Short sleep duration as a contributor to racial disparities in breast cancer tumor grade
Kevin Allan, Nathan A. Berger,2, Li Li, Cheryl L. Thompson
Short sleep duration as a contributor to racial disparities in breast cancer tumor grade
Kevin Allan1, Nathan A. Berger1,2, Li Li2,3, Cheryl L. Thompson2,4
Although African Americans (AAs) are less likely to get breast cancer than European Americans(EAs), they get more aggressive forms. We previously showed that short sleep is associated with higher tumor grade. It is well documented that AAs get less sleep, on average, than EAs. We studied the contribution of short sleep to racial disparities in breast cancer aggressiveness among 809 invasive breast cancer patients who responded to a survey on their lifestyle. Multivariable regressions and mediation analyses were performed to assess the effect of sleep duration on the association of race with tumor grade. AAs reported shorter average sleep (mean [standard deviation] 6.57 [1.47] h) than EAs (mean [standard deviation] 7.11 [1.16] h; P<0.0001) and were almost twice as likely to report less than 6 h of sleep per night (48.0% vs. 25.3%, P<0.0001). AA patients were more likely to have high-grade tumors (52.6% vs. 28.7% in EAs, P=0.0002). In multivariate analysis, race was associated with tumor grade (P<0.0001). On adjustment for sleep duration, the effect of race was reduced by 7.1%, but remained statistically significant (P=0.0006). However, the Sobel test did not indicate statistical significance (z=1.69, P=0.091). In other models accounting for these and additional confounders, we found similar results. Because of the conservative nature of the mediation analysis and smaller sample size, replication of our results in larger studies with more AA patients is warranted.
Breast cancer; sleep; race; disparities
Introduction
Breast cancer is the most common cancer among women in the United States [1]. In 2017 alone,nearly a quarter of a million women will receive a diagnosis of breast cancer, and more than 40,000 women will die of the disease [1]. Despite the persistent lower incidence of breast cancer,African American (AA) women had a higher mortality rate than European American (EA)women [2]. The 5-year relative survival for EA breast cancer patients is 92.0%, whereas it is only 80.0% for AA breast cancer patients [3]. The causes of these disparities are likely multifactorial, including differences in socioeconomic status (SES), access to health care (including both breast cancer screening and treatment services), genetics, lifestyle factors, and tumor characteristics [4]. A recent study using a large sample of AA and EA breast cancer patients from the Surveillance, Epidemiology, and End Results registry matched on demographics showed a 12.9% overall difference in overall 5-year survival favoring EAs. Intriguingly, the study noted that a large portion (8.5% of the 12.9%) of racial differences in breast cancer survival were accounted for by clinical characteristics at presentation, including tumor grade, whereas only 0.8%were due to treatment differences [5].
It is well established that AA breast cancer patients present with more aggressive types of breast cancer [6, 7]. Tumor grade,the widely used clinical measure of tumor aggressiveness, is strongly correlated with clinical outcomes among breast cancer patients. AA breast cancer patients are significantly more likely to have high-grade tumors than EA breast cancer patients [7, 8].
There is emerging evidence for a role of short sleep duration, a potentially modif i able risk factor, in breast cancer tumor aggressiveness [9]. We recently demonstrated that short sleep before diagnosis is associated with a more aggressive tumor[10, 11]. We found that fewer hours of sleep before diagnosis was correlated with a higher Oncotype DX recurrence score(R=−0.41 P=0.0011) [10], which is associated with a higher likelihood of recurrence. We also found that breast cancer patients who reported sleeping less than 6 h per night before diagnosis were about twice as likely to have high-grade (grade 3) tumors, particularly in postmenopausal patients [11]. This association remained with adjustment for race, age, hormone replacement therapy, body mass index (BMI), physical activity, smoking, and alcohol consumption (P=0.049) [11]. It is well documented that AAs get less sleep, have lower sleep efficiency, and have higher sleep latency than their EA counterparts [12, 13], even with socioeconomic factors controlled for[13]. Given these observations, the goal of the present study was to evaluate if shorter sleep durations among AA breast cancer patients may account for part of the disparities in tumor grade observed among AA and EA breast cancer patients.
Methods
Study population
Newly diagnosed breast cancer patients (n=1263) were recruited from University Hospitals Cleveland Medical Center(UHCMC) and aff i liated clinics between 2007 and 2014.Patients were recruited before nonsurgical treatments, and were ineligible if they were known BRCA1 or BRCA2 mutation carriers or had a history of cancer.
Data collection
Breast cancer patients were surveyed for demographic, risk factor, and lifestyle data via telephone survey, and were asked to report on lifestyle variables, including average nightly sleep duration, before diagnosis. BMI was calculated from selfreported height and weight. Clinical data were abstracted from medical records, and included tumor stage, grade, and estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 status. All tumor grades were assigned by UHCMC staff breast pathologists. Self-reported sleep duration was both used continuously and coded as less than 6 h of sleep per night, 6 h to less than 7 h of sleep per night, and 7 h or more of sleep per night. These categories were chosen to compare them with standard recommendations of 6 or 7 h of sleep per night as well as compare to compare the findings with those of previously published studies [11].
Patients were coded for SES with use of census tract data for income bracket at their location of residence at diagnosis(low, moderate, middle, or upper). For statistical analyses, lowand moderate-income tracts were grouped together because of their small sample size.
For the current analysis, patients with a diagnosis of ductal carcinoma in situ were excluded (n=424), as were those for whom tumor grade could not be found (n=18). Because the goal of this study was to understand the contribution of sleep duration to differences in tumor grades observed between AA and EA women, those reporting a race other than EA or AA were also excluded (n=12). This resulted in a fi nal sample size of 809.
Statistical analyses
All continuous variables were compared between AA and EA patients by a standard t test. Categorical variables were analyzed by a χ2test. Analysis of differences in individual variables by sleep category (as defined earlier) was done by an ANOVA or χ2test, as appropriate.
Tumor grade may be associated with a number of other factors associated with race, such as age, physical activity,reproductive factors, and SES. To evaluate the effect of race on tumor grade accounting for these potential confounders, multivariable regressions were performed with an unconditional multinomial logistic regression with tumor grade (1, 2, or 3)as the outcome. We first evaluated the effect of race on tumor grade, accounting for other potential contributors, including age, BMI, physical activity, menopausal status, parity, age at menarche, use of hormone replacement therapy, and SES by adding these other variables to the model. Individual pairwise correlations between variables were calculated with a Pearson correlation coefficient, and multicollinearity was tested for by a variance inflation factor (VIF) analysis.
We evaluated the effect of sleep on the effect estimates for race and then conducted a meditation analysis using a Sobel test. We did this for two models: a complete model incorporating all variables we considered as potential confounders and the most parsimonious model. The most parsimonious model was derived via both forward and backward stepwise modeling. An F statistic greater than 0.5 would indicate the stopping of forward selection, and F<0.1 is needed for removal of a variable in the backward selection. To assess the effect of short sleep on the association of race with breast cancer tumor grade, we then added sleep duration into the most parsimonious regression model and compared the estimated effect of race (via the β coefficient) in the two models. All statistical analyses were done with SAS version 9.4, and P<0.05 was considered statistically significant.
Results
The distribution of EA and AA patients with regard to demographic factors is shown in Table 1. This distribution reflects the distribution of EA and AA patients in our sample population. The EA and AA patients in our sample have similar age, and there is no difference in menopausal status between the two groups (Table 1). AA patients reported a significantly higher BMI than EA patients (P<0.0001), and report exercising less (P<0.0001). In addition, a much greater percentage of the AA patients resided in low/moderate-income census tracts compared with the EA patients (P<0.0001).

Table 1. Patient demographics and clinical characteristics by race
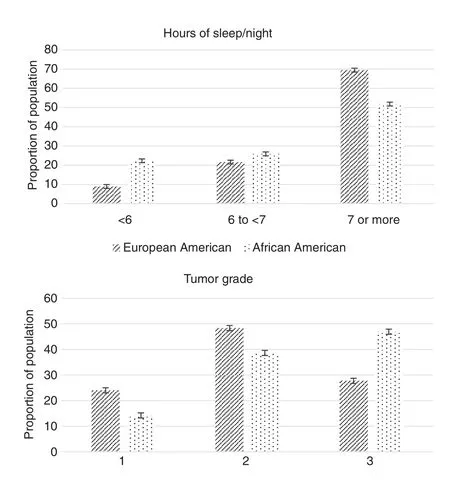
Fig. 1. Sleep, race, and tumor grade. Hours of sleep per night and tumor grade by race as percentage of patient population.
AA breast cancer patients reported sleeping approximately a half hour less, on average, than their EA counterparts(P<0.0001), and there was a significantly greater percentage of AAs than EAs in the “less than 6 h of sleep” category (22.3%vs. 8.9%, P<0.0001, Fig. 1). In addition, a smaller percentage of AA patients reported 7 h or more of sleep (51.8% vs. 69.0%,P<0.0001). As expected, a greater percentage of AA patients than EA patients received a diagnosis of grade 3 and triple negative breast cancer (Table 1, Fig. 1).
Variables such as SES, BMI, and physical activity are typically highly correlated. In our dataset this was true, with all correlation coefficients (R) ranging from 0.11 to 0.20 (P values all less than 0.002). VIFs were assessed in the full logistic model, and no VIF was greater than 1.3; thus we believe it is safe to include all in the initial model (before stepwise selection) together. The most parsimonious multinomial regression,obtained via both forward and backward stepwise selection,included only age (P<0.0001) and race (P=0.0009). In this model the effect estimate for race is β=0.362 (standard error 0.099, P<0.0001). When average sleep duration, as a continuous variable, was added into the model, the effect of race was reduced to β=0.340 (standard error 0.099, P=0.0006), representing an approximately 7.1% reduction in effect (measured through the β coefficient, Table 2). However, the Sobel test resulted in a nonsignificant P value (0.091).
Similar results were observed for the full model including all variables (Table 2), although, as expected, with additional variables the effect was reduced and Sobel test indicated less significance (P=0.11).
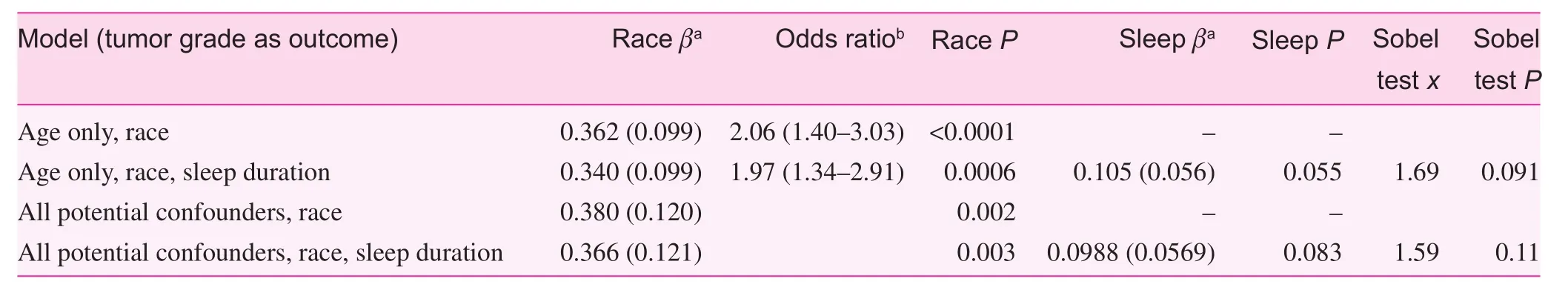
Table 2. Results of multinomial regressions and mediation analyses
Discussion
In this study, our goal was to investigate if shorter sleep duration may contribute to some of the observed racial variation in tumor grade. Indeed, we noted that the magnitude of race-tumor grade association was reduced by approximately 7.1% after we had accounting for sleep duration. This suggests that short duration of sleep is a factor mediating some of the association of race with tumor grade, and thus a small but potentially significant contributor to the observed racial disparities in breast cancer aggressiveness. Although this represented a small fraction of the overall difference in aggressiveness of AA patients, given the higher breast cancer mortality among AA patients and the modifiable nature of sleep, as well as that nearly one in four of the AA patients in our sample reported sleeping less than 6 h per night, the population attributable risk and impact would be substantial if the racial disparities in tumor aggressiveness were reduced by 7.1% by sleep interventions. However, this finding is diminished by our mediation analysis suggesting that this is not statistically significant in our sample.
In earlier studies, we noted an association of sleep duration before diagnosis and aggressiveness of breast tumors among newly diagnosed breast cancer patients. In these studies, we found that patients with fewer than 6 h of sleep per night on average had more aggressive tumors than those regularly getting at least 7 h per night [10, 11]. There is an established strong association between tumor grade and race among breast cancer patients, and it is well known that AAs report less sleep compared with individuals of other races. In our sample, we found that 22.3% of AAs reported sleeping less than 6 h per night compared with only 8.9% of the EAs in our sample.
The results of our Sobel test suggest that sleep is not a statistically significant mediator of the association between race and tumor grade. However, the Sobel test is known to be conservative,and our sample was limited. We feel it is important to explore this association in larger samples, particularly with more AA patients.
In the United States, AAs, as a group, differ from EAs in many ways, including culturally, genetically, and in their environment. Unfortunately, AAs also experience a number of disparities in health from EAs, and teasing out the underlying factors contributing to these disparities is often a challenge because of the complexity of the differences in populations. A number of factors have been proposed to account for the established disparities in outcomes among AA breast cancer patients, including health care costs and access to care, SES and stresses due to limited resources, genetic variations, and differences in lifestyle. One of the challenges in this field is to tease out the complex interrelation among these factors, each of these accounting for a relatively modest but significant portion of the observed racial disparities in breast cancer aggressiveness. Here we presented data suggesting that short sleep, a potentially modifiable risk factor, may be one novel factor that contributes to this disparity. However, further studies will need to be done to see if other variables related to sleep, such as stress or genetics, which we were unable to assess here, also contribute to disparities in breast cancer aggressiveness.
As with all studies, ours is not without limitation. Although we had a large number of individuals, because of the small fraction of AA patients in our sample population, we had a relatively small number (n=113) of AA patients, which limits our power, particularly for interaction and mediation analyses.Further, this study is a cross-sectional study of breast cancer patients in northeast Ohio, and it will be important to replicate these findings in other patient populations to see if these findings are generalizable to other populations. Further, because we included only newly diagnosed breast cancer patients, it is possible that overreporting or underreporting of sleep duration may have occurred. As such, caution must be exercised in inferring causality, and confirmation in large cohort studies is warranted.In this study, we were able to adjust the data for many of these potential confounding factors, but could not adjust the data for all of them, because with the data we have, we were unable to asses some of them, such as genetics or current occupation.Another potential limitation is recall bias. It is possible that individuals with higher-grade tumor reported sleeping less before diagnosis. However, we think that this would have a minimal effect on our study because recall bias has been mostly observed with respect to differences in case/control status, although it could be differentially reported by tumor grade. In addition,we do not think there would be differential recall bias by race,which was our primary analysis in this article.
In summary, sleep is increasingly recognized as an important and modif i able lifestyle factor that impacts our overall health. Our data here suggest that sleep duration contributes to racial disparities in tumor grade and that reducing racial differences in sleep duration may reduce disparities in breast cancer outcomes. Further work will need to be done to replicate the findings and to assess if sleep interventions in highrisk women confer improvements in clinical outcomes among these women. It is also important to acknowledge that to fully understand the complicated relationship between race and breast cancer aggressiveness, and the factors underlying the disparities in tumor aggressiveness, we need a very large sample with more detailed data on sleep as well as other potentially associated factors that we were unable to assess here,such as environment, stress, and genetic factors.
Acknowledgments
The authors thank all the breast cancer patients who participated in this study.
conflicts of interest
The authors declare no conflict of interest.
Funding
Funding for this study was provided from the National Cancer Institute (K07CA136758) and the Case Comprehensive Cancer Center (P30CA043703).
Author contributions
CLT and LL contributed to the concept and design of the study,acquisition of data, or analysis and interpretation of data. CLT,KA and NAB contributed to critically drafting and revising the manuscript for important intellectual content. KA, LL,NAB and CLT contributed to the final approval of the version to be published.
Significance Statement
African American (AA) women get more aggressive breast cancer compared to Caucasian women. It is thought that there are many contributors to this disparity, including genetics as well as lifestyle. We previously showed that women who slept less got more aggressive breast cancer. It is known that AAs get less sleep, on average, than Caucasians. This study is the first study to attempt to quantify the role of sleep in racial disparities in breast cancer aggressiveness. We showed that accounting for short sleep reduced the differences in aggressiveness by race about 7%. Although not a large fraction, due to the number of women with breast cancer and modifiable nature of sleep duration, it represents a potential large public health impact.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017;67:7—30.
2. Whitman S, Ansell D, Orsi J, Francois T. The racial disparity in breast cancer mortality. J Community Health 2011;36:588-96.
3. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5—29.
4. Ademuyiwa FO, Edge SB, Erwin DO, Orom H, Ambrosone CB,Underwood W 3rd. Breast cancer racial disparities: unanswered questions. Cancer Res 2011;71:640—4.
5. Silber JH, Rosenbaum PR, Clark AS, Giantonio BJ, Ross RN,Teng Y, et al. Characteristics associated with differences in survival among black and white women with breast cancer. J Am Med Assoc 2013;310:389—97.
6. Cunningham JE, Walters CA, Hill EG, Ford ME, Barker-Elamin T, Bennett CL. Mind the gap: racial differences in breast cancer incidence and biologic phenotype, but not stage, among lowincome women participating in a government-funded screening program. Breast Cancer Res Treat 2013;137:589—98.
7. Corsini C, Henouda S, Nejima DB, Bertet H, Toledano A,Boussen H, et al. Early onset breast cancer: differences in risk factors, tumor phenotype, and genotype between North African and South European women. Breast Cancer Res Treat 2017;(in press).
8. Furberg H, Millikan R, Dressler L, Newman B, Geradts J. Tumor characteristics in African American and white women. Breast Cancer Res Treat 2001;68:33—43.
9. Soucise A, Vaughn C, Thompson CL, Millen AE, Freudenheim JL, Wactawski-Wende J, et al. Sleep quality, duration, and breast cancer aggressiveness. Breast Cancer Res Treat 2017;164:169—78.
10. Thompson CL, Li L. Association of sleep duration and breast cancer OncotypeDX recurrence score. Breast Cancer Res Treat 2012;134:1291—5.
11. Khawaja A, Rao S, Li L, Thompson CL. Sleep duration and breast cancer phenotype. J Cancer Epidemiol 2013;2013:467927.
12. Adenekan B, Pandey A, McKenzie S, Zizi F, Casimir GJ, Jean-Louis G. Sleep in America: role of racial/ethnic differences.Sleep Med Rev 2013;17:255—62.
13. Hale L, Do DP. Racial differences in self-reports of sleep duration in a population-based study. Sleep 2007;30:1096—103.
1. Department of1Medicine,Case Western Reserve University, 10900 Euclid Ave., Cleveland, OH 44106, USA
2. Case Comprehensive Cancer Center, Case Western Reserve University, 10900 Euclid Ave.,Cleveland, OH 44106, USA
3. Department of Family Medicine and Community Health,Case Western Reserve University, 10900 Euclid Ave., Cleveland, OH 44106, USA
4. Department of Nutrition, Case Western Reserve University,10900 Euclid Ave., Cleveland,OH 44106, USA
Cheryl L. Thompson, PhD Case Comprehensive Cancer Center, Case Western Reserve University, 10900 Euclid Ave.,Cleveland, OH 44106, USA; and Department of Nutrition, Case Western Reserve University,10900 Euclid Ave., Cleveland,OH 44106, USA
Tel.: +1-216-3583956
E-mail: cheryl.l.thompson@case.edu
23 March 2017;
Accepted 6 September 2017
 Family Medicine and Community Health2017年2期
Family Medicine and Community Health2017年2期
- Family Medicine and Community Health的其它文章
- Primary care and cancer
- The association of inherited variation in the CLOCK gene with breast cancer tumor grade
- Use of prostate-specific antigen testing in Medicare beneficiaries:Association with previous evaluation
- Symptoms predicting health-related quality of life in prostate cancer patients treated with localized radiation therapy
- Complex multimorbidity and health outcomes in older adult cancer survivorsa
- The relationship between anxiety about prostate cancer among patients with biochemical cancer recurrence and the use of complementary and alternative medicines, diet, and exercise
