Online sample focusing technique in capillary electrophoresis-amperometric detection for biogenic amines
WANG Guan, TANG Wan-rong, GE Shu-li, HAN Ruo-bing, WANG Qing-jiang, HE Pin-gang, FANG Yu-zhi
(Department of Chemistry,East China Normal University,Shanghai 200062,China)
Online sample focusing technique in capillary electrophoresis-amperometric detection for biogenic amines
WANG Guan, TANG Wan-rong, GE Shu-li, HAN Ruo-bing, WANG Qing-jiang, HE Pin-gang, FANG Yu-zhi
(Department of Chemistry,East China Normal University,Shanghai 200062,China)
An online focusing method,based on field-enhanced sample injection coupled with transient moving substitution boundary,was developed for the separation and capillary electrophoresis(CE)-amperometric detection(AD)of biogenic amines under acidic conditions for the first time.The proposed method was based on the substitution reaction between 18-crown-6-bonded biogenic amine complexes and Na+cations at the substitution boundary.By using this method,electrode fouling was prevented in electrochemical detection.The factors affecting extraction efficiency,separation,and detection were investigated.Under the optimum conditions, the limits of detection of five analytes in a sample,which was prepared in a low-conductivity matrix,ranging from 2.2 to 9.8 nmol/L(signal-to-noise ratio,S/N=3),and about 160-to 300-fold enhancement in concentration was achieved compared to the conventional CE-AD method. This method has great potential for the detection of biogenic amines.
field-enhanced sample injection; moving substitution boundary; capillary electrophoresis; amperometric detection; biogenic amines
0 Introduction
Biogenic amines(BAs)are low-molecular-weight organic bases,formed by the enzymaticdecarboxylation of amino acids or by the amination and transamination of aldehydes and ketones in living organisms[1].They have powerful physiological effects and are commonly found in various organisms,plants,foods,and beverages.Despite their important roles in biological systems,some of the BAs cause food poisoning when present in significant amounts[2].For example,tyramine(Tyr)and phenylethylamine(Phe)have been associated with migraines and hypertension,whereas serotonin(5-HT)serves as the neurotransmitter in the central and peripheral nervous systems[2-3].Moreover,some studies have indicated a higher concentration of putrescine(Put)and spermine(Spm)in cancer patients compared to healthy subjects[4].Furthermore,the quantity of BAs is considered as a marker for the microbiological contamination level in food[5].Therefore,it is important to use an efficient and reliable analytical method to identify and quantify the BAs in food and environment.
Several analytical methods such as high-performance liquid chromatography (HPLC)[6]and capillary electrophoresis(CE)[7]have been developed for the analysis of BAs in food and environment.Among these methods,CE has been proved to be an efficient separation technique for the analysis of BAs.The most common detection methods coupled to CE are ultraviolet(UV)absorbance and laser-induced fluorescence(LIF)spectroscopy[8-9].However,they require a cumbersome pretreatment or derivatization step.In this study,capillary electrophoresis-amperometric detection(CE-AD)method with high selectivity and sensitivity was used for the analysis of BAs,without prior pretreatment or derivatization of the samples.
Moving reaction boundary(MRB)is a well-developed method to improve the sensitivity of separation in analytical chemistry[10],and consists of four parts:oving precipitate boundary(MPB),moving neutralization boundary(MNB),moving chelation boundary (MCB),and moving interaction boundary(MIB)[11-12].Crown ethers can form complexes with molecules containing primary amine groups.Furthermore,some metal cations form more stable complexes with crown ethers than with analytes containing primary amine groups[13].In CE,18-crown-6(18C6H4)has been used for chiral separation(CS)[14]. Therefore,the combination of crown ethers with MRB systems in CE has great potential to improve the separation and sensitivity of analytes simultaneously.
In this study,an online sample focusing technique based on field-enhanced sample injection(FESI)coupled with moving substitution boundary(MSB)(FESI-MSB)similar to our previous study was used for the analysis of five BAs,including Spm,Tyr,Put,Phe, and 5-HT[15].18C6 H4 was used as the pseudo stationary phase(PSP)to transport,release,and accumulate the analytes at the substitution boundary.We applied the FESIMSB method to identify and separate the BAs under acidic conditions,which also maintained the sample matrix and running buffer at a constant p H.Separations were performed in a fused silica capillary(Polymicro Technologies,Phoenix,AZ,USA)with 25μm i. d.,360μm o.d,and 75 cm length.This FESI-MSB method could determine BAs at very low concentrations under the optimized conditions,thus resulting in an about 160-to 300-fold enhancement compared to the normal CE-AD method.
1 Experimental
The CE-AD system was constructed inhouse as described in our previous study[16].E-lectrophoresis was driven by a high-voltage supply(±30 k V,Shanghai Institute of Nuclear Research,Shanghai,China).Electrochemistry experiments were performed using a CH-2 amperometric detector(Jiangsu Electrochemical Analytical Instruments Factory, China).All the electropherograms were recorded using a ZF-10B data recorder(Shanghai Zhengfang Instrument Company,China).
For CE-AD,a three-electrode system was used,consisting of a copper disk(300μm i.d.) working electrode,a saturated calomel reference electrode(SCE),and a platinum wire counter electrode.The entire system was housed in a plexiglass box equipped with an interlock for safety.All the solutions were degassed using an ultrasonic cleaning system (Branson Soest,NL).Deionized water was prepared using a Milli-Q water purification system(Millipore,Bedford,MA,USA).
Spm and Tyr were purchased from Tokyo Chemical Industry Co.,Ltd.5-HT was purchased from J&K Scientific Inc.Ltd.Put and Phe were purchased from Alfa Aesar. Sodium hydroxide(NaOH),boric acid(H3BO3),phosphoric acid(H3PO4),acetic acid (CH3COOH),18C6 H4,and all other chemicals were purchased from Shanghai First Reagent Factory(Shanghai,China).All the reagents were of analytical grade and used as received without further purification unless otherwise stated.
The running buffer consisted of 60 mmol/L CH3COOH,60 mmol/L H3PO4,and 80 mmol/L H3BO3in deionized water,and the p H of the solution was adjusted to 3.50 with 2 mol/L NaOH.The PSP matrix was prepared with 100 mmol/L 18C6 H4 in the running buffer.The stock solutions of each BA were prepared with a concentration of 5×10-4mol/L in deionized water and stored at 4°C.In the CE-AD experiments,a fresh working standard solution was prepared for injection by diluting appropriate amounts of stock solution with 150 mmol/L 18C6 H4 aqueous solution(p H 3.81).Prior to use,all the solutions were filtered through 0.22μm polypropylene Acrodisc syringe filters(XinyaPurification Instrument Factory,Shanghai,China)and sonicated for 5 min.The concentrations of the analyzed BAs are given in the text or figures,and their chemical structures are shown in Fig.1.
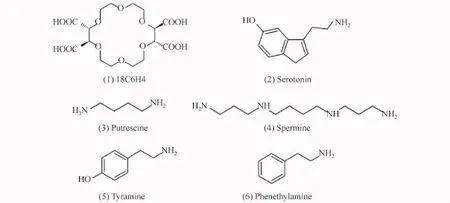
Fig.1 Chemical structures of FESI-MSB model
Fig.2 shows the FESI-MSB model in this experiment.First,a short plug of PSP matrix prepared with 18C6H4 in the background electrolyte(BGE)and a long sample plug prepared in a lower conductivity buffer were sequentially injected into the capillary filled with BGE(p H 3.50)(Fig.2A).After applying voltage,the effective electrophoretic mobility of the 18C6 H4-BA complexes containing positive charges was found to be equal to those of analytes and electroosmotic flow(EOF).Under the electrokinetic injection mode, the electric field strength in the sample zone was enhanced,thus introducing large sample volumes into the capillary.Simultaneously,the sample was focused into a narrow zone (Fig.2B).Then,the 18C6H4-BA complexes in the sample zone permeated the substitution boundary between the sample and PSP matrix,forming a sharp MSB zone(Fig.2C). In the transient MSB zone,the BAs were released from the 18C6 H4-BA complexes because of the substitution reaction between the 18C6H4-BA complexes and Na+cations.Finally,the focused analytes were brought to the detector point and separated according to general CE principles(Fig.2D).
2 Results and discussion
To identify the optimum conditions,several factors such as the p H and concentration of the running buffer,separation voltage,18C6H4 concentration in both sample and PSP matrices,and the injection time were investigated.Under the optimum conditions,five analytes were separated at a separation voltage of 16 k V and with separation buffer of mixed acid(60 mmol/L CH3COOH,60 mmol/L H3PO4,and 80 mmol/L H3BO3,p H 3.50),while maintaining 20 mmol/L NaOH as the buffer in the detection cell.The potential applied to the working electrode was 0.7 V(vs.SCE).The PSP matrix injection time was 20 s,followed by 90 s sample injection.All the experiments were performed at room temperature.
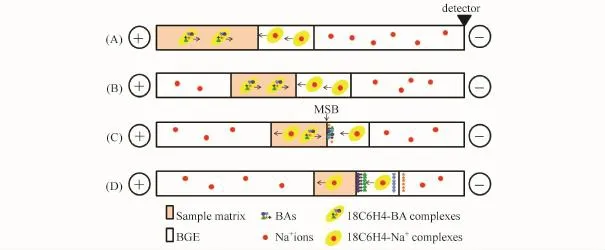
Fig.2 FESI-MSB model
2.1 Optimization of separation conditions
To select an optimum working electrode,copper-and carbon-disc electrodes were investigated.In terms of the peak height of the BAs,the copper-disc electrode was better than the carbon-disc electrode under the same conditions.Because the copper-disc electrode is usually used in alkaline solutions,20 mmol/L NaOH solution was selected as the detection buffer.
The behavior of BAs in a 20 mmol/L NaOH solution was studied using cyclic voltammetry (CV)to obtain the optimized potential.The results showed that the analytes generated recognizable anodic peaks at~0.5 V(vs.SCE).Moreover,the anodic peak was not observed in the blank NaOH solution.Fig.3 shows the results of hydrodynamic voltammetry(HDV)from 0.55 to 0.75 V range for the BAs.To obtain the best signal-to-noise(S/N)ratio,0.7 V was selected as the optimum detection potential.
Buffer composition plays a crucial role in the migration time and separation efficiency in CE.To find the optimum separation buffer for the BAs,different buffer systems(such as phosphate,citrate,acetate,and boric acid buffer)at various p H values(from 3.20 to 5.60)were investigated.The experimental results showed that mixed acid buffer solutions (CH3COOH,H3PO4,and H3BO3)provided better resolution and higher peak current.Moreover,the separation resolution increased with decreasing p H value.To balance the resolution,sensitivity,and stability,the mixed acid solution(CH3COOH,H3PO4,and H3BO3)at p H 3.50 was selected as the optimal BGE.
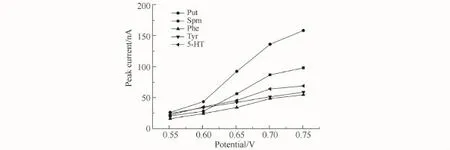
Fig.3 Hydrodynamic voltammograms of BAs
The running buffer concentration is another important parameter affecting peak height and theoretical plate number because it can influence the EOF and viscosity of electrolyte. After a detailed comparison,the optimum running buffer concentration was determined to be 60 mmol/L CH3COOH,60 mmol/L H3PO4,and 80 mmol/L H3BO3,p H 3.50 considering migration time,separation efficiency,and baseline noise.Fig.4 shows the separation of the analytes prepared in BGE.
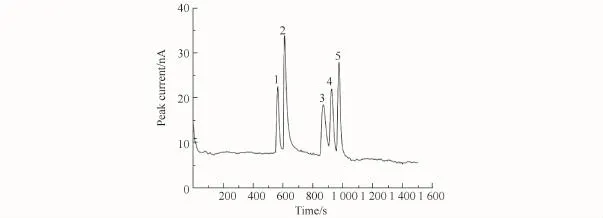
Fig.4 Electropherogram of BAs diluted with BGE
To investigate the separation efficiency of CE,12~18 k V separation voltage was used.A separation voltage of 16 k V was selected as the optimum separation voltage. Optimization of FESI experimental conditions
In FESI,the 18C6H4 concentration in sample matrix is an important factor for stacking.Therefore,the effect of 18C6H4 concentration in the sample solution was also investigated without the injection of a PSP matrix plug by varying the amount of 18C6H4(50~200 mmol/L)added to the sample solution.As shown in Fig.5A,the BAs diluted with deionized water stacked together.As shown in Fig.5B~E,evidently,the resolution of the separation increased with increasing 18C6H4 concentration,and the baseline separation was achieved when the 18C6 H4 concentration was 150 mmol/L.Therefore,150 mmol/L 18C6H4 sample solution was selected as the optimal 18C6H4 concentration.
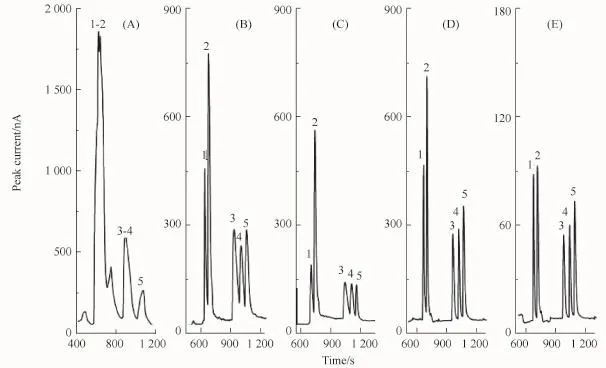
Fig.5 Effect of 18C6H4 concentration in sample matrix
The injection time can affect both peak current and peak shape as well as the overall precision of CE.To improve the sensitivity,the injection time was optimized from 50 to 90 s,as shown in Fig.6.The greatest enhancement in the total peak height was obtained at 80 s injection time.Therefore,80 s was selected as the injection time for FESI. Optimization of the MSB experimental conditions
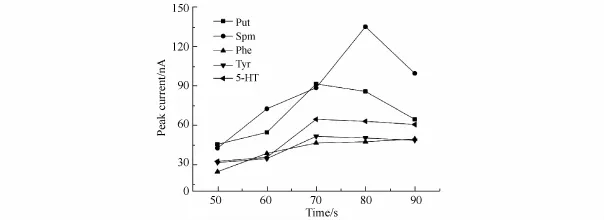
Fig.6 Effect of FESI injection time
18C6 H4 concentration in the PSP matrix played a significant role in achieving the enrichment efficiency.The effect of the concentration of 18C6H4 solution on peak height was investigated between 50 and 150 mmol/L 18C6 H4 diluted with BGE in the PSP matrix.As shown in Fig.7,the maximum enrichment efficiency was obtained at a 18C6H4 concentration of 100 mmol/L in the PSP matrix.Therefore,100 mmol/L was selected as the optimum concentration of the PSP matrix,maximizing the focusing efficiency.
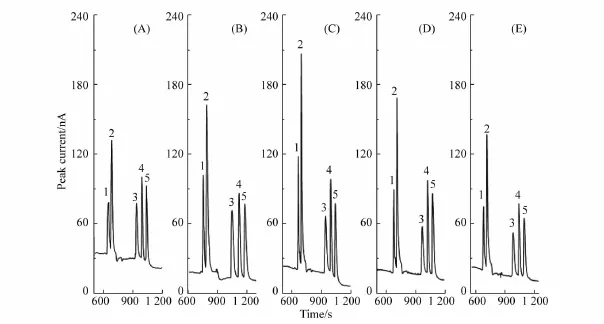
Fig.7 Effect of 18C6H4 concentration in PSP matrix
The ratio of the PSP matrix to the sample injection time(TPSPmatrix:Tsamplesolution)can affect both the injected volume of sample and focus efficiency.As shown in Fig.8,the results show that the maximum enrichment efficiency was obtained when the injection ratio was 2:9(Figs.8A-F).After the injection of a PSP matrix plug,the sample injection time increased from 80 s to 90 s(Figs.8A and B).Furthermore,a peak broadening caused by overloading appeared(Fig.8C and D).When the PSP matrix was injected longer than 20 s,peaks 3—5 were stacked together(Fig.8H).To maintain a stable MSB boundary and maximize the enrichment efficiency,the optimal PSP matrix injection time was selected as 20 s,followed by 90 s sample injection.
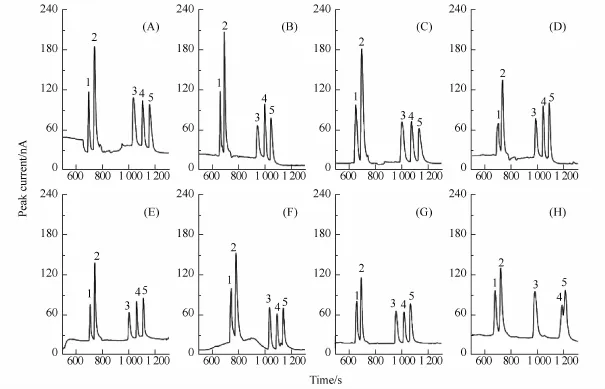
Fig.8 Effect of the PSP matrix to sample injection time ratio(TPSPmatrix:Tsamplesolution)
2.2 FESI-MSB method validation
From the above mentioned experiments,the optimum separation and focusing conditions were established.A series of standard mixture solutions were tested under the optimum conditions for normal CE and FESI-MSB methods to determine the linearity of this method.The linearity range and detection limits of CE and FESI-MSB are listed in Table 1.For FESI-MSB,the linear range covered more than three orders of magnitude of concentrations,and all the curves exhibited two segments at different concentrations.As listed inTable 1,the limits of detection(LODs)of FESI-MSB(S/N=3)were 2.2~9.8 nmol/L.In comparison,the FESI-MSB method has a much lower detection limit,indicating that approximately 160-to 300-fold enhancement in the FESI-MSB method was achieved.The sensitivity in the detection of the five BAs using FESI-MSB was improved by two orders of magnitude over those of previously reported CE-AD methods[16].
The relative standard deviations(RSDs)for FESI-MSB method are shown in Tab.1 (n=5).For FESI-MSB,the RSDs of migration time and peak height were about 1.7%~4.1%and 4.5%~6.3%,respectively,indicating good reproducibility and high precision.
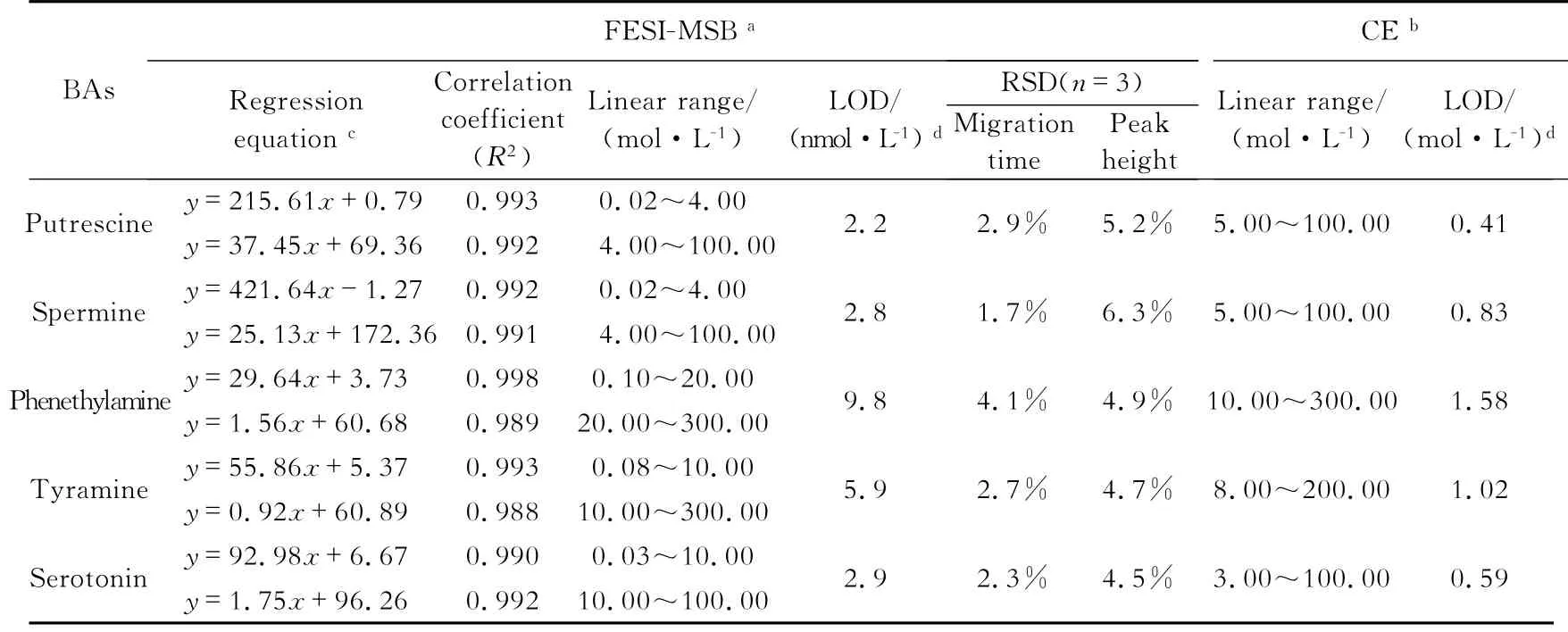
Tab.1 Performances of normal CE and FESI-MSB methods
3 Conclusion
We developed a sensitive method for the analysis of BAs based on the hyphenation of FESI and transient MSB in CE-AD.The FESI increased the introduction of large sample volumes into the capillary,and the MSB facilitated the substitution reaction between the 18C6H4-BA complexes in the sample matrix and the Na+cations in BGE.Using this method,five BAs were simultaneously focused and separated within 20 min.Compared to the conventional CE-AD method,the method could determine these BAs at very low concentrations(nmol/L),resulting in about 160-to 300-fold enhancement in peak height.The main advantage of this method is that it does not require cumbersome sample pretreatment or derivatization steps prior to the analysis.Furthermore,the method could prevent the electrode fouling in electrochemical detection.The FESI-MSB technique developed in this study can be used under both acidic and basic conditions.
[1] HUANG Y,HUANG C,CHANG H.Capillary electrophoresis-based separation techniques for the analysis ofproteins[J].ELECTROPHORESIS,2006,27(23):4792-4807.
[2] SANTOS S,HORENSIA M.Biogenic amines:Their importance in foods[J].International Journal of Food Microbiology,1996,29(2):213-231.
[3] NGUYEN L,RIGO J M,ROCHER V.Striatal PSA-NCAM(+)precursor cells from the newborn rat express functional glycine receptors.[J].Cell and Tissue Research,2001,305(2):187-202.
[4] NAIRN L M,LINDSAY G S,WOSTER P M.Cytotoxicity of novel unsymmetrically substituted inhibitors of polyamine biosynthesis in human cancer cells.[J].Journal of Cellular Physiology,2000,182(2):209-213.
[5] LI S S,WU H L,LIU Y J.Simultaneous determination of methotrexate and its eight metabolites in human whole blood by capillary zone electrophoresis[J].Chin Chem Lett,2010,24(2013):239-242.
[6] ZOTOU A,LOUKOU Z,SOUFLEROS E.A comparative survey of the simultaneous ultraviolet and fluorescence detection in the RP-HPLC determination of dansylated biogenic amines in alcoholic beverages[J].Chromatographia,2003,57(7):429-439.
[7] HERREO M,GARCIA-CANAS V,SIMO C.Direct analysis of biogenic amines in water matrix by modified capillary zone electrophoresis with 18-crown-6[J].Electrophoresis,2010,31(1):205-228.
[8] KANG L,YOU J,SUN Z.LC determination of trace biogenic amines in foods samples with fluorescence detection and MS identification[J].Chromatographia,2011,73(1):43-50.
[9] LOUKOU Z,ZOTOU A.Determination of biogenic amines by capillary electrophoresis using a chameleon type of fluorescent stain[J].Chromatographia,2003,58(9):579-585.
[10] CAO C X,FAN L Y,ZHANG W.Novel moving reaction boundary-induced stacking and separation of human hemoglobins in slab polyacrylamide gel electrophoresis[J].Analyst,2008,113(9):1139-1157.
[11] FAN Y,LI S,FAN L.Visual offline sample stacking via moving neutralization boundary electrophoresis for analysis of heavy metal ion.[J].Talanta,2012,(95):42-49.
[12] ZHANG W,CHEN J F,FAN L Y.Effective pre-concentration and analysis of heavy metals using ligand step gradient focusing in combination with isotachophoresis[J].Analyst,2010,135(1):140-148.
[13] KUHN R.Chiral electromigration techniques in pharmaceutical and biomedical analysis[J].Electrophoresis, 1999,20(13):2605-2613.
[14] XIA S,ZHANG L,LU M.Enantiomeric separation of chiral dipeptides by CE-ESI-MS employing a partial filling technique with chiral crown ether[J].Electrophoresis,2009,30(16):2837-2844.
[15] GE S L,TANG W R,HAN R B.Sensitive analysis of aminoglycoside antibiotics via hyphenation of transient moving substitution boundary with field-enhanced sample injection in capillary electrophoresis[J].Journal of chromatography A,2013,(1295):128-135.
[16] ZHANG S,DONG S,CHI L.Methods of extraction,preconcentration,and determination of quercetin[J].Talanta,2008,76(4):780-784.
(责任编辑 张 晶)
毛细管电泳安培检测在线富集分析生物胺研究
王 冠, 唐菀融, 葛淑丽, 韩若冰, 王清江, 何品刚, 方禹之
(华东师范大学化学系,上海 200062)
将场放大进样-移动取代边界与毛细管电泳-安培检测联用技术在酸性条件下进一步发展,成功实现了生物胺的在线富集与检测.该技术利用18-冠-6-四羧酸与带伯胺基团的生物胺分子结合的络合物与缓冲溶液中Na+发生的取代反应,在释放生物胺分子的同时实现瞬时富集.通过对样品溶液、伪稳定相、进样时间等影响富集与分离的重要参数进行研究,在最优实验条件下,5种被测生物胺分子的检测限可达到2.2~9.8 nmol/L(S/N=3),灵敏度较传统方法相比提高了160~300倍.且无需复杂的预处理步骤.实验结果证明,该法快速高效,能有效避免电极被污染,亦可进一步用于实际样品的分析检测.
毛细管电泳; 安培检测; 场放大进样; 移动取代边界; 冠醚; 生物胺
2014-12
仪器设备开发专项项目(国家级)(2011YQ150072)
王冠,女,硕士研究生,研究方向为毛细管电泳.E-mail:wang_guan_108@163.com.
王清江,男,博士,教授,研究方向为微纳分离分析.E-mail:qjwang@chem.ecnu.edu.cn.
O657.8 Document code:A
10.3969/j.issn.1000-5641.2016.01.016
1000-5641(2016)01-0123-11

