The relationship between anxiety about prostate cancer among patients with biochemical cancer recurrence and the use of complementary and alternative medicines, diet, and exercise
Richard T. Lee, Joshua A. Hemmerich, Nancy Kwon, Kathryn Bylow, Walter M. Stadler, Supriya G. Mohile,William Dale
The relationship between anxiety about prostate cancer among patients with biochemical cancer recurrence and the use of complementary and alternative medicines, diet, and exercise
Richard T. Lee1, Joshua A. Hemmerich2,†, Nancy Kwon3, Kathryn Bylow4, Walter M. Stadler2, Supriya G. Mohile5,William Dale2
Objective: We aimed to explore associations between anxiety and specific health behaviors such as complementary and alternative medicine (CAM), diet, and exercise among prostate cancer patients.
Methods:PCa patients enrolled in a prospective cohort study of men with biochemical cancer recurrence were surveyed about use of CAM, diet, and exercise. Anxiety was measured with the Memorial Anxiety Scale for Prostate Cancer (MAX-PC) and the anxiety subscale of the Hospital Anxiety and Depression Scale.
Results:Nearly 70% (44 of 67) of the original cohort of patients completed the supplementary CAM survey. The mean age was 68 years. Eighty percent of respondents reported engaging in a relevant health behavior, and 64% reported doing so in direct response to their PCa diagnosis. Overall,the most prevalent specific behaviors were exercising (56%), making dietary changes (50%), taking calcium supplements (41%), and taking vitamin D supplements (39%). Elevated baseline PCaspecific anxiety (MAX-PC score >16) after biochemical cancer recurrence was associated with use of any CAM (P=0.01), use of herbs/supplements (P=0.01), and dietary changes (P=0.04).
Conclusion:PCa patients commonly use CAM, dietary changes, and exercise in response to their diagnosis, and these changes are associated with elevated general and PCa-specific anxiety.
Prostate cancer; complementary and alternative medicine; health behaviors; anxiety
Introduction
Since the 1990s the use of complementary and alternative medicine (CAM) has grown in the United States, and a report from the 2012 cohort of the National Health Interview Survey found that 33% of American adults had used CAM in the previous 12 months [1].CAM includes a variety of therapies, including herbs/supplements, acupuncture, yoga, and other nonconventional approaches. The rate of CAM use among cancer patients appears to be equal to or higher than the rate in the general adult population, with an increasing trend in the past several decades from 25%to 49% [2]. Prostate cancer (PCa) patients have also been characterized as commonly using CAM, with prevalence estimates ranging from 8% to 90% with a mean of 30% in studies of patients with disease of different stages [3]. Additionally, PCa patients have been reported to incorporate other health behaviors such as diet and exercise behaviors after their diagnosis [4—6].
Studies have identified several correlates of the use of CAM, including higher income and education levels, younger ages, larger social networks, and specific psychological conditions [7—10]. Studies of cancer patients’ psychological state have revealed a variety of potential factors that may influence CAM use, including anxiety, fear of recurrence, depression,emotional distress, and coping behavior [11—13]. Studies among PCa patients have found that anxiety can be an important factor in patients’ decision-making process regarding treatment decisions [14—16]. This is increasingly recognized in patients with a biochemical recurrence (BCR) of PCa,which is an asymptomatic rise in prostate-specific antigen(PSA) level following initial therapy. Cancer-specific anxiety importantly influences those with BCR with regard to the timing of their decision to start androgen deprivation therapy(ADT), with patients with greater PCa-specific anxiety tending to start ADT earlier [17]. specifically, identifying reasons for CAM use is important because these therapies could have potential risks as demonstrated by the SELECT trial, which found an increased risk of PCa among those randomized to receive vitamin E supplementation [18]. The influence of anxiety on patients’ decisions to pursue use of CAM such as herbs and supplements, dietary changes, and exercise has not been investigated among men with BCR.
Aware of the effect anxiety can have on a patient’s decision-making process, we hypothesized that among PCa patients with a BCR, those with higher PCa-specific anxiety levels would be likelier to report CAM use, dietary changes,and exercise in the hope of improving their health or prognosis. The aims of this study were (1) to measure the prevalence of an array of different health behaviors (CAM, diet, and exercise) among a sample of men with BCR, and (2) to investigate the specific relationship between PCa-specific anxiety and the use of CAM, diet, and exercise.
Methods
Men who had undergone primary therapy (prostatectomy or radiation therapy) for PCa and then experienced BCR but who had not yet started ADT were approached to participate in a prospective cohort study at the time of their initial appointment at the University of Chicago’s Genitourinary Medical Oncology Clinic [17]. Patients were eligible if they had at least two increases in their PSA level a minimum of 2 weeks apart and had no radiographic evidence of PCa [19].Informed consent was obtained according to institutional guidelines, and the protocol was approved by the University of Chicago Biological Sciences Division Institutional Review Board.
For the current study, an approved amendment was added to the original protocol so as to collect additional information regarding health behaviors and CAM use. On enrollment in the original study, patients completed a baseline questionnaire, and additional questionnaires were completed after each follow-up physician appointment until initiation of ADT [20].We surveyed patients in the original cohort to complete an additional questionnaire regarding health behaviors, including CAM use, dietary changes, and exercise. This questionnaire was completed at follow-up appointments or by mail if the patient had already completed the study. Details on the participants and methods of the original study have been published elsewhere [17]. This analysis was not designed as part of the original study, and thus is secondary in nature.
Measurements
The patients’ demographic characteristics were available from the original study [17], as were the patients’ psychological state and physical functioning scores, which had been assessed with validated instruments, including the Memorial Anxiety Scale for Prostate Cancer (MAX-PC) and the anxiety subscale of the Hospital Anxiety and Depression Scale (HADS-A) [21—24]. A MAX-PC PCa-specific anxiety subscale score of more than 16 was considered to be elevated and a HADS-A score of more than 8 was considered to represent an elevated general anxiety level [21—23, 25]. Clinical data were extracted from the medical records, including the patients’ Gleason scores,PSA values, previous treatments, comorbid conditions, and medications.
The CAM questionnaire was designed on the basis of the frequent use of CAM and other health behaviors reported in previous studies of PCa patients [20, 26—28]. specifically,patients were asked about (1) use of herbs and supplements(e.g., use of echinacea, saw palmetto, garlic, lycopene, soy,green tea, megadose vitamins, selenium, vitamin E, calcium,vitamin D, or other supplements), (2) changes in their diet (e.g.,change to a low-fat diet, increased soy intake, increased green tea intake, or other changes), (3) use of alternative medical systems (e.g., traditional Chinese medicine, naturopathic medicine, homeopathic medicine, or other systems), and (4) use of other CAM therapies and health behaviors (e.g., exercise,chiropractor, acupuncture, massage, prayer, support group, or other therapies). The questionnaire also asked about the timing and reasons for these health behaviors to understand if they were used before the original cancer diagnosis, following PCa diagnosis, and/or following BCR. Patients were also asked if they engaged in these health behaviors as a response to their PCa diagnosis or BCR. Finally, the questionnaire surveyed patients’ communication patterns, decision-making processes,and monthly costs related to health behaviors.
Statistical analysis
We analyzed use of CAM as follows: (1) use of any health behavior, including CAM, diet, and exercise; (2) use of any CAM therapies (excluding diet, exercise, and support groups);(3) use of herbs and supplements (e.g., vitamins, minerals,herbs, nutraceuticals); (4) use of other CAM therapies excluding herbs and supplements (e.g., acupuncture, massage); (5)making of dietary changes (e.g., increased green tea intake,low-fat diet); and (6) changes in exercise pattern. Participants were considered to have an elevated anxiety level if the MAX-PC PCa anxiety subscale score was more than 16 and if the HADS-A score was more than eight at the baseline as cutoffs as reported in previous publications [17, 24]. Associations between elevated anxiety and use of CAM, diet, and exercise were analyzed by a chi-squared test for univariate analysis.We considered P<0.05 as significant. All data analyses were performed with STATA SE 10.0 (StataCorp, College Station,Texas, United States).
Results
Patient characteristics
Sixty-nine percent (44/67) of the patients from the original study completed the questionnaire on CAM use and other health behaviors (Table 1). The respondents’ mean age was 68 years (range 50—84 years, standard deviation 9.1 years),and approximately one-quarter (21%) of respondents were African American. BCR was diagnosed in the patients a median of 58 months (range 1—175 months) before this study,and the patients had a median PSA level of 1.7 ng/mL (range 0.3—27.9 ng/mL) at the time of enrollment. Ninety-three percent of patients reported that their general health was good to excellent, and the patients had a MAX-PC PCa-specific anxiety subscale score of 7.7±5.8 and a HADS-A score of 4.6±3.2,consistent with scores previously reported [21].
Health behaviors and CAM therapies
Most respondents (80%) had engaged in at least one health behavior at some point in the past, and 61% did so before their original PCa diagnosis, which declined to 43% after BCR. Approximately two-thirds (64%) stated that at least one of these health behaviors was in direct response to their PCa diagnosis (rather than a therapy they used for other reasons before their PCa diagnosis). About two-thirds of patients (65%) were incorporating herbs and supplements,which was the most common type of CAM therapy. The use of herbs and supplements increased initially after PCa diagnosis but then declined after BCR, from 39% to 27%(Table 2).
With regard to specific therapies, an exercise program(56%), dietary changes (50%), taking of calcium supplements(41%), and prayer (33%) were the most prevalent (Table 2).The behaviors engaged in by most patients after PCa diagnosis were dietary changes (20%), taking of selenium supplements(18%), and engagement in an exercise program (16%). After BCR, dietary changes (23%), increasing dietary green tea intake (18%), and engagement in an exercise program (16%)were the most common behaviors. Overall, use of CAM,diet, and exercise was stable or declined after BCR. The only increase in health behavior after BCR was incorporation of green tea in the diet, but even this was a marginal increase in one participant. The behaviors most often used in response to PCa diagnosis in any timeframe were exercise (30%), use of calcium supplements (23%), drinking of green tea (23%), and taking of selenium supplements (18%). The mean monthly outof-pocket cost for CAM use was $46 dollars (range $0—600).Supplements (e.g., green tea and garlic) and dietary changes were the modif i cations most often considered (22% and 11%respectively) but not actually taken by patients, primarily because of the unclear benefits, lack of information, and the low interest in these therapies.

Table 1. Patient characteristics and comparison with nonresponders
Associations between anxiety and health behaviors
Generalized anxiety, as indicated by an elevated HADS-A score greater than 8, correlated with an increased use of herbs and supplements (P=0.04), dietary changes (P=0.04), and other CAM therapies (P=0.04) (Table 3). After PCa diagnosis, an elevated general anxiety level was associated with increased use of any health behavior (P=0.04), the making dietary changes (P=0.01), the starting of an exercise program(P<0.01), and use of other CAM therapies (P=0.01). In regard to PCa-specific anxiety (MAX-PC score >16), after the initial PCa diagnosis, no correlations were found with overall prevalence of any of the health behaviors, and it correlated only with dietary changes (P<0.01). However, after BCR, an elevated PCa-specific anxiety level was associated with increased health behavior of any type (P=0.05), CAM use in general(P=0.01), use of herbs and supplements (P=0.01), and dietary changes (P=0.04) (Tables 4 and 5). In regard to demographic analyses, those with a college degree or higher were likelier to engage in an exercise program at any time than those with less than a college education (75% vs 25%, P=0.04). No statistically significant correlations were found with income, race, or age.

Table 2. Prevalence of health behaviors and use of complementary and alternative medicine

Table 3. Correlations between anxiety and overall use of any health behavior

Table 4. Correlations between anxiety and health behaviors after original prostate cancer diagnosis
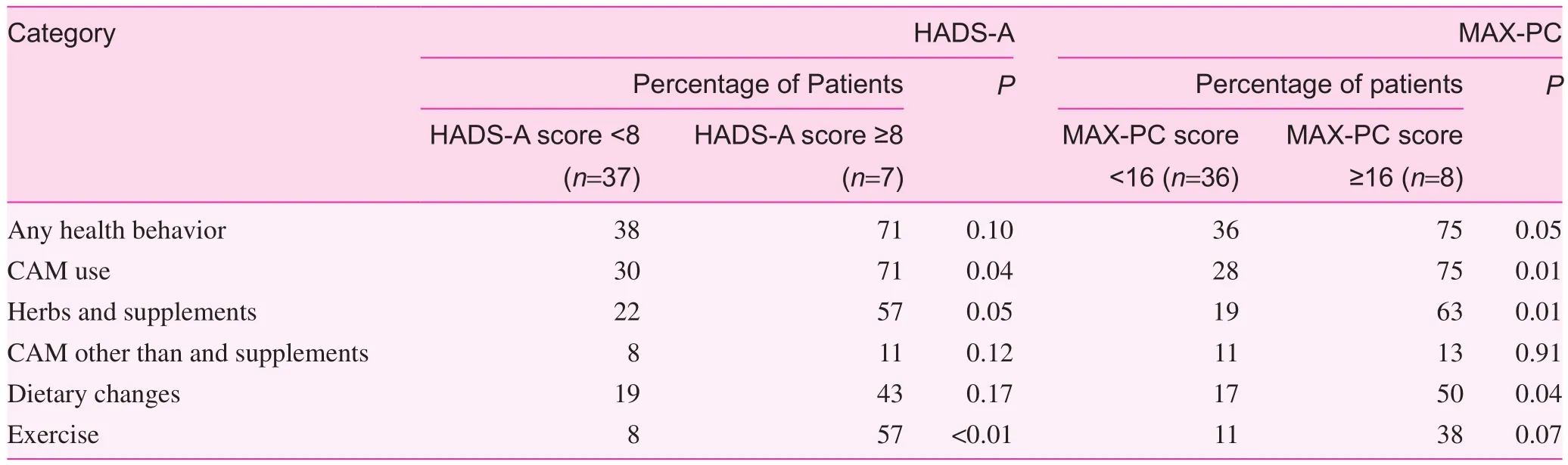
Table 5. Correlation between anxiety and health behaviors after biochemical relapse
Decision making and communication regarding health behaviors and CAM
The most commonly reported sources of information about CAM use and other health behaviors were physicians (64%),print media (50%), and friends (33%). Although physicians were often reported as information sources, approximately two-thirds of patients (67%) indicated that the decision to pursue these interventions was entirely their own. The most commonly reported reasons for pursuing health behaviors and CAM use were “a desire to improve overall health” (88%),a desire to “do everything possible to treat cancer” (58%),and a belief that these therapies had few side effects (50%)(Table 6). Other reasons given for CAM use and engaging in other health behaviors included a desire to use a more holistic approach to health care (29%), a desire to have a sense of control over medical care (29%), and the hope that the therapies would help with treatment-related side effects (28%). Half of the participants told at least one physician about some or all of their health behaviors and CAM therapies. The reasons most commonly given for nondisclosure were that the physician never asked the patient (44%) and a lack of time to discuss the topic (28%).

Table 6. Reasons reported for health behaviors and use of complementary and alternative medicine
Discussion
We found that PCa patients with BCR commonly incorporate health behaviors including CAM, diet, and exercise, with many doing so specifically in response to their PCa diagnosis and recurrence. Many of these health behaviors declined after PCa diagnosis and BCR, with only dietary changes, vitamin D supplementation, selenium intake, and green tea intake in the diet increasing after diagnosis. Another important finding is that patient anxiety, as measured by the MAX-PC (PCa specific) and HADS-A (general), correlated with a significantly increased use of several health behaviors, including use of CAM therapies in this population.
While several articles have reported the use of CAM in PCa patients, ours is the first to report on its use in patients with a BCR along their cancer clinical continuum — before PCa diagnosis, after PCa diagnosis ,and after BCR [8, 20, 26—38]. Most studies have examined CAM use overall or at a single point in time, usually after cancer diagnosis. One study examined urologic patients before and after surgery and found an increase in the utilization of CAM after surgery, from 30 to 53 among a total of 172 participants. Conversely, we found overall use of health behaviors and CAM declined after initial diagnosis, and further investigation is needed to assess these differences.
Only one prior study included a psychometrically validated instrument for anxiety in patients with localized PCa [8], and no prior study of these health behaviors among PCa patients used a PCa-specific anxiety scale, such as the MAX-PC. In that study, anxiety and depression were measured with the Profile of Mood States, and it did not predict CAM use among patients with localized PCa who underwent therapy [39]. Our results indicate that anxiety could potentially play a role in increasing PCa patients’ pursuit of specific health behaviors such as CAM use, dietary change, or exercise initiation. This study is unique in that our patients represent a carefully defined clinical scenario — patients in whom a BCR has just been diagnosed — a time when patients are often anxious and uncertain,and therefore likelier to take action as a consequence [17].
We found that PCa patients typically chose to pursue health behaviors including CAM use on their own, and only half of them talked with their physicians about these decisions. Many of these behaviors, especially dietary and exercise changes, are beneficial for improving overall health, and growing evidence in PCa, breast cancer, and colon cancer indicates that these lifestyle changes may lead to decreased disease recurrence [40—45]. The use of calcium and vitamin D is common advice for patients receiving ADT because of the risk of osteoporosis. However,some CAM therapies, such as supplementation with vitamin E,selenium, β-carotene, and multivitamins, have unproven benefits in PCa treatment, and in some instances can have potential harmful effects [46—49]. If patients are interested in improving their overall health, which was the reason most commonly cited for pursuing health behaviors such as CAM use, then improving diet and improving exercise patterns are the most important behavior changes that should be addressed. A lack of information was a common reason not to pursue these health behaviors,and thus such a discussion about health behaviors could help patients to make a better decision to improve their health.
Patients with a BCR of PCa and high PCa-related anxiety levels may be particularly eager to discuss modif i cation of their lifestyle with their physicians. Health care professionals should take this opportunity to provide education on how diet and exercise will impact the patient’s overall health and possibly the course of the cancer. Additionally, PCa patients who start ADT are at risk of long-term side effects, such as sarcopenia, diabetes, and heart disease, and CAM and lifestyle changes may help prevent the development of these conditions[50, 51]. However, clinicians should also counsel patients that interventions, such as use of multivitamins and other supplements, may carry risks [46, 48, 52].
There are limitations to our pilot study. Because the sample was small, with data collected at a single academic center, the generalizability of our findings, while intriguing,is limited. This study was conducted among a cohort of men with BCR of PCa, which also limits generalization to other cancer types and to women. Our survey was based on retrospective self-report, and is at risk of recall biases; therefore prospective studies following patients’ CAM use longitudinally are needed. The response rate of 69% is reasonable but not ideal; fortunately, the nonresponders do not appear to be substantially different than those who responded (Table 1).In fact, they appear to be slightly more anxious, making our findings conservative.
In summary, we found that PCa patients with a BCR of PCa commonly engage in health behaviors such as CAM use,dietary changes, and exercise initiation in response to their diagnosis. We also found that those with elevated general anxiety and PCa-specific anxiety have higher rates of health behaviors, including CAM use. Signs of anxiety may also present a cue to physicians that there is an important opportunity to educate patients about health behaviors that will lead to improved overall health, such as exercise, calcium,and vitamin D use. Using validated cancer-specific anxiety instruments such as the MAX-PC in larger, prospective studies will help provide more accurate information regarding a possible causal role of anxiety in the decision to pursue health behaviors and CAM therapies. Oncologists should ask about patients’ health behaviors and CAM use that may impact their clinical outcomes.
Acknowledgement
Grant Support: Paul Beeson Career Development Award, NIA(WD); Center for Health Administration Studies, University of Chicago; Cancer Research Center, University of Chicago.We thank Dr. Farr Curlin for his assistance with the questionnaire, study design, and his feedback on an earlier draft of this paper.
conflict of interest
The authors declare no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Significance statement
This study among prostate cancer patients highlights the high prevalence of complementary and alternative medicine (CAM),diet, and exercise. These prevalence rates appear to vary depending on when patients are diagnosed and have a recurrence of their prostate cancer. An elevated anxiety level may be a factor in a patient’s decision to utilize CAM, diet and exercise.For clinicians treating patients with prostate cancer, inquiries into these specific health behaviors are important. Increasing research is showing that diet and exercise may improve clinical outcomes including recurrence and survival in many cancer types including prostate cancer. Thus, there may be an opportune time after diagnosis and/or after recurrence of prostate cancer to advise patients about these health behaviors.
1. Clarke TC, Black LI, Stussman BJ, Barnes PM, Nahin RL. Trends in the use of complementary health approaches among adults:United States, 2002—2012. Natl Health Stat Report 2015;(79):1—16.
2. Horneber M, Bueschel G, Dennert G, Less D, Ritter E, Zwahlen M. How many cancer patients use complementary and alternative medicine: a systematic review and metaanalysis. Integr Cancer Ther 2012;11:187—203.
3. Bishop FL, Rea A, Lewith H, Chan YK, Saville J, Prescott P,et al. Complementary medicine use by men with prostate cancer:a systematic review of prevalence studies. Prostate Cancer Prostatic Dis 2011;14:1—13.
4. Satia JA, Walsh JF, Pruthi RS. Health behavior changes in white and African American prostate cancer survivors. Cancer Nurs 2009;32:107—17.
5. Hunt-Shanks TT, Blanchard CM, Baker F, Hann D, Roberts CS, McDonald J, et al. Exercise use as complementary therapy among breast and prostate cancer survivors receiving active treatment: examination of exercise intention. Integr Cancer Ther 2006;5:109—16.
6. Avery KN, Donovan JL, Gilbert R, Davis M, Emmett P, Down L, et al. Men with prostate cancer make positive dietary changes following diagnosis and treatment. Cancer Causes Control 2013;24:1119—28.
7. Boon H, Brown JB, Gavin A, Westlake K. Men with prostate cancer: making decisions about complementary/alternative medicine. Med Decis Making 2003;23:471—9.
8. Diefenbach MA, Hamrick N, Uzzo R, Pollack A, Horwitz E,Greenberg R, et al. Clinical, demographic and psychosocial correlates of complementary and alternative medicine use by men diagnosed with localized prostate cancer. J Urol 2003;170:166—9.
9. Ohlen J, Balneaves LG, Bottorff JL, Brazier AS. The influence of significant others in complementary and alternative medicine decisions by cancer patients. Soc Sci Med 2006;63:1625—36.
10. Singh H, Maskarinec G, Shumay DM. Understanding the motivation for conventional and complementary/alternative medicine use among men with prostate cancer. Integr Cancer Ther 2005;4:187—94.
11. Rakovitch E, Pignol JP, Chartier C, Ezer M, Verma S, Dranitsaris G, et al. Complementary and alternative medicine use is associated with an increased perception of breast cancer risk and death. Breast Cancer Res Treat 2005;90:139—48.
12. Sollner W, Maislinger S, DeVries A, Steixner E, Rumpold G,Lukas P. Use of complementary and alternative medicine by cancer patients is not associated with perceived distress or poor compliance with standard treatment but with active coping behavior:a survey. Cancer 2000;89:873—80.
13. Burstein HJ, Gelber S, Guadagnoli E, Weeks JC. Use of alternative medicine by women with early-stage breast cancer. N Engl J Med 1999;340:1733—9.
14. Dale W, Bilir P, Han M, Meltzer D. The role of anxiety in prostate carcinoma: a structured review of the literature. Cancer 2005;104:467—78.
15. Latini DM, Hart SL, Knight SJ, Cowan JE, Ross PL, DuChane J, et al. The relationship between anxiety and time to treatment for patients with prostate cancer on surveillance. J Urol 2007;178:826—31; discussion 831—2.
16. Mahal BA, Chen MH, Bennett CL, Kattan MW, Sartor O, Stein K, et al. High PSA anxiety and low health literacy skills: drivers of early use of salvage ADT among men with biochemically recurrent prostate cancer after radiotherapy? Ann Oncol 2015;26:1390—5.
17. Dale W, Hemmerich J, Bylow K, Mohile S, Mullaney M, Stadler WM. Patient anxiety about prostate cancer independently predicts early initiation of androgen deprivation therapy for biochemical cancer recurrence in older men: a prospective cohort study. J Clin Oncol 2009;27:1557—63.
18. Klein EA, Thompson IM Jr., Tangen CM, Crowley JJ, Lucia MS,Goodman PJ, et al. Vitamin E and the risk of prostate cancer:the Selenium and Vitamin E Cancer Prevention Trial (SELECT).JAMA 2011;306:1549—56.
19. Bubley GJ, Carducci M, Dahut W, Dawson N, Daliani D, Eisenberger M, et al. Eligibility and response guidelines for phase II clinical trials in androgen-independent prostate cancer: recommendations from the Prostate-specific antigen Working Group.J Clin Oncol 1999;17:3461—7.
20. Chan JM, Elkin EP, Silva SJ, Broering JM, Latini DM, Carroll PR. Total and specific complementary and alternative medicine use in a large cohort of men with prostate cancer. Urology 2005;66:1223—8.
21. Dale W, Hemmerich J, Meltzer D. Extending the validity of the Memorial Anxiety Scale for Prostate Cancer (MAX-PC) at the time of prostate biopsy in a racially-mixed population. Psychooncology 2007;16:493—8.
22. Roth A, Nelson CJ, Rosenfeld B, Warshowski A, O’shea N,Scher H, et al. Assessing anxiety in men with prostate cancer: further data on the reliability and validity of the Memorial Anxiety Scale for Prostate Cancer (MAX-PC). Psychosomatics 2006;47:340—7.
23. Roth AJ, Rosenfeld B, Kornblith AB, Gibson C, Scher HI,Curley ST, et al. The Memorial Anxiety Scale for Prostate Cancer: validation of a new scale to measure anxiety in men with prostate cancer. Cancer 2003;97:2910—8.
24. Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the hospital anxiety and depression scale. An updated literature review. J Psychosom Res 2002;52:69—77.
25. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361—70.
26. Boon H, Westlake K, Stewart M, Gray R, Fleshner N, Gavin A,et al. Use of complementary/alternative medicine by men diagnosed with prostate cancer: prevalence and characteristics. Urology 2003;62:849—53.
27. Eng J, Ramsum D, Verhoef M, Guns E, Davison J, Gallagher R. A population-based survey of complementary and alternative medicine use in men recently diagnosed with prostate cancer.Integr Cancer Ther 2003;2:212—6.
28. Kao GD, Devine P. Use of complementary health practices by prostate carcinoma patients undergoing radiation therapy. Cancer 2000;88:615—9.
29. Lerner IJ, Kennedy BJ. The prevalence of questionable methods of cancer treatment in the United States. CA Cancer J Clin 1992;42:181—91.
30. Lee MM, Chang JS, Jacobs B, Wrensch MR. Complementary and alternative medicine use among men with prostate cancer in 4 ethnic populations. Am J Public Health 2002;92:1606—9.
31. Lippert MC, McClain R, Boyd JC, Theodorescu D. Alternative medicine use in patients with localized prostate carcinoma treated with curative intent. Cancer 1999;86:2642—8.
32. Wiygul JB, Evans BR, Peterson BL, Polascik TJ, Walther PJ,Robertson CN, et al. Supplement use among men with prostate cancer. Urology 2005;66:161—6.
33. Nam RK, Fleshner N, Rakovitch E, Klotz L, Trachtenberg J,Choo R, et al. Prevalence and patterns of the use of complementary therapies among prostate cancer patients: an epidemiological analysis. J Urol 1999;161:1521—4.
34. Ponholzer A, Struhal G, Madersbacher S. Frequent use of complementary medicine by prostate cancer patients. Eur Urol 2003;43:604—8.
35. Porter M, Kolva E, Ahl R, Diefenbach MA. Changing patterns of CAM use among prostate cancer patients two years after diagnosis: reasons for maintenance or discontinuation. Complement Ther Med 2008;16:318—24.
36. Mani J, Juengel E, Arslan I, Bartsch G, Filmann N, Ackermann H, et al. Use of complementary and alternative medicine before and after organ removal due to urologic cancer. Patient Prefer Adherence 2015;9:1407—12.
37. McDermott CL, Blough DK, Fedorenko CR, Arora NK, Zeliadt SB, Fairweather ME, et al. Complementary and alternative medicine use among newly diagnosed prostate cancer patients.Support Care Cancer 2012;20:65—73.
38. Ramsey SD, Zeliadt SB, Blough DK, Fedorenko CR, Fairweather ME, McDermott CL, et al. Complementary and alternative medicine use, patient-reported outcomes, and treatment satisfaction among men with localized prostate cancer. Urology 2012;79:1034—41.
39. Usala PD, Hertzog C. Measurement of affective states in adults.Evaluation of an adjective rating scale instrument. Res Aging 1989;11:403—26.
40. Chlebowski RT, Blackburn GL, Thomson CA, Nixon DW,Shapiro A, Hoy MK, et al. Dietary fat reduction and breast cancer outcome: interim efficacy results from the Women’s Intervention Nutrition Study. J Natl Cancer Inst 2006;98:1767—76.
41. Pierce JP, Stefanick ML, Flatt SW, Natarajan L, Sternfeld B,Madlensky L, et al. Greater survival after breast cancer in physically active women with high vegetable-fruit intake regardless of obesity. J Clin Oncol 2007;25:2345—51.
42. Meyerhardt JA, Giovannucci EL, Holmes MD, Chan AT, Chan JA, Colditz GA, et al. Physical activity and survival after colorectal cancer diagnosis. J Clin Oncol 2006;24:3527—34.
43. Meyerhardt JA, Heseltine D, Niedzwiecki D, Hollis D, Saltz LB,Mayer RJ, et al. Impact of physical activity on cancer recurrence and survival in patients with stage III colon cancer: findings from CALGB 89803. J Clin Oncol 2006;24:3535—41.
44. Bonn SE, Sjolander A, Lagerros YT, Wiklund F, Stattin P,Holmberg E, et al. Physical activity and survival among men diagnosed with prostate cancer. Cancer Epidemiol Biomarkers Prev 2015;24:57—64.
45. Kenfield SA, Stampfer MJ, Giovannucci E, Chan JM.Physical activity and survival after prostate cancer diagnosis in the health professionals follow-up study. J Clin Oncol 2011;29:726—32.
46. Lawson KA, Wright ME, Subar A, Mouw T, Hollenbeck A,Schatzkin A, et al. Multivitamin use and risk of prostate cancer in the National Institutes of Health-AARP Diet and Health Study.J Natl Cancer Inst 2007;99:754—64.
47. Lippman SM, Klein EA, Goodman PJ, Lucia MS, Thompson IM, Ford LG, et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 2009;301:39—51.
48. Stevens VL, McCullough ML, Diver WR, Rodriguez C, Jacobs EJ, Thun MJ, et al. Use of multivitamins and prostate cancer mortality in a large cohort of US men. Cancer Causes Control 2005;16:643—50.
49. Omenn GS, Goodman G, Thornquist M, Barnhart S, Balmes J,Cherniack M, et al. Chemoprevention of lung cancer: the β-Carotene and Retinol efficacy Trial (CARET) in highrisk smokers and asbestos-exposed workers. IARC Sci Publ 1996:67—85.
50. Schwandt A, Garcia JA. Complications of androgen deprivation therapy in prostate cancer. Curr Opin Urol 2009;19:322—6.
51. Bylow K, Mohile SG, Stadler WM, Dale W. Does androgendeprivation therapy accelerate the development of frailty in older men with prostate cancer?: a conceptual review. Cancer 2007;110:2604—13.
52. Lee AH, Ingraham SE, Kopp M, Foraida MI, Jazieh AR.The incidence of potential interactions between dietary supplements and prescription medications in cancer patients at a Veterans Administration hospital. Am J Clin Oncol 2006;29:178—82.
1. Department of Medicine, Case Western Reserve University and University Hospitals, Cleveland,OH, USA
2. Department of Medicine, University of Chicago, Chicago, IL,USA
3. Department of Medicine,Northwestern University, Chicago, IL, USA
4. Department of Medicine,Medical College of Wisconsin,Milwaukee, WI, USA
5. Department of Medicine, University of Rochester, Rochester,NY, USA
†Joshua A. Hemmerich passed away suddenly before the completion of the manuscript.
Richard T. Lee
Associate Professor, Division of Hematology/Oncology, Case Western Reserve University and University Hospitals, Director of Supportive and Integrative Oncology Seidman Cancer Center,Parker Hannifin-Helen Moss Cancer Research Foundation Professor of Integrative Oncology,Cleveland, OH, USA
Tel.: +1-216-3682415
E-mail: richard.t.lee@case.edu
18 April 2017;
Accepted 14 June 2017
 Family Medicine and Community Health2017年2期
Family Medicine and Community Health2017年2期
- Family Medicine and Community Health的其它文章
- Primary care and cancer
- The association of inherited variation in the CLOCK gene with breast cancer tumor grade
- Use of prostate-specific antigen testing in Medicare beneficiaries:Association with previous evaluation
- Symptoms predicting health-related quality of life in prostate cancer patients treated with localized radiation therapy
- Complex multimorbidity and health outcomes in older adult cancer survivorsa
- Short sleep duration as a contributor to racial disparities in breast cancer tumor grade
