Assessment of food toxicology
Alexander Gosslau
a Department of Science(Biology),City University of New York,BMCC,New York,NY 10007,United States
b Department of Chemistry and Chemical Biology,Rutgers University,Piscataway,NJ 08854-8087,United States
Abstract The interest in food toxicology is evident by the dependency of humankind on nutrition by virtue of their heterotrophic metabolism.By means of modern biochemistry,molecular and cell biology,computer science,bioinformatics as well as high-throughput and high-content screening technologies it has been possible to identify adverse effects and characterize potential toxicants in food.The mechanisms of toxicant actions are multifactorial but many toxic effects converge on the generation of oxidative stress and chronic inflammation resulting in cell death,aging and degenerative diseases.Integration of food toxicology data obtained throughout biochemical and cell-based in vitro,animal in vivo and human clinical settings has enabled the establishment of alternative,highly predictable in silico models.These systems utilize a combination of complex in vitro cell-based models with computer-based algorithms.A decrease of rodent animal testing with its limitations of high costs,low throughput readouts,inconsistent responses,ethical issues and concerns of extrapolability to humans have led to an increased use of these but also alternative lower hierarchy surrogate animal models (e.g.Drosophila melanogaster; Caenorhabditis elegans or Danio rerio) and efforts to integrate organotypic systems and stem cell-based assays.Despite those achievements,there are numerous challenges in various disciplines of food toxicology.
Keywords: Food toxicology;Oxidative stress;Inflammation;In vitro,in vivo and in silico models;Alternative models
1.Overview
The history of food toxicity might have started as early as Hippocrates made the statement “Let food be thy medicine and medicine thy food” which presaged the modern science by over two millennia ago.With the development of modern biochemistry,molecular biology,cell culture techniques,computer science and bioinformatics,it has been possible to identify and characterize potential toxicants in food[1–7].Mechanistic insights gained by toxicity assessment of food using different models ranging fromin vitrobiochemical,cell-basedin vitro,animalin vivoto clinical settings have led to a better food safety.The growing interest in this area is reflected by a stunning 6280 publications in PubMed as of February 2016 when combining“food,toxicity,review”in searches and the exploding numbers of around 200 reviews per year on these topics starting from 2002(Fig.1).
There are two different related areas in the measurement of toxicants and toxicity in food:(1) actual measurements of the effects of toxicants in different models ranging fromin vitrobiochemical systems,cell-basedin vitrosystems,animalin vivomodels to clinical settings analyzing systemic or organ-specific toxicity and(2)assessment and/or predictions of potential toxicants in food.These two are interrelated since the mechanistic knowledge gained by the actual assessment of the effects of toxicants can lead to the identification of other potential toxicants in food.The majority of assessment systems for food toxicology were developed in the field of pharmacology[5,6,8,9].Pharmacology and nutritional science share common roots since many of the world’s most commonly used drugs are derived from natural products as illustrated by the term“nutraceutical”[10].

Fig.1.Reviews referenced in PubMed(www.PubMed.gov)as of February 2016 when combining“food,toxicity,review”in search.
The mechanisms of toxicant effects are multifactorial interacting intrinsically and extrinsically with key molecules which play major roles in cell integrity,metabolism,signaling pathways,gene expression and translation.For a variety of toxicants their effects appear to converge on the generation of electrophilic species(ES)leading to oxidative stress and chronic inflammation[11–15].Oxidative reactions induced by toxicants lead to an accumulation of damaged macromolecules thus harming cells,tissues and organs.Therefore,toxicants may play central roles in cell death,chronic inflammation,aging and degenerative diseases such as Alzheimers,Parkinsons and Huntingtons diseases,as well as multiple sclerosis,myocardial infarction,arteriosclerosis,diabetes,rheumatoid arthritis,sterility,cataracts and many others[13,14,16–23].
Forin vitroassessment a variety of biochemical systems have been developed to analyze damaging effects on integrity or activity of key biomolecules.Such molecules are important in cell integrity,metabolism,signaling pathways,as well as gene expression and translation.The list of affected molecules is extensive and includes enzymes,receptors,membrane lipids,nucleic acids and/or or factors involved in gene expression[3–6,24–29].On cellular level,a variety of viability assays are routinely used to quantify effects of potential food toxicants for extrapolation of range of dosages used for maximal tolerated concentrations forin vivoanimal models and also clinical settings [3–6,30–32].For more mechanistic insights,several cell-basedin vitrosystems were developed in combination with targetedin vitroanalyses which focus on cell-specific key enzymes and receptor-dependent pathways.In vivorodent models still appear to be the gold standard for toxicity assessment but there are limitations of such traditional testing such as high costs,low throughput readouts,inconsistent responses,ethical issues and concerns of extrapolability to humans[2,5,6,8].Consequently,new strategies have been developed and the paradigm in toxicology has switched from the traditional apical endpoint approach as determined in animal models to a mechanism-based approach byin silicomethods[6,7,29,33,34].
In silicoscreening systems,a combination of focusedin vitrocell-based models and computer based algorithms employ a variety of different high-throughput and highcontent screening technologies.Cell-specific biomarkers on gene,protein or metabolite levels can be measured by toxicogenomics,toxicoproteonomics or toxicometabonomics,respectively [6,27,35–40].The integration of food toxicology data obtainedvia in vitrobiochemical,cell-based,in vivoanimal models andin silicosystems have led to a mechanistic knowledge of systemic or organ-specific toxicity in humans and the identification and use of specific surrogate biomarkers in clinical settings.
Although complexin vitrocell culture systems integrated within silicosystems provide unique mechanistic insights intoin vivotoxicology more relevant to humans,they will never completely model the higher level complexity of cross-talk throughout different pathways present in an intact organism [1,2,4–6,8,41].Another refinement in toxicity assessment is the installation of alternative lower hierarchy surrogate animal models such as zebrafish (Danio rerio),fruit flies(Drosophila melanogaster)or nematodes(Caenorhabditis elegans).These models offer an advantage in terms of ethical concerns,high throughput and genetic manipulation over traditional rodent models[4–6,42,43].The value of using alternative sub-mammalian vertebrate and invertebrate models became evident by the surprising discovery of the high degree of homology of genes between humans and zebrafish,fruit flies or nematodes[5,43–49].
Overall,the achievements in food toxicology have significantly improved the prediction rate of drug and food safety in dimensions as unimagined only a decade ago [4–6,8,50–52].The deeper understanding of the molecular mode of action on key targets of biological pathways have enhanced the predictivity and robustness ofin vitrocell-based toxicity models and thus led to the improvement of food safety.Moreover,although in early development,stem cell-based screening or three-dimensional organotypic models will further increase the predictivity of acute toxicity and help to answer fundamental biological questions and/or enable testing of novel therapeutic approaches[6,7,53–57].
Despite those achievements,at present there are still huge challenges to increase the rate of predictivity in various areas such as reproductive and developmental toxicity,neurotoxicity,genotoxicity,carcinogenicity,immunotoxicity,food allergy,and endocrine disruption[4–6,8,27,58–60].
2.Molecular effects of toxicants
Although the mechanisms leading to toxic effects in humans are multifactorial the majority of toxic effects appear to converge on the generation of free radicals.Different electrophilic species (ES) such as reactive oxygen species (ROS) or reactive nitrogen species (RNS) are capable of oxidizing virtually all biomolecules.Whereas a variety of toxicants generate ES directly,others induce a secondary response leading indirectly to generation of ES by immunocompetent leukocytes which play a key role in the inflammatory cascade[14,15,61].ES are also involved in the modulation of gene expression by interfering with transcription factors and/or DNA which can lead to mutations and carcinogenesis.The accumulation of damage to membrane lipids,cellular proteins,carbohydrates as well as nucleic acids harm the functioning of cells,tissues and organs[11–15,62,63].These and other observations strengthen the hypothesis that toxicants leading to oxidative stress and chronic inflammation play central roles in cell death,aging and degenerative diseases[13,14,16–23].
ROS comprise differently reduced oxygen species such as the superoxide anion radical(•O2−),hydrogen peroxide(H2O2)and the highly reactive hydroxyl radical(•OH)[12,13,63–65].Dismutation of these radicals leads to hydrogen peroxide as a fairly stable ROS member.Heavy metals (iron,but also copper,chromium or vanadium),as an important group of toxicants,can generate the highly toxic hydroxyl radical by wayoftheFentonreaction(H2O2+Fe2+→•OH+OH−+Fe3+)[12,13,63,66].Therefore,the amount of hydroxyl radicals formed in a cell depends on endogenous ROS generation,but also on the amounts of reduced metal ions for the Fenton reaction to occur[66,67].The other highly reactive group of molecules consists of reactive nitrogen species(RNS).The signal molecule nitric oxide(NO)exists as NO+,NO•and NO–while the peroxynitrite ion(ONOO−)is generated by the reaction of NO with the superoxide anion radical[62,63,68].
The oxidation oflipids,proteins,nucleic acids and carbohydrates generate a variety of damaging breakdown products which thus can lead to the onset of many degenerative diseases [11–15,62,63,69].Lipid peroxidation of cell structures containing lipids can lead to the generation of different toxic products,including alcohols,ketones,alkanes,aldehydes and ethers which have the potential to contribute to cell damage,necrosis or apoptosis[26,63,70–72].For proteins thiolgroups of cysteine residues are the most sensitive targets of ES.Redoxdependent modifications ofintra- and intermolecular disulfide bonds can lead to structural/functional changes and protein aggregation [12,24,62,73–76].Altogether these ROS-induced damages may cause malfunctioning enzymes,transporters,signal transducers or structural proteins.Nucleic acids are delicate targets of ES leading to mutations.Damage of nucleic acids by ES may result in single and double strand breaks,DNA–DNA,DNA–protein,DNA–lipid adducts or numerous base modifications such as 8-hydroxy-deoxyguaonosine,5-hydroxylmethyluracil,8-hydroxydeoxyadenine and thyminglycol [12,19,24,77–80].Mitochondrial DNA (mtDNA) is particularly susceptible to oxidative damage because of the absence of associated histones,an incomplete mitochondrial DNA repair system and the generation of free radicals through electron leakage from the respiratory chain [78–80].Interestingly,carbohydrate oxidation may also be involved in DNA damage,as oxidation and fragmentation of deoxyribose fragments produced from DNA by free-radical attack are believed to play a major role in mutations by blocking the action of DNA polymerase and DNA ligase[19,27,58,81].
3.In vitro biochemical assessment of toxicity
Severalin vitrobiochemical assessment systems are focused either on the measurements of primary or secondary products derived from oxidized lipids,proteins,nucleic acids and carbohydrates or the integrity or activity of a variety of key biomolecules which play major roles in cell integrity,metabolism,signaling pathways,gene expression and translation[3,11–15,62,63].Testing whether a chemical can modulate the activity of particular enzyme or binding affinities to a particular receptor or other biomolecule is the most direct way to gain mechanistic insights into action at the molecular level.There are different biochemicalin vitroassays which analyze the integrity or mutation of DNA and RNA,membrane lipids,as well as the binding and activity of various receptors,enzymes involved in signaling transduction,drug or neurotransmitter metabolism and manyothers[3–6,24,25,27–29].Theriskassessmentofgenotoxicity by DNA-reactive toxicants in food is of particular interest by virtue of the close correlation with carcinogenesis[24,82,83].To measure the potential for genotoxic activity of food compounds which might lead to mutations traditionally the Ames bacterial reverse mutation test is used [84].The Ames test is based on the growth of several histidine dependent Salmonella strains carrying different mutations in various genes of the histidine operon [6,27,85].Other methods measuring genotoxic potential in cell-based systems are discussed below.
A variety of enzyme and receptor-binding assays have been developed to examine specific mechanisms of action at the molecular level of different receptors (e.g.ion channels,G-protein coupled receptors,tyrosine kinases,nuclear receptors),signaling transduction enzymes(kinases,proteases,phosphatases,phosphodiesterases),and enzymes metabolizing drugs (e.g.cytochrome P450 monooxygenases) or neurotransmitters(e.g.acetylcholinesterase).Asin vitroprescreening tools the human ether-a-go-go-related gene (hERG) potassium ion channel [86–88]or acetylcholinesterase activity assay [89,90]are routinely used for a global assessment of cardiotoxicity or neurotoxicity,respectively.Other assays monitor the potential effects of toxicants which interfere with anti-inflammatory drugs.These may utilize a high-throughput screening for microsomal prostaglandin E synthase activity[91].
Xenobiotic metabolism is a commonly encountered problem during development of new drug candidates thus applicable also for potential toxicants in food [4,9,25,28,34,92].By far the most important class of metabolic enzymes are cytochrome P450 (CYPs) monooxygenase drug metabolizing phase I enzymes but there are several other classes of biotransformation enzymes including uridine diphosphate glucuronosyltransferases (UGTs),glutathione S-transferases(GSTs),N-acetyltransferases(NATs),sulfotransferases(SATs)and methyltransferases (MTs) which are referred to phase II enzymes [25,28,93–95].The majority ofinhibition studies are using fluorescent or luminescent substrates and recombinant CYPs and UGTs but also various kinases.They employ high-throughput formats which involve protein-based microarrays [25,94–98].Other approaches use recombinant CYP450s metabolizing enzyme toxicology assay chips (MetaChips)which assess the toxicity of the generated metabolites by coupling with cell-based screening which thus enable a cell-specific screening[25,99].The abundance ofin vitrobiochemical testing platforms for CYP450 enzymes is understandable sincein vivothe liver is dependent on these enzymes for detoxification of xenobiotics.Many hepatoma-derived HepG2 cell lines used for hepatotoxicity screening lack functional expression of almost all the relevant human xenobiotic metabolizing enzymes in this family[28,92,100].
Lipid peroxidation characterized by radical chain reactions with the production of a variety of breakdown products frequently serves as marker for oxidative stress and inflammation in different biochemicalin vitroassays [26,63,66,70–72].Detection of generated aldehydes,particularly malondialdehyde,is used by the thiobarbituric acid test to determine the degree oflipid peroxidation;these are designated as“thiobarbituric reactive substances”(TBARS)[101].Because ofits sensitivity and simplicity this method is considered as a first global measure oflipid peroxidation in a variety of chemical as well as biological material [26,102].Other reliable and stable indicators of oxidative stress include F2-isoprostanes which are generated during lipid peroxidation [103,104].Tert-butyl hydroperoxide initiated chemiluminescence is another method which has been successfully utilized to detect oxidative damage associated to experimental or pathological situations in subcellular fractions,tissue homogenates,or different organs[105,106].
4.Cell-based in vitro assessment of toxicity
The use of cellular models provides a much higher level of complexity than simple biochemical assays.A huge number of humancelllinesareavailableandavarietyofdifferentcell-basedin vitroassays have been developed for screening of food toxicants[3–6,30–32,41].Usually two basic approaches are applied:(1)a universal screening approach using one or a few cell lines to assess cell viability and (2) a target-organ-based approach,using a panel of different cell types with more specialized functions,such as representative cell lines from different organs such as liver,heart,kidney lung,brain or others.
4.1.Viability assays
For general assessment of cytotoxicity an indirect measure of cell viability is usually performed and several cellular bioassays are routinely used integrating different cytotoxicity endpoints such as membrane leakage or cellular activity [6,30,31].Whereas the trypan blue,propidium iodide,crystal violet,or lactate dehydrogenase assays are analyzing membrane integrity based on exclusion,other viability assays such as the neutral red,alamar blue or MTT assay are metabolic measures of cellular activity.Inhibitory concentrations(IC values)obtained by viability assays are then used for initial dose selection in testing on animals and humans [3,5,30–32].Inhibitory effects on cell viability as a measure of cytotoxicity due to necrosis,apoptosis or autophagy can be further discriminated by the use of assay systems using a variety of specific multiplexed panels[30,31,69].
A prominent and reproducible method to analyze cell viability is the trypan blue exclusion method based on the ratio of stainedversusunstained cells in a sample as a reflection of membrane damage[107].Usually,the method is performed by cell counting but,an elegant quantitative measure of trypan blue staining has been introduced[108].Similiar to the trypan blue exclusion assay,the crystal violet assay is based on the growth rate reduction reflected by stained cells through reaction of crystal violet with negatively charged cell components such as nucleic acids or peptidoglycans [109,110].In addition to the measurement of dyes,activity analysis of enzymes is also an established technique used to determine membrane integrity.Leakage ofintracellular enzymes such as lactate dehydrogenase(LDH)or others into the extracellular medium is thus employed as indicator of cell membrane damage[30,31,111].
Other frequently used viability assays are based on cellular activity such as the neutral red (NR),MTT or alamar blue assay.Whereas the NR assay determines cell viability by endocytic uptake of neutral red into lysosomes of uninjured cells [112],the MTT viability assay is based on mitochondrial activity by conversion of the yellow tetrazolium salt MTT (3,(4,5-dimethylthiazol-2-yl)-2,5,-diphenyl-tetrazoliumbromide) by mitochondrial dehydrogenase activity to form a blue formazan product [113].Similiar to the MTT-method the alamar blue assay is based on redox-reactions converting blue,oxidized resazurin to reduced resorufin which is red and highly fluorescent [114].It has been reported that the MTT-method based on intracellular redox reactions may yield false-positive results for certain cell types when treated with different antioxidants [115].Therefore,it appears to be advisable to include other viability assays besides the MTT- but also the alamar blue method for cell-based toxicity screening[116].Damage of mitochondria is a major contributor to organ toxicity,such as of the liver,kidney,heart,muscle,and the central nervous system,and mitochondrial dysfunction is increasingly implicated in a growing list of degenerative diseases[117–119].Therefore,the impact of toxicants on mitochondria is of particular importance for toxicity assessment[6,118,119].
4.2.Genotoxicity,nutrigenomic and immunotoxicity testing
Due to the nature of many toxicants to generate electrophilic species it is not surprising that many of them induce oxidation and damage of DNA or RNA thus leading to genotoxicity[24,27,82,83,120].Since there is a strong correlation between genotoxicity and carcinogenicity,one aim of genotoxicity testing is to identify potentially carcinogenic food ingredients[6,27,120].Additionally,chronic inflammation is widely recognized as a major underlying contributor to carcinogenesis as well as various other degenerative conditions including cardiovascular-,Alzheimer’s disease,arthritis,and diabetes.Therefore,cell-based models have been developed which analyze the impact of food ingredients on inflammation using nutrigenomic screening,a discipline to analyze the influence of nutritional compounds on gene expression[61,121–123].
For cell-based genotoxic screening,various exploratory genetic toxicity assays are used to measure chromosomal damage in eukaryotic cells.Frequently used models are the mouse lymphoma tk gene mutation assay,the Chinese hamster ovary(CHO) chromosomal aberration assay,the micronucleus clastogenicity assay and the Comet assay [2,6,27].Interestingly,there has been a high false positive rate (low specificity) in genotoxicity testing which might be due to the recent discovery of non-covalent DNA interaction and interference of toxicants with critical DNA metabolizing proteins such as topoisomerase and DNA polymerases[27,58,79–81].The linkage between cellbasedin vitro,ex vivoandin vivoevaluation is evident by the micronucleus test used for genotoxic screening of chromosomal damage in bone marrow polychromatic erythrocytes [6,124].Also for immunotoxicity there are severalex vivotest models available.Functional immunological tests include activity of macrophages,natural killer cells and the immune responses of T-and B-cells[2,125,126].As for other human organ-basedex vivotissue or organ toxicology studies on heart,brain,lung,kidney or liver there is an advantage with thein vitrotoin vivocomparison/extrapolation.
4.3.Complex cellular toxicity assays
For viability,genotoxicity,immunotoxicity or nutrigenomic testing usually one cell line is employed.Recently,novel,more complexin vitrohuman cell-based systems are being increasingly used to model mammalian tissues in a more holistic approach [5,6,29,51].A variety of parameters such as key signaling pathways,gene and protein expression levels,receptor activity,cytoskeletal and membrane integrity,energy status,morphology of cell organelles,cell movement,cell cycle status,and cell differentiation can be quantified on single cell level.Complex humanin vitrocell-based models are well suited to predict thein vivoresponse by comparison throughout the panel of different cells by quantitativein vitrotoin vivoextrapolation thus decomposing complex toxicological pathways of particular organs[4–6,9,25,27,32,34,41,127].Correlation analysis with animal and clinical studies have led to a high predictivity and robustness of those complex cell-basedin vitromodels which do not have issues with interspecies extrapolation[5,7,34,128].Consequently,high content as well as high-throughput complex cellularin vitromodels focusing on organ-specific toxicity such as hepatotoxicity[127,129,130],nephrotoxicity[131,132],neurotoxicity [133–135],cardiotoxicity [59,136],and respiratory toxicity[137,138]are routinely used.An even higher level of complexity is achieved by three-dimensional organotypic models.Although still in its infancy organotypic systems have a very high potential for predicting acute toxicity and can be used to answer fundamental biological questions,and enable testing of novel therapeutic approaches,often using patient-derived cells[7,53,54].
4.4.Stem cell models
Recently,stem cell-based assays are being discussed as source for various toxicological applications [6,55–57]thanks to the Nobel Prize-winning discovery of how to reprogram ordinary somatic cells to behave like embryonic stem cells [139].Human-induced pluripotent stem cells (iPSCs) allow assays to consider an individual’s genetic background and potential epigenetic influences that affect the variability of the toxicity response [56].By the use of comprehensive profilingviagenomics,proteomics,transcriptomics,and metabolomics stem cellin vitromodelsofferan unprecedented opportunity to testthe effects of potential food toxicants in a very predictive and personalized manner [6,55–57].Stem cell-based models are also of particular interest for toxicity measurements which either lack extrapolability in rodent models such as for genotoxicity,cardiotoxicity,respiratory toxicity or for different stages of disorders which largely remain unknown such as neurological disorders(depression,anxiety,Alzheimer’s and Parkinson’s disease),autoimmune diseases(multiple sclerosis,type I diabetes,asthma),systemic infection,cancer and others [8,27,58–60].Although in early development,human stem cell models may further reduce the usage of animals in safety and risk assessment studies and offer the potential to dramatically enhance our understanding and thus prediction of the molecular basis of toxicity[6,55–57].
5.In vivo toxicity assessment in animals
While complex cell culture systems can provide unique insights intoin vivotoxicology,they will never completely model the higher level interactions present in an intact organism[1,2,4–6,8,41,140].Therefore,the gold standard for toxicity assessment has beenin vivotoxicology,where a particular molecule or complex food ingredients are given to animals to evaluate acute,subacute,and chronic effects.A large body ofinformation about their responses have led to the development of various specific high-content animal models that may have the ability to simulate the genetic heterogeneity of the human population and evaluate possible reproductive and developmental toxicity,neurotoxicity,genotoxicity,carcinogenicity,immunotoxicity,food allergy,and endocrine disruption[1,2,4–6,8,9].
The majority of animals used are rodents and to derive statistically significant results the numbers of animals needed for testing are enormous with an estimation of 7000 animals and tens of millions of dollars for each test compound in the pharmaceutical industry [141].Although the numbers of animals involved in food toxicity screening are decreasing,the numbers of compounds or food ingredients to be tested as well as the costs of the currentin vivoassessment systems are exploding[2,5–7,41,42,51,142].Traditionalin vivotoxicity testing is also characterized by low-throughput readouts and ethical concerns of using such large quantities of animals from animal protection groups.Lastly,there are debates on whether the data is readily translatable to humans due to differences in species sensitivity but also because of the heterogeneity of the human population as reflected by a steady increase of reports on adverse events [8,143].The high costs,low-throughput readouts,ethical and extrapolability concerns have urged calls for alternative strategies in toxicity testing methods[5,7,142].
Consequently,the paradigm of the high use of rodent forin vivotoxicity testing shifted in recent years with an approximate 50% reduction in the number of animals required for toxicological tests[4–6,144].This was paralleled by a focused use of specific animal models due to a better understanding of mechanisms.For example,the knowledge gained on the effects of toxicants on lipids stems from the similarity oflipid metabolism between mini pigs and humans,whereas the rat appears to be an unsuitable model for the study of cholesterol levels[2].Higher specificity was also achieved by the use of new genetically diversein vivohigh-content models simulating the genetic heterogeneity of humans or transgenic animals modeling common diseases and/or genetic polymorphisms considering specific groups within the general population that may be at particular risk following exposure to a food component[2,6,7,42,51].
In addition to enhancing the specificity of traditional animal models,the value of using alternative sub-mammalian vertebrate and invertebrate models became evident by the significant discoveries of homologous genes related to development,immune response,cancer or related pathways in humans [5,43–49].Majorly three test platforms of model organisms are adapted to high-throughput screening.These include the fruit flyD.melanogaster; the nematodeC.elegansand the zebrafishD.rerio.The use of these but also other alternative lower hierarchy surrogate animal models offers an advantage in the ease of ethical concerns in terms of high throughput and genetic manipulation over traditional rodent models[4–6,42,43].However,as with all animal models,alternativein vivomodels also have the caveat of extrapolation issues related to species differences.
The use of the fruit flyD.melanogasterhas been recognized as a model organism in studies of genetics and developmental biology for over 100 years [145].The genome is fully sequenced showing that nearly 75%of human disease-causing genes have a functional homolog inD.melanogaster[43,45,47].Extensive genetic manipulation of the fruit flyviaknockout or knockdown by RNAi is available for modifying fruit fly genetics [43,45,146].Interestingly,fruit flies have the potential to be used for chemical-toxicity screens particularly for neurotoxicity due to the high degree of orthologs associated to genes known to be involved in neurodegenerative diseases[43,47,147–149].The nematodeC.elegansis easily cultured in the lab and widely used for biomedical research.More than 50% of human genes have functional orthologs inC.elegansand all 959 somatic cells of the worms have been characterized with respect to lineage [46,150,151].As forD.melanogaster,a variety of molecular tools provide the availability of a large number of transgenic strains suited for a differential toxicity screening by high-throughput genomic studies [46,150–152].SinceC.elegansis capable of rudimentary learning and many neurotransmitters are well conserved it is also well suited for neurotoxicitytesting[151,153,154].Morerecently,thezebrafishD.reriohas been used as a vertebrate model organism for a wide variety of research including drug discovery and toxicology.The increased usage of zebrafish asin vivomodel system reflects the striking similiar toxicity profile between humans and zebrafish due to substantial physiological,anatomic,and genetic homology [44,49,155,156].The zebrafish model is also amenable to gene manipulation,is low in cost,has a short generation time,and is particular well suited for high-throughput screening as well as microarray and proteomic studies [48,157,158].Since zebrafish larvae are transparent they are ideal for studies on organ morphology byin vivoimaging techniques in addition to more detailed studies by immunohistochemistry orin situhybridization[49,155,158].
6.Toxicity assessment in humans
Toxicity assessment in humans involves different fields such as clinical,forensic,environmental,and regulatory toxicology.A systemic determination of toxicants in body tissues is usually obtained by biopsy or by analyzing body fluids such as blood and urine.Clinical toxicology is mainly based on analyzing genotoxicity,neurotoxicity,cardiotoxicity,hepatotoxicity,nephrotoxicity,carcinogenicity,immunotoxicity,food allergy,and/or endocrine disruption as they affect a variety of disorders[13,14,16–23].
A great deal of knowledge on toxicity in humans has been obtained by post mortem molecular and anatomic analysis of cells,tissues and organs[159,160].Forensic toxicology is very related to toxicologic pathology but focusing more on the application to the purposes of the law [160,161].The discipline of environmental toxicology is related to studies of various chemical,biological and physical agents which are harmful to humans,whereas regulatory toxicology is concerned with risk assessment of food and potential toxicants [51,162].By virtue of advances in nanotechnology and its application in food industry,the newly created discipline of nanotoxicology investigates safety or potential hazards of nanoparticles[52,163–165].Another dimension refers to genetically modified organisms(GMO)or genetically modified food(GMF)as potential source of toxicity [52,166,167].All the different disciplines of toxicity assessment in humans are not mutually exclusive but rather highly interconnected.The goal is to identify and understand the molecular mechanisms of toxicants causing adverse effects in order to ultimately prevent their intake thus increasing food safety[52,168].
A major tool in clinical toxicology is the use of surrogate biomarker molecules as specific indicators of organ and tissue damage.As a consequence of the rapid development in biotechnology an increase of specificity as well as sensitivity of detection levels has led to a better predictivity of those biomarkers.Toxicological assessment of organ and tissue damage can be grouped in two basic types of biomarkers which indicate different adverse biological effects:(1)biomarkers assessing functionand integrity of cells and tissues which are typically tissuespecific cytoplasmic enzymes that leak from damaged or dying cells and can be monitored in blood or urine.Usually,a comprehensive clinical chemistry profile is performed employing a variety of tissue-specific biomarkers as indicators for organspecific damage such as liver,kidney,brain,heart,vascular system and muscles(Table1)and(2)biomarkers as indicators of damage responses of cells and tissues based on the inducible cellular defense systems.Representative members for the group ofinducible biomarkers such as antioxidant enzymes,antioxidants,metal-chelating proteins,repair enzymes,transcription factors,inflammatory factors or xenobiotic factors are listed in Table2.Integration of data obtained by these two approaches as first line of toxicity assessment is usually validated by histology which may potentially lead to causative relationships relevant to degenerative diseases[3,169–173].
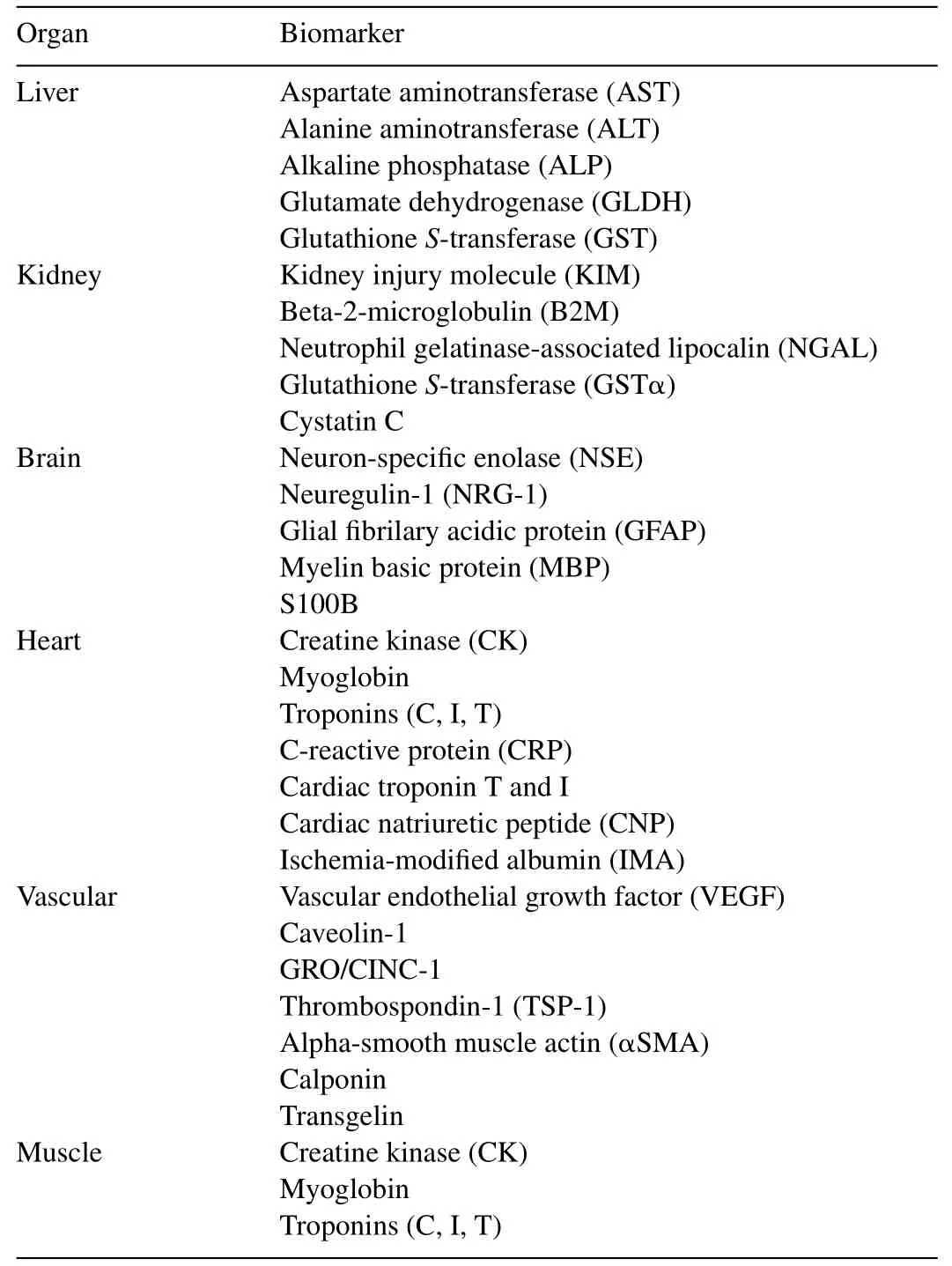
Table1 Organ-specific biomarkers.Different biomarkers indicating organ-specific damage are listed.For reference see:[1,3–6,8,9,23,52,59,86,130–136,168–173,191–194].
As a result of recent developments in metabolomics the clinical chemistry profile is enhanced by NMR technology to identify intermediary metabolites associated with a variety of pathologies and functional alterations,including renal and hepatic toxicity[170,171,174].Furthermore,modern non-invasive techniques such as positron emission tomography (PET),fluorescence magnetic resonance imaging (fMRI),computerized tomography (CT),or single-photon emission computed tomography (SPECT) are increasingly proposed for monitoring molecular biomarkers because of possibilities for relatively direct clinical translation[6,169–171,175–177].

Table2 Inducible biomarkers.Different damage-responsive biomarkers are listed.For reference see:[1,3–6,8,9,11–16,22,28,39,52,61–67,70,76–80,91,170,171,173,195–197].
7.In silico toxicity models
The integration of food toxicology data obtained throughout biochemical and cell-basedin vitro,animalin vivoand human clinical settings enabled the establishment of alternative,highly predictablein silicomodels employing new focused cell-based bioassays as valuable tool for toxicity risk assessment in food.In silicomodels are being used to study pathways of subsequent cellular events,starting from a molecular initiating event,through a sequential series of higher order effects using complexin vitrocell-based models and computer algorithms[7,33,34,178].As a basic principle,quantitative structure–activity relationships (QSAR) between a chemical structure and the biological effects give valuable insights into the molecular mechanisms of action of toxic substances.The predictive value of QSARs can be greatly enhanced by quantitativein vitrotoin vivoextrapolation when toxicokinetic data on xenobiotic biotransformation,chemical–chemical interactions,absorption,distribution,bioavailability,metabolism and/or excretion of the substance under study are available[2,7,32,34,172,179,180].Advances of thesein silicotools to assess toxicity in food has led to a wealth of mechanistic information of adverse effects of food toxicants and a significant reduction in the number of animals required for toxicological tests for a new active substance [5–8,27,33].Therefore,in silicomodels are being increasingly recognized as predictive tools to analyze hepatotoxicity,cardiotoxicity and nephrotoxicity[9,33,34,39,172,181–183].
7.1.High-throughput and high content screening methods
Thanks to significant advances made in biotechnology during the last two decades the majority of toxicity test systems are now analyzed by high-throughput or high content screening technology[4,5,9,25,27,127].These newly emerged screening systems were originally developed by the pharmaceutical industry to identify bioactive compounds from huge corporate compound libraries [5,184,185].Whereas the high-throughput approach is based on quick screening of the biological activity of numerous compounds,high-content screening includes the measurement of many parameters in a single cell or tissue setting[4–7,9,186,187].Most of those systems are strongly driven by fluorescence-based bioassays or biosensor systems employing image analysis algorithms.These systems allow multiplexing fluorescent endpoints by the use of robotic based screening in multiwell plates and automated liquid handling equipment settings[4–6,40,188].Due to the establishment of the different-omics technologies the impact of toxicants on genes,proteins,or metabolites can be analyzed by toxicogenomics,toxicoproteomics,or toxicometabolomics,respectively[6,27,35–39].Whereas toxicogenomics and toxicoproteomics are fairly established screening methods,toxicometabolomics has started to be integrated as profiling method in the panels for toxicity assessment only in the past few years [6,174].Now,highthroughput and high-content technologies in combination with-omics technologies are routinely used throughout biochemical and cell-basedin vitro,animalin vivomodels as well as clinical and pathologic analyses.These new techniques allow very quick screens of a huge number of compounds to yield important mechanistic information on numerous critical cell signaling pathways and cell health parameters with unprecedented quality and reproducibility.Former trade-offs of high-throughput and high-content technology due to high rates of false-positives as well as false-negatives have been recently addressed by the use of quantitative high-throughput screening[5,189,190].
8.Concluding remarks
Overall,the achievements in food toxicology in the last decades have been significant gaining a deeper understanding of the molecular mode of action by which toxic effects are induced.These mechanistic insights have helped to identify potential toxicants thus enhancing food safety.The list of toxicants is growing and comprises a heterogeneous groups of simple or complex molecules which play different roles in toxicological pathways.In Table3 representative toxicants derived from different sources are listed.Major advances in biotechnology in the use of high-throughput,high content testing programs,-omics technologies,computational toxicology,as well as the establishment of prediction models focusing on quantitative structure–activity relationships (QSAR) have augmented our knowledge of the molecular mechanisms of how food molecules affect targets of key biological pathways thus inducing toxicity.
Mining,integration and correlation analysis of toxicological data throughoutin vitrobiochemical and cell-based models,in vivoanimal as well as clinical settings are leading to a better predictivity of complexin vitrocell-based models as essential part ofin silicosystems.Evaluation of complex data obtained by high-throughput and high-content screening technologies utilizing algorithms-based software is now possible through major accomplishments in bioinformatics.Humanin silicomodels have the advantage that they do not have issues with interspecies extrapolation and complex toxicological end points.These models can often be analyzed to yield a few specific pathways in specific target organs.
Another positive trend in food toxicology is the increased usage of alternative lower surrogate animal models such as the zebrafish (D.rerio),the nematodeC.elegansand the fruit fly(D.melagonaster).The discovery of a higher degree of evolutionary conservation of homologous genes between humans and lower vertebrate or invertebrates than assumed decades ago has allowed for a decrease of rodent animal models in favor of alternative animal models for use in food toxicology.The use of these non-traditional organisms offers advantages in the absence of ethical concerns with genetic manipulation,organ toxicology,as well as providing higher throughput and lower costs over mammalian models,in particular rodents.
Due to the positive developments in food toxicology assessment in the last two decades,but also because of ethical and extrapolability concerns as well as an increase of test candidates,there has been a paradigm shift to reduce animal testing.By virtue of the establishment of predictivein silicotoxicity assessment tools the traditional endpoint testing moved toward a mechanism-based approach.Although evaluation of toxicants through complexin vitrohuman cell-based models embedded inin silicomethods are now being increasingly recognized as predictive tools,there will be a continuous need for comparison with traditionalin vivotesting.In particular,testing of new food ingredients by rodents or larger vertebrates will beinevitable to identify whole-animal and mechanistic organ level responses to integrate new data intoin silicosoftware systems.Recently,organotypic systems and stem cell-based assays are being discussed as very promising sources for various toxicological applications.Although still in early development the long-term potential for these approaches to predict acute toxicity is very high.The application of these or other models will help to further answer fundamental biological questions and pave the road toward the goal of higher specificity and accuracy aiming for a reduction of animal toxicological testing.

Table3 Toxicants derived from different sources.Representative toxic substances are listed.For reference see:[1–6,8,9,17,19,20,24,28,36,37,52,78,81,94,120,123,168,196,198,199].
Despite the achievements in many areas of food toxicology,there are numerous challenges in predicting and/or translating the effects of specific toxicants in food due to:(1)crosstalk of toxicants by interacting with different endogenous and exogenous molecules (such as drugs and food matrixes) throughout different toxicity pathways; (2) the fact that toxicity pathways will be perturbed differently due to cell-,tissue-,organ- and even organisms-type dependent variability in gene expression;(3)the fact that some forms of toxicity are dependent on higher order interactions of cells in tissues or organs;(4)the possible modifications during digestion and absorption; (5) the aspects ofimmune sensitization or desensitization; (6) the difference in solubility,bioavailability,biotransformation and bioconversion; (7) human variability such as heterogeneity,epigenetics,gender,size,health and age; (8) the impact of toxicants on different nutritional health,disease status or other environmental factors; (9) xenometabolisms in the liver and other organs; and (10) the lack of standardization in synthesis and chemical characterization as well as the normalization of test compounds.
- 食品科学与人类健康(英文)的其它文章
- Genetically modified foods:A critical review of their promise and problems
- Genetically modified foods in China and the United States:A primer of regulation and intellectual property protection
- Risk factors in street food practices in developing countries:A review
- Review of Finger millet(Eleusine coracana(L.)Gaertn):A power house of health benefiting nutrients
- Prevalence of fumonisin producing Fusarium verticillioides associated with cereals grown in Karnataka(India)
- Investigation of potential Shiga toxin producing Escherichia coli(STEC)associated with a local foodborne outbreak using multidisciplinary approaches

