Prevalence of fumonisin producing Fusarium verticillioides associated with cereals grown in Karnataka(India)
N.Deepa,H.Nagaraja,M.Y.Sreenivasa
Department of Studies in Microbiology,University of Mysore,Manasagangotri,Mysore 570 006,Karnataka,India
Abstract A total of 135 cereal samples were collected from different districts of Karnataka state,India in which 69 samples were infected with Fusarium species.Amongthese51sampleswerehavingFusariumverticillioidesinfectionandamongthem42sampleswerepositiveforfumonisinproduction.Per cent incidence and frequency were high in maize samples with 33.12%and 47.54%,respectively followed by paddy and sorghum,while pearl millet was free from F.verticillioides infection.Relative density of F.verticillioides association was 59.50%among the screened samples.A total of 326 Fusarium species were isolated by screening 135 cereal samples and among these 194 isolates of F.verticillioides scored positive for VERTF-1 and VERTR species-specific pair of primers.Further amplification with VERTF-1 and VERTF-2 pair of primers recorded 176 isolates of fumonisinproducingF.verticillioides.Thestudyrevealedhighincidence,frequencyandrelativedensityoffumonisinproducingF.verticillioidesand production of fumonisins in cereals.It was amplified using one forward and two reverse primers to discriminate fumonisin producing from fumonisin non-producing F.verticillioides which stresses the need for the development of managemental stratergies before they enter into the food chain.© 2016 Beijing Academy of Food Sciences.Production and hosting by Elsevier B.V.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Keywords: Fumonisin;Fusarium;IGS;Cereal;Maize;Paddy;Sorghum
1.Introduction
Cereals are the basic staple food of India and provide much of the energy and protein for majority of the population.They also have known to contain a range of micronutrients such as vitamin E,some of the B vitamins,sodium,magnesium and zinc.Contamination of cereals due to poor agricultural practices and intermittent rain at the time of harvest by fungal species ofAspergillus,FusariumandPenicilliumare often unavoidable and it is worldwide problem[1,2].The most common mycotoxins present in cereals are aflatoxins,fumonisins,zearalenone,ochratoxins,T2 toxin and deoxynivalenol[3].Contaminantion ofcerealsandcerealbasedproductswithfumonisinsposesthreat to agriculture and food safety throughout the globe.Food and Agriculture Organization (FAO) estimated that each year 25%–50%of the world’s food crops are contaminated by mycotoxins[4].Ofthe fungiinvolved,themostcommon areFusariumspecies which are associated with cereals all over the world.Totally 70 differentFusariumspecies were isolated and identified from many substrates throughout the world[2].Fusarium verticillioidesis an important fungal pathogen with a wide range of plant hosts such as maize,paddy,sorghum,etc.[5].The risk of contamination by fumonisins is related to the association ofF.verticillioidesspecies with cereals[6,7].Fumonisins are considered as agriculturally important environmental toxins produced byF.verticillioidesand otherFusariumspecies in the field or during storage [8].Fumonisins cause several diseases such as blind staggers and leukoencephalomalacia in horses [9],pulmonary edema in swine [10]and hepatic cancer in rats [11],esophageal cancer,liver cancer [12],skin lesions [13],wound[14],keratitis and polycystic kidney disease in humans.More than ten types of fumonisins have been characterized among which B1,B2 and B3 are the major types produced [15].The International Agency for Research on Cancer(IARC)has indicated that FB1 is a possible carcinogen to humans.
Rocha et al.[42]reported high frequency(96%)ofF.verticillioidesin maize grains collected from four different regions of Brazil.F.verticillioidesand otherFusariumspecies are reported to cause ear rot in maize.F.proliferatumwas reported along withF.verticillioidesfrom Italy[16],Southern Europe[17]and Iran[18].F.subglutinanswas the species most frequently recovered from asymptomatic host tissue and was more frequent thanF.verticillioides[19].Many instances of asymptomatic infection ofF.verticillioidesin corn have been reported [20,21].Levic et al.,[39]reported dominance and frequency ofFusariumspecies isolated from corn kernels over years;F.subglutinanspredominated in some years.High prevalence ofF.verticilliodesassociated with cereals consistently proved by molecular based study with species specific primers when compared to conventional methods.
The most reliable method to distinguish betweenF.verticillioidesand closely related species is DNA sequence comparison.DNA used included nuclear ribosomal DNA intergenic spacer(IGS),the nuclear ribosomal DNA internal transcribed spacer,genes encoding the translation elongation factor 1α (TEF),b-tubulin,calmodulin,cytochrome P450 reductase,and 28S ribosomal RNA [22,23].One set of species specific primer VERTF-1 [24]and IGS based VERTR primer [25]have been used to differeniateFusarium verticillioidesfrom otherFusariumspecies.The other set of primer included VERTF-1 and VERTF-2todiscriminatefumonisinproducingfromnonfumonisin producing isolates.Aim of the present work was to study the per cent incidence,frequency and relative density ofF.verticillioidesassociated with maize,sorghum,paddy and pearl millet using conventional and PCR methods.Further,to know their ability to produce fumonisin by LC MS method.
2.Material and methods
2.1.Collection of samples
A total of 135 cereal samples (61 maize,42 paddy,24 sorghum and 8 pearl millet)were collected from different districts of Karnataka state during November 2012–May 2014.All the collected samples(0.5 kg)were packed in sterile polythene bags,labeled appropriately and maintained at 4◦C.They were subjected to mycological analysis.
2.2.Isolation of Fusarium species
Sampling was done by hand halving method according to International Seed Testing Association(ISTA 2003).The incidence ofFusariumspecies was analyzed using both standard blotter and agar plating methods[26].Two hundred grains from each sample were placed on moist blotting material as well as on agar media.Melachite Green Agar 2.5(MGA-2.5)was used as the selective isolation medium[11].The plates were incubated with alternating periods of 12 h darkness/light at 25±2◦C for seven days.After incubation,plates were visualized forFusa-riumspecies by micro-morphological studies.Fusariumspecies were transferred onto Potato Dextrose Agar(PDA),(Himedia,India) to identify at the species level using fungal taxonomic keys [2,27].All fusarium isolates were maintained on Czapek Dox Agar slants at 4◦C for further studies.
Percent incidence,frequency and relative density were calculated according to the following formula;

2.3.DNA Isolation from Fusarium species
Based on the morphological characters,a total of 372Fusariumisolates were inoculated to 500 μL of potato dextrose broth in 2 mL microfuge tubes and incubated with alternating periods of 12 h darkness/light at 25±2◦C for 4 days.From the resulting mycelium DNA was extracted[28].The mycelial mat was pelleted by centrifugation at 5000 r per minute for 5 min.The pellet was ground in microfuge tubes with blunt ends of sterile disposable pipette tips in 500 μL oflysis buffer(20%SDS,PVP,0.1 mol/L EDTA,2 mol/L Tris–HCl,lithium chloride,pH 8.0)and incubated at 65◦C for 15 min.During incubation,the mixture was briefly vortexed 2–3 times.The samples were then treated with 500 μL of phenol:chloroform (1:1,v/v) and vortexed for 1 min and the supernatant was collected after centrifugation at 3000 r per minute for 5 min at 4◦C.DNA was precipitated with an equal volume ofice-cold isopropanol,and incubated at −20◦C for 60 min and centrifuged at 8000 r per minute for 8 min at 4◦C.The pellet obtained was rinsed with 70% ethanol,air-dried,resuspended in 50 μL of nuclease free water and for PCR.
2.4.Primers for PCR
Isolates were confirmed asFusarium verticillioideswith the use of forward primer VERTF-1(5′-GCG GGA ATT CAA AAG TGG CC-3′)designed by Patino et al.[24]and the reverse primer VERT-R(5′-CGA CTC ACG GCC AGG AAA CC-3′)designed by Sreenivasa et al.[29].These were used to identifyF.verticillioidesstrains at the species level.The isolates were tested using the PCRspecific assay forfumonisin-producingF.verticillioideswith primers VERTF-1(5′-GCG GGA ATT CAA AAG TGG CC-3′) and VERTF-2 (5′-GAG GGC GCG AAA CGG ATC GG-3′) as described by Patino et al.[24].The expected PCR amplicon sizes were 1016-bp and 400-bp for the primers VERTF-1/VERT-R and VERTF-1/VERTF-2,respectively.
2.5.PCR amplification
The isolated 372 DNA samples were subjected to PCR(Sure cycler 8000 Agilent technologies)using VERTF-1 and VERT-R set of primers with the PCR conditions 95◦C for 2 min ofinitial denaturation,94◦C for 30 s of denaturation,61◦C for 30 s of annealing,primer extension at 72◦C for 1 min and final extension at 72◦C for 13 min.VERTF-1 and VERTF-2 set of primers were used to discriminate fumonisin producing and non producingF.verticillioideswith the PCR conditions 95◦C for 2 min ofinitial denaturation,94◦C for 30 s of denaturation,60◦C for 30 s of annealing,primer extension 72◦C for 45 s and final extension at 72◦C for 13 min.PCR reaction included 12 μL of master mix(Genei PCR Master mix),1 μL DNA,0.6 μL of each primer and 10.8 μL of water in a total volume of 25 μL reaction mixture.PCR products were analyzed in 1.5% agarose gel (50×TAE-Tris base,glacial acetic acid,0.5 mol/L EDTA,pH 8)and image was documented with a gel documentation system(Vilber Lourmat-Lab India,Hyderabad)after staining with ethidium bromide.
2.6.Sequencing and construction of phylogenetic tree
Randomly selected six PCR amplified products(fourF.verticillioidesisolates confirmed up to species level with VERTF-1 and VERT-R primer and twoF.verticillioidesisolates amplified with VERTF-1 and VERTF-2 primer for the presence of gene coding for the production of fumonisin) were sequenced(Amnion Sequencing,Bangalore,India).The sequences were deposited at NCBI,GenBank,USA.Phylogenetic tree was constructed by online software MEGA 5.1 using neighbor joining method for the sequenced isolates.
2.7.Analysis of fumonisin-producing ability of Fusarium isolates by LC/MS
Randomly selected nine isolates ofF.verticillioideswhich were identified up to species level as well as fumonisin producingF.verticillioidesconfirmed by PCR(three isolates each from maize,paddy and sorghum) were tested for their ability to synthesize fumonisin B1.Each isolate was artificially inoculated with the concentration of 1 mL of 106spores/mL into 5 g autoclaved each cereal in culture tubes.The tubes after incubation for 27 days were finely ground using liquid nitrogen,with sterile pestle and mortar and used for fumonisin extraction.Ground sample weighing 0.4 g was taken in a sterile glass vial and suspended with 2 mL acetonitrile/water(1:1,v/v)extraction solvent and allowed for equilibration overnight in a gel rocker at 28±2◦C.The extracts were syringe filtered using 0.45 μm nylon membrane filters and liquid chromatography/mass spectrometry (LC/MS Waters Acquity Synapt G2,United States)was performed for the sample extracts.A column C18 was used at 50◦C with sample temperature being 24◦C for a run time of 8 min and mobile phase was water/acetonitrile.MassLynx SCN781 software was used to validate the LC/MS results.All sample extractions were carried out in triplicate.

Table1 Cereal samples collected all over Karnataka,India.
3.Results
Mycological analysis using standard blotter and agar plate methods showed that,among 135 cereal samples (maize-61,paddy-42,sorghum-24,pearl millet-8) collected (Table1),69 samples(maize-37,paddy-22,sorghum-9,pearl millet-1)were positive forFusariuminfection and 51 samples were havingF.verticillioidescontamination.Among these 42 samples were fumonisin producingF.verticillioides(Table2).
Among the samples,maize showed maximum incidence ofF.verticillioideswith 8.5%incidence in Mysore,3.5%in Madikeri and 3.6%in Bellary samples.Further 2%incidence was in paddy from Hassan and 1.4% incidence was in sorghum from Mysore samples (Table3).Pearl millet was free from infection.Frequency ofF.verticillioidesamong screened samples was high (47.54%) with maize followed by paddy (42.85%)and least in sorghum (16.66%).Among the screened samples maize (40.98%),paddy (33.33%) and sorghum (12.5%) samples were found contaminated with fumonisin producingF.verticillioides(Table2).Of the 326Fusariumisolates obtained from 69 infected cereal samples,194 wereF.verticillioideswhich were isolated from 51 cereal samples.The relative density of the isolatedF.verticillioidesspecies was 59.50%whereas otherFusariumspecies screened had relative density of 40.5%(Fig.1).

Table2 Frequency of cereal samples infected with Fusarium and its species with fumonisin production.

Table3 Percent incidence(%)of Fusarium verticillioides in cereal samples.
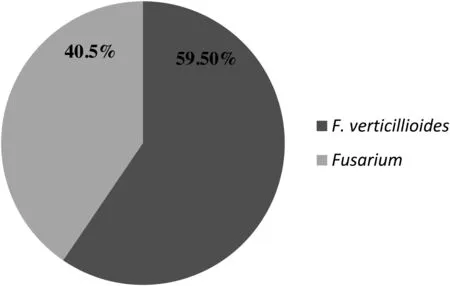
Fig.1.Relative density of the F.verticillioides isolated from cereal samples.
When all the 326Fusariumspecies isolates were subjected to PCR amplification with VERT primers,194 isolates scored positive with species specific VERTF-1 and VERTR(1016 bp)primers,and 176 isolates scored positive for VERTF-1 and VERTF-2 (400 bp) primers (Fig.2).Hence,among the 194 isolates,176 isolates were fumonisin producingF.verticillioidesand 18 isolates were non-fumonisin producing.F.verticillioides(MTCC 156)strain was used as positive control andF.graminerium(MTCC 1893)was used as negative control(Fig.3).
For reconfirmation of PCR results the randomly selected PCR amplified products of VERTF-1/VERTR and VERTF-1/VERTF-2 were subjected to sequencing.Sequencing reads of PCR analyzed products were tested using nucleotide megablast.Thesequenceswere99%similartoGiberrellamoniliformisstrains.The same sequences were deposited at NCBI and obtained the accession numbers KJ410046,KJ410047,KR061317,KR061318 with VERTF-1/VERTR primers and KJ410767,KJ410768 with VERTF-1/VERTF-2.The sequencing result provides as reconfirmation for PCR carried out for theF.verticillioidesstrains isolated from the cereals.The sequenced isolates are represented by phylogenetic tree analysis(Fig.4).

Fig.2.Incidence of Fusarium and its species with fumonisin production based on PCR confirmation.

Fig.3.Agarose gel differentiating fumonisin and non-fumonisin producing isolates.Lane 1&7-Positive control(MTCC 156),Lane 2,3,4,5-Positive isolates of F.verticillioides amplified with VERTF-1 and VERTR primers,Lane6&12-Negative isolate(MTCC 1893),M-250bp ladder,Lane 8,9&11-Positive isolates of fumonisin producing F.verticillioides amplified with VERTF-1 and VERTF-2 primers,Lane 10-Negative isolate of fumonisin producing F.verticillioides.
The fumonisin producing ability was reconfirmed through LCMS results and three randomly selected representative isolates from maize,paddy and sorghum were compared with toxin standard of fumonisin B1 with retention time of 1.76 min and molecular weight of 722.535 g/mol.Six isolates(two each from maize,paddy,sorghum) recorded as fumonisin producing and three isolates were non fumonisin producers (one each from maize,paddy and sorghum)when compared to standard graph(Fig.5).
4.Discussion
During the last decade,fumonisin producingF.verticillioidesand related species have received worldwide attention.The non-scientific methods of agricultural practices,poor storage facilities and unfavorable environmental conditions from the time of harvest to storage/marketing have led to colonization of fumonisin producing fungi [30].Moulds,besides depleting the nutrients,also produce toxic substances that have potential health hazards to animals and in turn to humans[31,32].In this study 29 maize samples exhibited the association ofF.verticillioidesout of 37 samples of maize screened forFusariumspecies and this was followed by paddy and sorghum but pearl millet was devoid ofF.verticillioidesinfection(Table2).F.verticillioideswas considered as predominant species on maize with significant levels of fumonisin[33]andG.fujikuroiwas the dominant species that would probably be the main source for fumonisin production in cereals [34,35].In the present study 194F.verticillioideswere isolated(maize-108,paddy-80,sorghum-6)from 326Fusariumspecies (maize-158,paddy-137,sorghum-29,pearl millet-2)screened from 135 cereal samples(Fig.2).
Fig.4.Represenation of phylogenetic tree for sequenced isolates of F.verticillioides.

Fig.5.Isolate representing production of fumonisin toxin with molecular weight of 722.548 g/mol when compared to standard graph.
Even after certain measures taken for the control of fumonisin production,the association of fumonisin producingF.verticilloidesis increasing day by day.One hundred and seventy six fumonisin producingF.verticillioides(maize-103,paddy-68,sorghum-5) were isolated from screened 326Fusariumspecies in the present work(Fig.2).Among the 45 maize samples collected from south Karnataka,25 samples were found to be highly infected with FB1 producingF.verticillioides[36].Among the 22 fumonisin producing isolates screened,18 wereF.verticillioidesin which 17(94.4%)isolates produced fumonisins(FB1+FB2) at concentration range of 0.07–121.45 μg/g [37].An epidemiological survey conducted in Karnataka and Andhra Pradesh during 1997 revealed that consumption of mouldy grains affected 1424 persons in 27 villages[38].Greater attention is bestowed to investigateFusariumspecies worldwide as they reduce the value of cereals used as food and feed.Cereals contaminated with toxigenic species cause acute and chronic poisoning and allergic symptoms to animals and human.Fungi causing deterioration of cereals especially in maize,paddy and sorghum are a major problem because they produce mycotoxins[40].
In the present study,the PCR assay used for the identification ofF.verticillioidesisolates was quick,accurate and more sensitive,as compared to conventional methods.It was shown that 194 out of 245 isolates were morphologically identified asF.ver-ticillioidesand this was further confirmed by VERTF1/VERT-R species-specific primers(Table4).Among the remaining 51 isolates ofFusarium,33 were identified asF.proliferatumand 18 asFusariumspecies.F.proliferatumandF.verticillioidesare having similar morphological characters.Fabrico Lanza et al.[41]did molecular identification ofFusariumisolates by PCR and species-specific primer pairs.Initially they identified five species asF.proliferatumbased on morphological criteria but based on PCR test they were identified asF.verticillioides.Molecular analysis using species-specific PCR primers makes possible the precise identification ofFusariumspecies in such cases where morphological identification is not possible.PCR method also differentiates morphologically similar but toxigenically differentF.verticillioidesisolates.Present study revealed the association of 53.98% of fumonisin producingF.verticillioideswith cereal samples collected from different regions of Karnataka state.
In conclusion,data on the per cent incidence,frequency and relative density ofF.verticillioideswould be of great sig-nificance for predicting the extent of post-harvest infection,colonization and subsequent deterioration of cereals.Further,it also helps us to know the dry matter loss,nutritional changes and the extent of fumonisin levels during storage.Such a data is ofimmense value for assessing the possible health hazards in humans and animals upon consumption of such contaminated food grains.The high incidence of mycotoxigenicF.verticillioidesis of primary concern for policy makers and food experts in this region to reduce the economic losses caused by these fungi and also to minimize the exposure of human and animal life to the potential risks of mycotoxins.

Table4 Conventional and Molecular identification of F.verticillioides from cereals.
Conflict ofinterest
The authors declare that there are no conflicts ofinterest
Acknowledgements
We thank the Department of Science and Technology(DST-SERB) India,for providing grants through Young Scientist FAST TRAK research project(No.SR/FT/LS-176/2009;30.04.2012)to Dr.M Y Sreenivasa,Principal Investigator.We thank the Institute of Excellence,University of Mysore,for their valuable support.
- 食品科学与人类健康(英文)的其它文章
- Assessment of food toxicology
- Genetically modified foods:A critical review of their promise and problems
- Genetically modified foods in China and the United States:A primer of regulation and intellectual property protection
- Risk factors in street food practices in developing countries:A review
- Review of Finger millet(Eleusine coracana(L.)Gaertn):A power house of health benefiting nutrients
- Investigation of potential Shiga toxin producing Escherichia coli(STEC)associated with a local foodborne outbreak using multidisciplinary approaches

