效数据。各样本有效读数在39 037 544~48 942 774之间,Q20均为99.99%;Q30在97.79%~98.39%之间。分别将各样品有效度数与桑树参考基因比对,比对效率为95.95%~96.99%,表明测序获得数据可靠,可用于后续分析。
2.3 基因差异表达分析
对不同发育阶段基因表达以p<0.05、|log2FC|≥1作为筛选标准进行差异基因分析,在W2 vs W1、W3 vs W2和W3 vs W1的比较中,分别鉴定出6063个差异基因,其中2082个上调,3981个下调;1923个差异基因,其中793个上调,1130个下调;9098个差异基因,其中2915个上调,6183个下调(图2-A)。对3个比较组合差异基因绘制韦恩图,其中共有的表达基因有762个,而特有差异基因W3 vs W1组最多,为2836个;其次W2 vs W1组,为499个;最少的是W3 vs W2组,为195个(图2-B)。由此可以推断,在果实发育初期(S2)大量的基因转录和翻译可能被激活,而在成熟期基因的转录和翻译可能被抑制。
2.4 差异基因KEGG富集分析
为进一步分析差异表达基因在桑葚发育过程中的作用,分别对3个比较组中的差异基因进行KEGG通路富集分析,在W2 vs W1和W3 vs W2组中差异基因富集到与糖酸代谢相关的通路,主要为淀粉和蔗糖代谢(starch and sucrose metabolism)和柠檬酸循环(TCA cycle)(图3)。其中,在W2 vs W1组中有52个上调的差异基因富集到淀粉和蔗糖代谢,27个上调的差异基因富集到柠檬酸循环,在W3 vs W2组中有27个上调的差异基因富集到淀粉和蔗糖代谢。此外,在W2 vs W1组和W3 vs W1中,差异基因数量富集较多的代谢通路还包括核糖体(ribosome)、激素信号转导(plant hormone signal transduction)、MAPK信号通路-植物(MAPK signaling pathway-plant)(图3-A、B)。在W3 vs W2组中,差异基因富集数量较多的代谢通路主要有黄酮类生物化合物的合成(flavonoid biosynthesis)31个,半乳糖代谢(galactose metabolism)28个、植物昼夜节律(circadian rhythm-plant)25个(图3-C),由此可以推断桑葚在S2阶段大量基因表达被激活,合成桑葚成熟的代谢物质。
2.5 代谢物与差异表达基因关联分析
采用Pearsons计算淀粉和蔗糖代谢及柠檬酸循环中差异表达基因与糖酸主要代谢物之间的相关性。在糖代谢物与差异基因相关性分析中共确定43个与蔗糖、葡萄糖、果糖成正相关的差异表达基因,基因与代谢物均随果实发育表达呈现不断积累的模式(图4-A)。在有机酸代谢与合成中共鉴定到24个与苹果酸、柠檬酸、琥珀酸显著相关的差异表达基因,其中负相关基因有8个,正相关的有16个基因(图4-B)。上述关键代谢物和差异表达基因可能是桑葚成熟过程中主要的物质和基因。
2.6 桑葚中可溶性糖和有机酸合成途径分析
结合差异基因鉴定、相关性分析,表明与可溶性糖和有机酸代谢相关的一些候选基因在桑葚中表达存在显著差异(图5)。NINV和SUSY可将蔗糖转化为果糖和葡萄糖,检测到4个差异表达的SUSY基因(LOC21391172,LOC21407811,
LOC21386815,LOC21402491),其中2个(LOC21386815,LOC21402491)在果实发育初期表达水平很高,而随着果实发育表达水平呈现大幅度下降的趋势;检测到3个差异表达的NINV基因(LOC21386769,LOC21401851,LOC21401285),其中2个(LOC21401851,LOC21401285)随着果实的发育表达呈现上升的趋势。葡萄糖和果糖可被HK和FRK磷酸化为葡萄糖-6磷酸(G6P)和果糖-6-磷酸(F6P)。鉴定到2个差异表达的FRK(LOC21409854,LOC21406385)在果实发育初期高表达;鉴定到1个差异表达的HK(LOC21408947)基因,其表达随着果实发育表达逐渐升高(图5)。三羧酸(TCA)循环中草酰乙酸经CS催化直接合成柠檬酸,柠檬酸被ACO降解为异柠檬酸,异柠檬酸被ICDH转运生成2-戊羟二酸。CS(LOC21399865)和ICDH(LOC21407110,LOC21391200,LOC21390016)基因在果实发育过程中表达量大幅升高,说明桑葚中柠檬酸代谢增强并受这些基因调控。MDH与果实中苹果酸的生物合成和降解有关。2个MDH基因(LOC21399030,LOC21401654)在果实成熟过程中表达量增加,而且MDH的表达与苹果酸含量显著相关。以上结果表明,这些基因在桑葚成熟过程中发挥着显著的调控作用。
2.7 差异基因qRT-PCR表达分析
对筛选获得的可溶性糖和有机酸代谢中关键调控基因NINV(LOC21401851)、HK(LOC21408947)、CS(LOC21399865)、ACO(LOC21409265)、MDH(LOC21399030)和ICDH(LOC21391200)进行qRT-PCR表达,并与各基因在不同发育时期的转录本表达比较。6个基因的表达水平与转录组数据一致(图6),表明6个基因在桑葚成熟过程中发挥关键调控作用。
3 讨 论
可溶性糖和有机酸含量是衡量果实品质和口感的重要指标。因此,揭示桑葚果实可溶性糖积累和有机酸代谢的分子机制具有重要意义。不同组学技术的结合深入地解析了枇杷、西瓜、杧果等成熟果实中糖积累和有机酸代谢的机制[11,17,22]。蔗糖几乎是低糖和高糖积累植物中总糖含量变化的全部因子[23]。笔者在本研究中共测定12种可溶性糖,通过分析仅发现蔗糖、葡萄糖和D-果糖含量差异显著,在桑葚发育过程中含量明显增加,并在W3达到峰值。在果实成熟的中后期,这3种糖的快速积累可能决定了桑葚的甜度。同样,在其他果实的研究中也观察到了类似的糖积累模式[24,14]。有机酸在水果营养中起着至关重要的作用,其含量取决于酸合成和降解之间的平衡[14]。中等浓度的有机酸可以增强水果的味道,但高酸含量往往会降低水果的品质。柠檬酸和苹果酸是甜瓜果实中的主要有机酸[25]。在桑葚中检测到丰富的苹果酸、柠檬酸和琥珀酸,苹果酸和琥珀酸的含量在桑葚果实成熟过程中呈先上升后下降的趋势,而柠檬酸的积累呈现持续上升趋势。这说明苹果酸、柠檬酸和琥珀酸为桑葚的主要酸,苹果酸和琥珀酸合成和降解之间的平衡影响着果实的口感。在笔者课题组的研究中4-氨基丁酸和莽草酸等有机酸随着果实的发育积累量呈现降低的趋势,马来酸在果实成熟前期未检测到积累,而在成熟时检测到其大量的积累。综上所述,丰富多样的糖和有机酸是随着桑葚的成熟呈现不同程度的积累与降解,这些变化影响着果实最终的口味。
蔗糖由叶片(源组织)的光合作用产生,随后转运到果实(汇组织)并储存在果实中[26]。蔗糖的这种远距离转运是由蔗糖转运蛋白和SWEET外排蛋白控制的,而SWEET在功能上具有底物偏好蔗糖、葡萄糖或果糖[27-28]的特点。蔗糖进入到水果细胞可以通过NINV转化为果糖和葡萄糖,SUSY也可以催化蔗糖转化为果糖和D-葡萄糖[29]。检测到2个SUSY(LOC21386815,LOC21402491)在果实发育初期中表达水平很高,而随着果实发育表达水平呈现大幅度下降;NINV基因则随着果实的发育表达呈现上升的趋势。结果表明桑葚中蔗糖转化为葡萄糖和果糖主要受NINV基因调控。葡萄糖和果糖被HK和FRK磷酸化为葡萄糖- 6磷酸(G6P)和果糖-6-磷酸(F6P)[30]。笔者在本研究发现,所鉴定到差异表达的2个FRK在果实发育初期高表达;而仅鉴定到1个差异表达的HK基因,且其表达随着果实发育逐渐升高,这表明桑葚中通过促进HK基因的表达,将葡萄糖转化为糖酵解等下游过程的中间化合物(图4)。在对甜瓜的研究中发现高甜度和低甜度的两个品种中,高甜度品种中抑制糖转化为中间化合物的基因HK和FK的表达[22]。
TCA循环在能量代谢、糖异生、脂肪生成和氨基酸合成中发挥重要作用。草酰乙酸经CS催化直接合成柠檬酸,然后柠檬酸被ACO降解为异柠檬酸,然后异柠檬酸被ICDH转运生成2-戊羟二酸[11]。本研究发现,CS和ICDH基因在果实发育过程中表达量大幅升高,说明桑葚中柠檬酸代谢增强并受这些基因调控。MDH与果实中苹果酸的生物合成和降解有关[30]。本研究中发现两个MDH基因在果实成熟过程中表达量增加,而且MDH的表达量与苹果酸含量显著相关,表明它们是苹果酸代谢的关键参与者。综上所述,桑葚果實在成熟过程中TCA循环得到了促进,影响了有机酸含量,最终影响了果实的口感差异。
4 结 论
研究共检测到64种代谢物,其中有机酸52种、可溶性糖12种。数据分析发现,蔗糖、葡萄糖和D-果糖为桑葚中主要可溶性糖类物质,苹果酸、柠檬酸和琥珀酸为桑葚中主要有机酸类物质。转录组分析共获得58.65 Gb Clean Data,W3 vs W1组获得的差异基因数量最多,高达9098个。KEGG富集分析表明,W2 vs W1和W3 vs W2组中差异基因富集到与糖酸相关代谢通路,主要为淀粉和蔗糖代谢和三羧酸循环通路,在W2 vs W1组中有52个上调的差异基因富集到淀粉和蔗糖代谢,27个上调的差异基因富集到柠檬酸循环,在W3 vs W2组中有27个上调的差异基因富集到淀粉和蔗糖代谢。代谢组和转录组关联分析表明,NINV、HK、CS、ACO、MDH和ICDH是桑葚糖酸积累的关键调控基因。笔者在本研究中鉴定出的丰富代谢物和差异基因不仅为桑葚的优质遗传改良提供大量信息,而且也为其他浆果类作物的有关研究提供有价值的参考。
參考文献 References:
[1] KIM I,LEE J. Variations in anthocyanin profiles and antioxidant activity of 12 genotypes of mulberry (Morus spp.) fruits and their changes during processing[J]. Antioxidants,2020,9(3):242.
[2] SHREELAKSHMI S V,NAZARETH M S,KUMAR S S,GIRIDHAR P,HARISH PRASHANTH K V,SHETTY N P. Physicochemical composition and characterization of bioactive compounds of mulberry (Morus indica L.) fruit during ontogeny[J]. Plant Foods for Human Nutrition,2021,76(3):304-310.
[3] 刘晴晴,李勇,张明霞,余向阳,杨晨晔,武国华. 紫色桑葚和白色桑葚总酚含量、抗氧化能力及代谢指纹图谱差异分析[J]. 江苏农业学报,2022,38(3):813-820.
LIU Qingqing,LI Yong,ZHANG Mingxia,YU Xiangyang,YANG Chenye,WU Guohua. Differences in total phenol content,antioxidant activity and metabolic fingerprint between purple mulberry and white mulberry[J]. Jiangsu Journal of Agricultural Sciences,2022,38(3):813-820.
[4] LIN C Y,LAY H L. Characteristics of fruit growth,component analysis and antioxidant activity of mulberry (Morus spp.)[J]. Scientia Horticulturae,2013,162:285-292.
[5] LEE Y,HWANG K T. Changes in physicochemical properties of mulberry fruits (Morus alba L.) during ripening[J]. Scientia Horticulturae,2017,217:189-196.
[6] MA M Z,YANG X T,YING X G,SHI C,JIA Z X,JIA B C. Applications of gas sensing in food quality detection:A review[J]. Foods,2023,12(21):3966.
[7] SALADI? M,CA?IZARES J,PHILLIPS M A,RODRIGUEZ-CONCEPCION M,LARRIGAUDI?RE C,GIBON Y,STITT M,LUNN J E,GARCIA-MAS J. Comparative transcriptional profiling analysis of developing melon (Cucumis melo L.) fruit from climacteric and non-climacteric varieties[J]. BMC Genomics,2015,16(1):440.
[8] LI Z Y,WANG J B,FU Y L,JING Y L,HUANG B L,CHEN Y,WANG Q L,WANG X B,MENG C Y,YANG Q Q,XU L. The Musa troglodytarum L. genome provides insights into the mechanism of non-climacteric behaviour and enrichment of carotenoids[J]. BMC Biology,2022,20(1):186.
[9] DAI N,COHEN S,PORTNOY V,TZURI G,HAREL-BEJA R,POMPAN-LOTAN M,CARMI N,ZHANG G F,DIBER A,POLLOCK S,KARCHI H,YESELSON Y,PETREIKOV M,SHEN S,SAHAR U,HOVAV R,LEWINSOHN E,TADMOR Y,GRANOT D,OPHIR R,SHERMAN A,FEI Z J,GIOVANNONI J,BURGER Y,KATZIR N,SCHAFFER A A. Metabolism of soluble sugars in developing melon fruit:A global transcriptional view of the metabolic transition to sucrose accumulation[J]. Plant Molecular Biology,2011,76(1):1-18.
[10] SU L Y,ZHANG T,WU M,ZHONG Y,CHENG Z M. Transcriptome and metabolome reveal sugar and organic acid accumulation in Rosa roxburghii fruit[J]. Plants,2023,12(17):3036.
[11] MAYUONI-KIRSHINBAUM L,PORAT R. The flavor of pomegranate fruit:A review[J]. Journal of the Science of Food and Agriculture,2014,94(1):21-27.
[12] OBANDO-ULLOA J M,EDUARDO I,MONFORTE A J,FERN?NDEZ-TRUJILLO J P. Identification of QTLs related to sugar and organic acid composition in melon using near-isogenic lines[J]. Scientia Horticulturae,2009,121(4):425-433.
[13] NU?EZ-PALENIUS H G,GOMEZ-LIM M,OCHOA-ALEJO N,GRUMET R,LESTER G,CANTLIFFE D J. Melon fruits:Genetic diversity,physiology,and biotechnology features[J]. Critical Reviews in Biotechnology,2008,28(1):13-55.
[14] ZHANG H,WANG H S,YI H P,ZHAI W Q,WANG G Z,FU Q S. Transcriptome profiling of Cucumis melo fruit development and ripening[J]. Horticulture Research,2016,3:16014.
[15] XING A S,WANG X Y,NAZIR M F,ZHANG X M,WANG X X,YANG R,CHEN B J,FU G Y,WANG J J,GE H,PENG Z,JIA Y H,HE S P,DU X M. Transcriptomic and metabolomic profiling of flavonoid biosynthesis provides novel insights into petals coloration in Asian cotton (Gossypium arboreum L.)[J]. BMC Plant Biology,2022,22(1):416.
[16] WANG R,REN C X,DONG S,CHEN C,XIAN B,WU Q H,WANG J,PEI J,CHEN J. Integrated metabolomics and transcriptome analysis of flavonoid biosynthesis in safflower (Carthamus tinctorius L.) with different colors[J]. Frontiers in Plant Science,2021,12:712038.
[17] LI L,WU H X,MA X W,XU W T,LIANG Q Z,ZHAN R L,WANG S B. Transcriptional mechanism of differential sugar accumulation in pulp of two contrasting mango (Mangifera indica L.) cultivars[J]. Genomics,2020,112(6):4505-4515.
[18] MEDEIROS P M,SIMONEIT B R T. Analysis of sugars in environmental samples by gas chromatography-mass spectrometry[J]. Journal of Chromatography. A,2007,1141(2):271-278.
[19] SUN S H,WANG H,XIE J P,SU Y. Simultaneous determination of rhamnose,xylitol,arabitol,fructose,glucose,inositol,sucrose,maltose in jujube (Zizyphus jujube Mill.) extract:Comparison of HPLC-ELSD,LC-ESI-MS/MS and GC-MS[J]. Chemistry Central Journal,2016,10:25.
[20] HE N J,ZHANG C,…,XIANG Z H. Draft genome sequence of the mulberry tree Morus notabilis[J]. Nature Communications,2013,4:2445.
[21] PERTEA M,PERTEA G M,ANTONESCU C M,CHANG T C,MENDELL J T,SALZBERG S L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads[J]. Nature Biotechnology,2015,33:290-295.
[22] ZOU S C,WU J C,SHAHID M Q,HE Y H,LIN S Q,LIU Z H,YANG X H. Identification of key taste components in loquat using widely targeted metabolomics[J]. Food Chemistry,2020,323:126822.
[23] CHENG H,KONG W P,TANG T X,REN K L,ZHANG K L,WEI H X,LIN T. Identification of key gene networks controlling soluble sugar and organic acid metabolism during oriental melon fruit development by integrated analysis of metabolic and transcriptomic analyses[J]. Frontiers in Plant Science,2022,13:830517.
[24] SCHEMBERGER M O,STROKA M A,REIS L,DE SOUZA LOS K K,DE ARAUJO G A T,SFEIR M Z T,GALV?O C W,ETTO R M,BAPTIST?O A R G,AYUB R A. Transcriptome profiling of non-climacteric ‘Yellow melon during ripening:Insights on sugar metabolism[J]. BMC Genomics,2020,21(1):262.
[25] ATKINSON R G,GUNASEELAN K,WANG M Y,LUO L K,WANG T C,NORLING C L,JOHNSTON S L,MADDUMAGE R,SCHR?DER R,SCHAFFER R J. Dissecting the role of climacteric ethylene in kiwifruit (Actinidia chinensis) ripening using a 1-aminocyclopropane-1-carboxylic acid oxidase knockdown line[J]. Journal of Experimental Botany,2011,62(11):3821-3835.
[26] CHEN L Q,CHEUNG L S,FENG L,TANNER W,FROMMER W B. Transport of sugars[J]. Annual Review of Biochemistry,2015,84:865-894.
[27] CHEN L Q,QU X Q,HOU B H,SOSSO D,OSORIO S,FERNIE A R,FROMMER W B. Sucrose efflux mediated by SWEET proteins as a key step for phloem transport[J]. Science,2012,335(6065):207-211.
[28] JIANG R,WU L F,ZENG J M,SHAH K,ZHANG R,HU G B,QIN Y H,ZHANG Z K. Identification of HuSWEET family in pitaya (Hylocereus undatus) and key roles of HuSWEET12a and HuSWEET13d in sugar accumulation[J]. International Journal of Molecular Sciences,2023,24(16):12882.
[29] KOCH K. Sucrose metabolism:Regulatory mechanisms and pivotal roles in sugar sensing and plant development[J]. Current Opinion in Plant Biology,2004,7(3):235-246.
[30] ETIENNE A,G?NARD M,LOBIT P,MBEGUI?-A-MB?GUI? D,BUGAUD C. What controls fleshy fruit acidity? A review of malate and citrate accumulation in fruit cells[J]. Journal of Experimental Botany,2013,64(6):1451-1469.

