Symptoms predicting health-related quality of life in prostate cancer patients treated with localized radiation therapy
Chao-Pin Hsiao, Mea-Kuang Chen, Kathy J. Meyers, Leorey N. Saligan
Symptoms predicting health-related quality of life in prostate cancer patients treated with localized radiation therapy
Chao-Pin Hsiao1, Mea-Kuang Chen2, Kathy J. Meyers1, Leorey N. Saligan3
Objective: Patient-reported health-related quality-of-life (HRQOL) measures can provide guidance for treatment decision making, symptom management, and discharge planning. HRQOL is often influenced by the distress experienced by patients from disease or treatment-related symptoms. This study aimed to identify symptoms that can predict changes in HRQOL in men undergoing external beam radiation therapy (EBRT) for nonmetastatic prostate cancer (NMPC).
Methods:Fifty-one men with NMPC scheduled for EBRT were assessed at the baseline, at the midpoint of EBRT, and at the end of EBRT. All participants received 38—42 daily doses of EBRT (five times a week), depending on the stage of their disease. Validated questionnaires were administered to evaluate depressive symptoms, urinary and sexual functions, bowel issues, symptom-related distress, fatigue, and HRQOL. Pearson correlations, repeated-measures ANOVA, and multiple regressions examined the relationships among variables.
Results:Intensification of symptoms and increased symptom-related distress, with a corresponding decline in HRQOL, were observed during EBRT in men with NMPC. Changes in symptoms and symptom distress were associated with changes in HRQOL at the midpoint of EBRT(r=—0.37 to —0.6, P=0.05) and at the end of EBRT (r=—0.3 to —0.47, P=0.01) compared with the baseline. The regression model comprising age, body mass index, Gleason score, T category,androgen-deprivation therapy use, radiation dose received, symptoms (urinary/sexual/bowel problems, fatigue), and overall symptom distress explained 70% of the variance in predicting HRQOL.Urinary problems and fatigue significantly predicted the decline in HRQOL during EBRT.
Conclusion:Identifying specific symptoms that can influence HRQOL during EBRT for NMPC can provide feasible interventional targets to improve treatment outcomes.
Symptoms; symptom distress; health-related quality of life; prostate cancer; radiation therapy
Introduction
With increased survival rates and advanced treatment techniques, general well-being and functional performance have become important outcomes for oncology treatments. For example, patient-reported health-related quality of life (HRQOL) has become a key outcome measure in evaluating the effectiveness of cancer treatments [1]. In addition, patientreported HRQOL measures can guide treatment planning as well as symptom management for oncology patients [2, 3].
In 2017, prostate cancer is the second most prevalent malignancy for American men, with 161,360 new diagnoses, and is the third leading cause of overall cancer death [4]. External beam radiation therapy (EBRT) is one of the standard and effective treatments offered as curative therapy for men with nonmetastatic prostate cancer [5, 6]. Although radiation techniques have improved and EBRT has increased survival for men with prostate cancer, these advances still adversely affect physical, urinary, bowel, and sexual functions during treatment, and into survivorship following treatment completion[7—9], they negatively impacting their HRQOL [10, 11].
A recent study reported significant urinary (i.e., frequency,nocturia) and bowel (i.e., diarrhea, urgency) issues experienced by men who had undergone or not undergone prior prostatectomy during the course of their EBRT [3]. These symptoms persist for 12—24 months following EBRT completion [12,13]. However, the reported short-term and long-term effects of these symptoms on HRQOL for this clinical population are inconsistent [3, 11, 13—16], most likely related to differences in the instruments and approaches used to measure HRQOL.
Clustering of symptoms related to cancer therapy has been associated with inflammation and an altered immune response [17]. EBRT alters expression of genes and proteins associated with mitochondrial bioenergetics and biogenesis in prostate cancer patients receiving EBRT [18, 19], which can impair cellular energy supply and possibly lead to inflammation under hypoxic conditions. Alterations in the expression of genes and proteins related to inflammation and mitochondrial function have been associated with symptoms related to EBRT[18—22]. Investigation of the association of the expression of these genes and proteins with HRQOL of men undergoing EBRT may help us further understand the biologic underpinning of the relationship of symptoms and HRQOL.
Understanding specific symptoms that can predict overall HRQOL during cancer treatments can help patients and their clinicians better plan care during treatment, as well as the timing and design of optimal treatment options. The primary goal of this study was to identify symptoms that can predict changes in overall HRQOL during EBRT in patients with localized prostate cancer.
Methods
A prospective, exploratory, and repeated-measures design was used to investigate predictors and biomarkers of HRQOL in men with nonmetastatic prostate cancer undergoing EBRT.This study was approved by the Institutional Review Board of the National Institutes of Health, Bethesda, MD, USA(NCT00852111). Patients who were 18 years or older with diagnosed nonmetastatic prostate cancer who had undergone or not undergone prior prostatectomy and scheduled to receive EBRT with or without concurrent androgen deprivation therapy (ADT) were enrolled from urology and radiation oncology clinics of the Hatfield Clinical Research Center, National Institutes of Health. Research participants were excluded from the study if they had progressive disease; had experienced major psychiatric illness within the previous 5 years;had uncorrected hypothyroidism or anemia; took sedatives,steroids, or nonsteroidal anti-inf l ammatory agents; or had a second malignancy. Research participants were enrolled from May 2009 to January 2015. After informed consent had been obtained, medical records were reviewed through electronic records, and demographic information was obtained by patient interview. Peripheral blood samples and questionnaires were obtained from each participant before EBRT (baseline, day 0),on day 19 to day 21 (midpoint of EBRT), and on day 38 to day 42 (completion of EBRT).
Study measures
Clinical and demographic measures: Sociodemographic and clinical data (e.g., age, race, employment status, stage of prostate cancer, EBRT dose, type of EBRT technique used, and laboratory test results) were obtained from medical record review.
Depressive symptoms: Participants were screened for depressive symptoms with use of the Hamilton Depression Rating Scale (HAM-D) at each time point. This is a 21-item, clinician-rated paper questionnaire with good internal reliability (α=0.81—0.98). The predefined cutoff score for depression is 15 in cancer patients, with higher scores indicating more symptoms of depression [23].
Urinary tract problems: The American Urological Association (AUA) has developed a seven-item symptom index to assess urinary problems, including frequency, nocturia, weak urinary stream, hesitancy, intermittence, incomplete emptying,and urgency. It consists of an overall score ranging from 0 to 35, with higher scores indicating worse lower urinary tract symptoms. The AUA symptom index is a validated, reliable,and clinically sensible measure of urinary problems [24].
Sexual f unction: The Sexual Health Inventory for Men(SHIM) is used to assess sexual function in men treated for prostate cancer. The SHIM is a validated, five-item shortened version of the International Index of Erectile Function [14,25]. It measures the erectile function with a total score ranging from 0 to 25 (22—25, no erectile dysfunction; 17—21, mild erectile dysfunction; 12—16, mild/moderate erectile dysfunction; 8—11, moderate erectile dysfunction; 1—7, severe erectile dysfunction) [10].
Symptoms and symptom-related distress: The Symptom Indexes (SI) is a disease-specific symptom measurement focused on symptoms of dysfunction in urinary, sexual, bowel,and symptom-related distress for men treated with prostate cancer [26]. The SI is a 22-item self-administered questionnaire,consisting of two subscales (symptom indexes of symptoms[SISYM] and symptom indexes of symptom-related distress[SISD]). The SI measures symptoms and symptom- related distress, including urinary incontinence and obstruction/irritation,bowel symptoms (i.e., diarrhea, urgency of bowel movements,pain, bleeding, and passing of mucus during bowel movements), and sexual dysfunction (i.e., difficulty in getting and keeping erections, and ability to ejaculate and reach orgasm),and parallel items assess symptom distress related to urinary,sexual, and bowel problems. Each item is rated on a five-point frequency scale ranging from 1 for not at all to 5 for very frequently [26]. The SI is a valid questionnaire with good reliability (α=0.64—0.89) when used in the prostate cancer population[26, 27].
Fatigue: Fatigue was evaluated by a valid questionnaire that is widely used in oncology — the revised Piper Fatigue Scale(rPFS). The rPFS is a 22-item paper and pencil, self-administered questionnaire that measures four dimensions of fatigue(behavioral/severity, sensory, cognitive/mood, and affective)using a 0—10 intensity rating scale (0, none; 10, worst intensity). Scores are categorized as mild fatigue (1—3), moderate fatigue (4—5), and severe fatigue (>6). The rPFS has demonstrated satisfactory reliability and validity when used in cancer patients receiving radiation therapy, with internal consistency ranging from 0.69 for the symptom dimension to 0.95 for the sensory dimension [28, 29].
Health-related quality of life : The Functional Assessment of Cancer Therapy—Prostate (FACT-P) was developed to specifically capture the HRQOL relevant to prostate cancer patients. The FACT-P was validated in numerous studies [30,31], and is a well-established instrument with good reliability and validity [32—34]. It has 39 items grouped into five subscales, including physical well-being, functional well-being,emotional well-being, social/family well-being, and prostatecancer-specific items. The total FACT-P score ranges from 0 to 156, and a higher score indicates better HRQOL.
Statistical analysis
Descriptive analyses were performed to describe the participants’ demographic and clinical characteristics. Repeatedmeasures ANOVA were used to compare the mean differences of HAM-D, AUA, SHIM, rPFS, SI, and FACT-P scores from all the patients at the baseline and at the midpoint and the end of EBRT. Hierarchical multiple regression models were used to investigate the important predictors of HRQOL (based on FACT-P scores). No replacement value was assigned for missing data. All statistical analyses were conducted with IBM SPSS Statistics version 24.0 (IBM, Armonk, NY, USA).Statistical significance is indicated by P<0.05.
Results
Study participants
Patient demographic and clinical characteristics are summarized in Table 1. The study cohort comprised 51 patients with an average age of 65 years (standard deviation 7.65 years),with most of the participants being Caucasian (64.7%) and married (78.4%) men. More than half of the patients had a clinical category T2 (a—c) tumor with a Gleason score of either 7 or 8. Forty of the 51 patients received ADT as neoadjuvant treatment before EBRT. Eighty percent of the patients received a total EBRT dose of 75.6 Gy, and the remaining participants who had previously undergone prostatectomy (n=10) received a total EBRT dose of 68.4 Gy. All but one participant scored 90 on the Karnofsky performance scale at the baseline, indicating that these patients were able to carry out normal activities with only minor signs or symptoms of disease, before the start of EBRT. The mean prostate-specific antigen (PSA) level was 16.9±20.1 ng/mL at the baseline, before EBRT or ADT. The
mean baseline testosterone, thyroid-stimulating hormone, and albumin levels were all within the reference ranges.

Table 1. Demographic and clinical characteristics of the 51 study participants at the baseline before external beam radiation therapy
Symptoms related to EBRT
Depressive symptoms: There were no significant changes in mean depressive symptom score at the midpoint of EBRT(2.12±3.14) and at the end of EBRT (1.44±1.66) compared with the baseline (1.06±1.64). Overall, HAM-D scores of all patients were below 15 and mean scores were below 3. None of the HAM-D scores at the three time points (days 0, 21, and 42) reached the clinical cutoff for depression [17].
Gastrointestinal and genitourinary symptoms: The mean AUA urinary symptom scores increased significantly from the baseline (7.82±5.24) to the midpoint of EBRT(11.6±6.83, P<0.001) and the end of EBRT (13.82±7.75,P<0.001). The mean SHIM score decreased significantly at the midpoint of EBRT (6.96±8.35, P=0.002) and at completion of EBRT (6.18±8.11, P=0.001) compared with the baseline (10.22±8.95), indicating severe erectile dysfunction in this population during EBRT [22]. Compared with the baseline (40.23±7.08), the mean symptom scores from the SISYM significantly increased at the midpoint of EBRT (47.71±8.43,P<0.001) and remained elevated at completion of EBRT(48.23±8.71, P<0.001). Similarly, the mean SISD scores increased significantly from the baseline (27.07±9.18) to the midpoint of EBRT (32.27±10.97, P=0.001) and the completion of EBRT (34.70±12.47, P<0.001). Increased SISYM and SISD scores indicate moderate symptoms and symptom-related distress associated with urinary tract problems, bowel problems,and sexual dysfunction. Figure 1 delineates the mean AUA,SHIM, SISYM, and SISD scores at the baseline, at the midpoint of EBRT, and at the end of EBRT.
Fatigue: Compared with the baseline (1.51±1.82, range 0—6.13), the mean rPFS scores increased significantly at the midpoint of EBRT (2.95±1.82, P=0.001, range 0—8.2) and at completion of EBRT (2.89±2.26, P=0.002, range 0—7.9), indicating mild fatigue during EBRT (Fig. 1). Although the mean rPFS scores are considered to be in the mild fatigue category[28, 29], the higher end of the rPFS score ranges increased to the severe fatigue category (rPFS score >7) from the baseline to the midpoint of EBRT and from the baseline to completion of EBRT.
Health-related quality of life: The mean FACT-P scores at the baseline, at the midpoint of EBRT, and at completion of EBRT are described in Fig. 1. Compared with the baseline(131.02±16.14), there were significant changes in FACTP scores at the midpoint of EBRT (122.58±18.07, P<0.001)and at the end of EBRT (122.93±18.95, P<0.001). Figure 2 describes the trajectory of HRQOL during EBRT as measured by FACT-P. A change of FACT-P score of more than 6 from the baseline (before cancer treatment) is considered clinically meaningful [35].
Predictors of HRQOL
Table 2 describes the predictive model of HRQOL at completion of EBRT. The regression model comprising age, body mass index, Gleason score, T category, ADT use, radiation dose, and AUA, SHIM, SISYM, SISD, and rPFS scores explained 70% of the variance in predicting HRQOL (FACT-P). However, only urinary symptoms (β=—0.86, P=0.025) and fatigue symptoms(β=—4.67, P=0.01) were significant predictors of self-reported HRQOL, while demographic and clinical variables, including prior prostatectomy, were poor predictors of HRQOL change at completion of EBRT.
Discussion
To our knowledge, this is the first study to identify symptoms that can predict self-reported overall HRQOL during EBRT. The major findings include (1) HRQOL of men with nonmetastatic prostate cancer declines during EBRT, and (2)the urinary and fatigue symptoms of study participants were significant predictors of their HRQOL at completion of EBRT.Recent advances in nonmetastatic prostate cancer therapy have used new modalities to deliver radiation to tumor cells,including proton therapy. Unfortunately, a comparative study concluded that hypofractionated radiation therapy using either the standard, widely used carbon ions or newer approaches such as the use of protons produces the same types of symptoms (e.g., urinary problems, fatigue, gastrointestinal disturbance), with a decline in HRQOL as a side effect of all these prostate cancer therapies [36]. A recent interventional study using a smartphone for early detection and management of symptoms revealed that targeting EBRT-related symptoms early (fatigue, urinary symptoms, insomnia, emotional issues)improves HRQOL in men receiving EBRT for prostate cancer[37]. That study further supports the importance of understanding the relationships of various symptoms and HRQOL and the need to target these symptoms to improve HRQOL and overall treatment outcomes of this clinical population.

Fig. 1. Changes in the American Urological Association (AUA), Sexual Health Inventory for Men (SHIM), symptom indexes of symptoms for urinary, bowel, and sexual function problems (SISYM), symptom indexes of symptom-related distress for urinary, bowel, and sexual function(SISD), revised Piper Fatigue Scale (rPFS), and Functional Assessment of Cancer Therapy—Prostate (FACT-P) scores during external beam radiation therapy (EBRT) from the baseline (day 0, D0) to the midpoint of EBRT (day 19 to day 21; D21) and from the baseline (D0) to the end of EBRT (day 38—day 42, D42). Two asterisks indicates P<0.001.
Lower urinary tract symptoms and fatigue significantly predicted better HRQOL at completion of EBRT in this study. Age, body mass index, Gleason score, T category,radiation dose, ADT use, and overall symptoms and symptom distress were found to be insignificant predictors of HRQOL at completion of EBRT. Lower urinary tract symptoms and fatigue can be used as an interventional target to improve treatment outcomes of patients during EBRT. As proposed criteria, it would be clinically important to closely follow up patients who have poor baseline urinary symptom scores (AUA score >7 indicating clinically significant urinary symptoms) and fatigue (rPFS score >4 indicating moderate to severe fatigue) to avoid worsening of HRQOL at completion of EBRT.

Fig. 2. Trajectory of health-related quality of life (HRQOL) measured by the Functional Assessment of Cancer Therapy—Prostate (lower scores corresponds to worse HRQOL) in prostate cancer patients during external beam radiation therapy (EBRT) at the baseline (day 0,D0), at the midpoint of EBRT (day 19—day 21, D21), and at the end of EBRT (day 38—day 42, D42). Mean HRQOL scores of all study participants worsened at the midpoint and at completion of EBRT compared with the baseline. Two asterisks indicates P<0.001.
Few studies that have investigated predictors of HRQOL during EBRT. Most studies reported predictors of HRQOL years after radiation therapy for nonmetastatic prostate cancer.One of those studies revealed that baseline bowel issues and a depression diagnosis were independent predictors of HRQOL decline following stereotactic body radiation therapy (commonly known as CyberKnife) in prostate cancer men [16]. One study clearly described the trajectories of common symptoms reported by patients before, during, and after radiation therapy for prostate cancer [12]. However, that study did not explore symptoms that can predict the HRQOL of men with prostate cancer during radiation therapy.
Moving forward, it will be clinically important to understand the biologic underpinnings of symptom intensification and increasing symptom burden that can affect HRQOL. The role of mitochondria has received renewed interest to explain the cause of symptoms, We previously reported fi ve genes that were upregulated (BCL2L1, COX6B1, FIS1, SLC25A25,and SLC25A37) and nine genes that were downregulated(AIFM2, BCL2, BCS1L, BNIP3, TIMM10B, IMMP2L, MIPEP,SLC25A23, and SLC25A4) during EBRT for nonmetastatic prostate cancer [19]. We conducted an exploratory, secondary analysis of our previous findings using Pearson correlation,and found four of the previously reported 14 mitochondrial genes to be significantly associated with changes in HRQOL either at the midpoint of EBRT (r=—0.49 to 0.49, P=0.05) or at the end of EBRT (r=—0.6 to 0.48, P<0.05) (Table 3). These genes were BCS1L (BCS1 homolog, ubiquinol—cytochrome c reductase complex chaperone), FIS1 (fission, mitochondrial 1), IMMP2L (inner mitochondrial membrane peptidase subunit 2), and SLC25A37 (solute carrier family 25 member 37).

Table 2. Predictive model of the Functional Assessment of Cancer Therapy—Prostate at the end of external beam radiation therapy using multiple regression
These four genes may play an important role in development of symptoms mediating changes in the patients’HRQOL. For example, decreased BCS1L expression is associated with decreased activity of complex III in the mitochondrial respiratory chain [38], which impairs ATP production[39, 40], potentially contributing to fatigue intensification and irritative symptoms, such as urinary disturbances during EBRT. SLC25A37 (MFRN or mitoferrin-1) regulates iron uptake into mitochondria and promotes heme synthesis [41].Alteration in iron uptake and heme synthesis during EBRT is a common side effect, and has been reported to result in mitochondrial dysfunction [42], which may intensify fatigue during EBRT [18, 19, 43]. Mitochondrial fission 1 (encoded by FIS1) is involved in dynamic processes (fission and fusion)and plays a key role in maintaining cellular metabolic homeostasis. Excessive mitochondrial fission is implicated in multiple human diseases; for example, symptoms in type 2 diabetes[44], neurodegenerative disease, and cancer [45]. However,no study has described the association between mitochondrial genes and HRQOL.
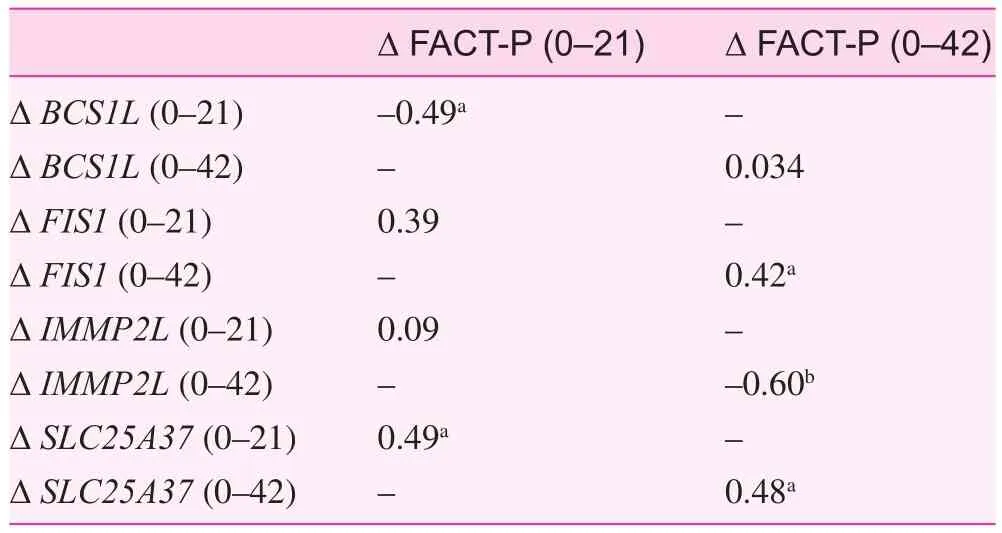
Table 3. Correlation (r) between changes in the differential expression of mitochondrial genes and changes in health-related quality of life scores (as measured by the Functional Assessment of Cancer Therapy—Prostate) from the baseline (day 0) to the midpoint of external beam radiation therapy (day 21) and the end of external beam radiation therapy (day 42)
Limitations of this study have been recognized. This study was conducted in a tertiary research setting with a semiselective patient population and convenience sampling; therefore the results may not be generalizable. Although this study investigated the effect of symptoms on HRQOL during EBRT,it is important to note that symptoms could last for 12—24 months after completion of EBRT. So, patients receiving this treatment should be followed up for longer after completion of EBRT to fully understand the association of symptoms and HRQOL in this clinical population. Another limitation of this study is the sample size, and a larger sample should be used to confirm the associations of specific symptoms and biomarkers with self-reported HRQOL.
Conclusion
The study findings provide information on the relationships between self-reported symptoms (urinary, bowel, and sexual problems, fatigue), symptom-related distress, and demographic and clinical factors and HRQOL in men with clinically localized prostate cancer undergoing EBRT. The findings revealed that urinary symptoms and fatigue are the best predictors of the decline in HRQOL at completion of EBRT. Furthermore,changes in the expression of mitochondria-related biomarkers (BCS1L, FIS1, IMMPL2, and SLC25A35) are associated with HRQOL and could be used for future investigations to help explain the biologic underpinning of the relationship of symptoms and HRQOL. Therefore assessment of symptoms,specifically urinary symptoms and fatigue at the beginning of EBRT, may enable clinicians to identify patients who need early and aggressive intervention to prevent a decline in HRQOL during EBRT.
Acknowledgment
The authors thank Barbara Daly for editing assistance.
Conflict of interest
The authors declare that they have no conflict of interest.
Funding
This study was fully supported by the Division of Intramural Research of the National Institute of Nursing Research of the National Institutes of Health, Bethesda, Maryland.
1. Sloan JA, Berk L, Roscoe J, Fisch MJ, Shaw EG, Wyatt G,et al. Integrating patient-reported outcomes into cancer symptom management clinical trials supported by the National Cancer Institute—sponsored Clinical Trials Networks. J Clin Oncol 2007;25(32):5070—7.
2. Schmidt S, Garin O, Pardo Y, Valderas JM, Alonso J, Rebollo P,et al. Assessing quality of life in patients with prostate cancer:a systematic and standardized comparison of available instruments. Qual Life Res 2014;23(8):2169—81.
3. Diao K, Lobos EA, Yirmibesoglu E, Basak R, Hendrix LH,Barbosa B, et al. Patient-reported quality of life during definitive and postprostatectomy image-guided radiation therapy for prostate cance. Pract Radiat Oncol 2017;7(2):e117—24.
4. American Cancer Society. American Cancer Society facts and fi gures. 2017 [cited 2017 March 10]. Available from: http://www.cancer.org/content/dam/CRC/PDF/Public/8793.00.pdf.
5. Pinkawa M, Gontero P. The motion: radiotherapy for prostate cancer preserves sexual function to a greater extent than nerve sparing radical prostatectomy. Eur Urol 2009;56(1):212—4.
6. Zaorsky NG, Shaikh T, Murphy CT, Hallman MA, Hayes SB,Sobczak ML, et al. Comparison of outcomes and toxicities among radiation therapy treatment options for prostate cancer.Cancer Treat Rev 2016;48:50—60.
7. Truong PT, Berthelet E, Lee JC, Petersen R, Lim JT, Gaul CA,et al. Prospective evaluation of the prevalence and severity of fatigue in patients with prostate cancer undergoing radical external beam radiotherapy and neoadjuvant hormone therapy. Can J Urol 2006;13(3):3139—46.
8. Langston B, Armes J, Levy A, Tidey E, Ream E. The prevalence and severity of fatigue in men with prostate cancer: a systematic review of the literature. Support Care Cancer 2013;21(6):1761—71.
9. Gray PJ, Paly JJ, Yeap BY, Sanda MG, Sandler HM, Michalski JM, et al. Patient-reported outcomes after 3-dimensional conformal, intensity-modulated, or proton beam radiotherapy for localized prostate cancer. Cancer 2013;119(9):1729—35.
10. Johnson ME, Zaorsky NG, Martin JM, Ruth K, Greenberg RE,Uzzo RG, et al. Patient reported outcomes among treatment modalities for prostate cancer. Can J Urol 2016;23(6):8535—45.
11. Shaikh T, Li T, Handorf EA, Johnson ME, Wang LS, Hallman MA, et al. Long-term patient-reported outcomes from a phase 3 randomized prospective trial of conventional versus hypofractionated radiation therapy for localized prostate cancer. Int J Radiat Oncol Biol Phys 2017;97(4):722—31.
12. Knapp K, Cooper B, Koetters T, Cataldo J, Dhruva A, Paul SM,et al. Trajectories and predictors of symptom occurrence, severity, and distress in prostate cancer patients undergoing radiation therapy. J Pain Symptom Manage 2012;44(4):486—507.
13. Banerji JS, Hurwitz LM, Cullen J, Wolff EM, Levie KE, Rosner IL, et al. A prospective study of health-related quality-of-life outcomes for patients with low-risk prostate cancer managed by active surveillance or radiation therapy. Urol Oncol 2017;35(5):234—42.
14. Cappelleri JC, Rosen RC. The sexual health inventory for men(SHIM): a 5-year review of research and clinical experience. Int J Impot Res 2005;17(4):307—19.
15. Chin S, Hayden AJ, Gebski V, Cross S, Turner SL. Long term patient reported urinary function following external beam radiotherapy for prostate cancer. Clin Oncol 2017;29(7):421—8.
16. Dess RT, Jackson WC, Suy S, Soni PD, Lee JY, Abugharib AE,et al. Predictors of multidomain decline in health-related quality of life after stereotactic body radiation therapy (SBRT) for prostate cancer. Cancer 2017;123(9):1635—42.
17. Lynch Kelly D, Dickinson K, Hsiao CP, Lukkahatai N,Gonzalez-Marrero V, McCabe M, et al. Biological basis for the clustering of symptoms. Semin Oncol Nurs 2016;32(4):351—60.18. Hsiao CP, Wang D, Kaushal A, Saligan L. Mitochondria-related gene expression changes are associated with fatigue in patients with nonmetastatic prostate cancer receiving external beam radiation therapy. Cancer Nurs 2013;36(3):189—97.
19. Hsiao C-P, Wang D, Kaushal A, Chen MK, Saligan L. Differential expression of genes related to mitochondrial biogenesis and bioenergetics in fatigued prostate cancer men receiving external beam radiation therapy. J Pain Symptom Manage 2014;48(6):1080—90.
20. Filler K, Lyon D, McCain N, Bennett J, Fernández-Martínez JL, deAndrés-Galiana EJ, et al. Relationship of mitochondrial enzymes to fatigue intensity in men with prostate cancer receiving external beam radiation therapy. Biol Res Nurs 2015;18(3):274—80.
21. Hsiao CP, Araneta M, Wang XM, Saligan LN. The association of IFI27 expression and fatigue intensif i cation during localized radiation therapy: implication of a para-inf l ammatory bystander response. Int J Mol Sci 2013;14(8):16943—57.
22. Feng LR, Suy S, Collins SP, Saligan LN. The role of TRAIL in fatigue induced by repeated stress from radiotherapy. J Psychiatr Res 2017;91:130—8.
23. Lydiatt WM, Denman D, McNeilly DP, Puumula SE, Burke WJ.A randomized, placebo-controlled trial of citalopram for the pre-vention of major depression during treatment for head and neck cancer. Arch Otolaryngol Head Neck Surg 2008;134(5):528—35.
24. Barry MJ, Fowler FJ, O’leary MP, Bruskewitz RC, Holtgrewe HL, Mebust WK, et al. The American Urological Association symptom index for benign prostatic hyperplasia. J Urol 2017;197(2 Suppl):S189—97.
25. Cappelleri JC, Siegel RL, Glasser DB, Osterloh IH, Rosen RC.Relationship between patient self-assessment of erectile dysfunction and the sexual health inventory for men. Clin Ther 2001;23(10):1707—19.
26. Clark JA, Talcott JA. Symptom indexes to assess outcomes of treatment for early prostate cancer. Med Care 2001;39(10):1118—30.
27. Clark JA, Bokhour BG, Inui TS, Silliman RA, Talcott JA. Measuring patients’ perceptions of the outcomes of treatment for early prostate cancer. Med Care 2003;41(8):923—36.
28. Piper BF, Dibble SL, Dodd MJ, Weiss MC, Slaughter RE, Paul SM. The revised Piper Fatigue Scale: psychometric evaluation in women with breast cancer. Oncol Nurs Forum 1998;25(4):677—84.
29. Piper BF, Cella D. Cancer-related fatigue: definitions and clinical subtypes. J Natl Compr Canc Netw 2010;8(8):958—66.
30. Esper P, Mo F, Chodak G, Sinner M, Cella D, Pienta KJ. Measuring quality of life in men with prostate cancer using the Functional Assessment of Cancer Therapy-prostate instrument.Urology 1997;50(6):920—8.
31. Wong CK, Choi EP, Tsu JH, Ho BS, Ng AT, Chin WY, et al. Psychometric properties of functional assessment of cancer therapyprostate (FACT-P) in Chinese patients with prostate cancer. Qual Life Res 2015;24(10):2397—402.
32. Stone PC, Murphy RF, Matar HE, Almerie MQ. Measuring the individual quality of life of patients with prostate cancer. Prostate Cancer Prostatic Dis 2008;11(4):390—6.
33. Stone PC, Murphy RF, Matar HE, Almerie MQ. Quality of life in patients with prostate cancer: development and application of a hybrid assessment method. Prostate Cancer Prostatic Dis 2009;12(1):72—7.
34. Choi EP, Wong CK, Wan EY, Tsu JH, Chin WY, Kung K, et al.The internal and external responsiveness of functional assessment of cancer therapy-prostate (FACT-P) and Short Form-12 Health Survey version 2 (SF-12 v2) in patients with prostate cance. Qual Life Res 2016;25(9):2379—93.
35. Cella D, Nichol MB, Eton D, Nelson JB, Mulani P. Estimating clinically meaningful changes for the functional assessment of cancer therapy—prostate: results from a clinical trial of patients with metastatic hormone-refractory prostate cancer. Value Health 2009;12(1):124—9.
36. Habl G, Uhl M, Katayama S, Kessel KA, Hatiboglu G, Hadaschik B, et al. Acute toxicity and quality of life in patients with prostate cancer treated with protons or carbon ions in a prospective randomized phase ii study — the IPI trial. Int J Radiat Oncol Biol Phys 2016;95(1):435—43.
37. Sundberg K, Wengström Y, Blomberg K, Hälleberg-Nyman M,Frank C, Langius-Eklöf A. Early detection and management of symptoms using an interactive smartphone application (Interaktor) during radiotherapy for prostate cancer. Support Care Cancer 2017;25(7):2195—204.
38. Borisov VB, Liebl U, Rappaport F, Martin JL, Zhang J, Gennis RB, et al. Interactions between heme d and heme b595 in quinol oxidase bd from Escherichia coli: a photoselection study using femtosecond spectroscopy. Biochemistry 2002;41(5):1654—62.
39. Hinson JT, Fantin VR, Schönberger J, Breivik N, Siem G,McDonough B, et al. Missense mutations in the BCS1L gene as a cause of the Björnstad syndrome. N Engl J Med 2007;356(8):809—19.
40. Lesnefsky EJ, Hoppel CL. Oxidative phosphorylation and aging.Ageing Res Rev 2006;5(4):402—33.
41. Lill R, Diekert K, Kaut A, Lange H, Pelzer W, Prohl C, et al. The essential role of mitochondria in the biogenesis of cellular ironsulfur proteins. Biol Chem 1999;380(10):1157—66.
42. Sripetchwandee J, Sanit J, Chattipakorn N, Chattipakorn SC.Mitochondrial calcium uniporter blocker effectively prevents brain mitochondrial dysfunction caused by iron overload. Life Sci 2013;92(4—5):298—304.
43. Hsiao C-P, Daly B, Hoppel C. Association between mitochondrial bioenergetics and radiation-related fatigue: a possible mechanism and novel target. Arch Cancer Res 2015;3(2):14.
44. Rovira-Llo pis S, Bañuls C, Diaz-Morales N, Hernandez-Mijares A, Rocha M, Victor VM. Mitochondrial dynamics in type 2 diabetes: pathophysiological implication. Redox Biol 2017;11:637—45.
45. Serasinghe MN, Chipuk JE. Mitochondrial fission in human diseases. Springer Berlin Heidelberg: Berlin, Heidelberg; 2016.p. 1—30.
1. The Frances Payne Bolton School of Nursing, Case Western Reserve University, Cleveland,OH, USA
2. University of Arizona, 3009 E 4th St. Tucson, AZ 85716, USA
3. National Institute of Nursing Research, Division of Intramural Research, National Institutes of Health, 9000 Rockville Pike,Building 3, Room 5E14, Bethesda, MD 20892, USA
Chao-Pin Hsiao
The Frances Payne Bolton School of Nursing, Case Western Reserve University, 2120 Cornell Road, Room 3120, Cleveland, OH 44106, USA
Tel.: +1-216-3683343
Fax: +1-216-3683542
E-mail: cxh416@case.edu
1 April 2017;
Accepted 5 July 2017
 Family Medicine and Community Health2017年2期
Family Medicine and Community Health2017年2期
- Family Medicine and Community Health的其它文章
- Primary care and cancer
- The association of inherited variation in the CLOCK gene with breast cancer tumor grade
- Use of prostate-specific antigen testing in Medicare beneficiaries:Association with previous evaluation
- Complex multimorbidity and health outcomes in older adult cancer survivorsa
- The relationship between anxiety about prostate cancer among patients with biochemical cancer recurrence and the use of complementary and alternative medicines, diet, and exercise
- Short sleep duration as a contributor to racial disparities in breast cancer tumor grade
