The Akt/glycogen synthase kinase-3β pathway participates in the neuroprotective effect of interleukin-4 against cerebral ischemia/reperfusion injury
Mei Li, Wen-Wei Gao , Lian Liu, Yue Gao, Ya-Feng Wang, Bo Zhao, Xiao-Xing Xiong
1 Department of Anesthesiology, Renmin Hospital of Wuhan University, Wuhan, Hubei Province, China
2 Department of Critical Care Medicine, Renmin Hospital of Wuhan University, Wuhan, Hubei Province, China
3 Department of Personnel, Renmin Hospital of Wuhan University, Wuhan, Hubei Province, China
4 Department of Neurosurgery, Renmin Hospital of Wuhan University, Wuhan, Hubei Province, China
Abstract
Abstract Interleukin-4 (IL-4) has a protective effect against cerebral ischemia/reperfusion injury. Animal experiments have shown that IL-4 improves the short- and long-term prognosis of neurological function. The Akt (also called protein kinase B, PKB)/glycogen synthase kinase-3β (Akt/GSK-3β) signaling pathway is involved in oxidative stress, the inf lammatory response, apoptosis, and autophagy. However, it is not yet clear whether the Akt/GSK-3β pathway participates in the neuroprotective effect of IL-4 against cerebral ischemia/reperfusion injury. In the present study, we established a cerebral ischemia/reperfusion mouse model by middle cerebral artery occlusion for 60 minutes followed by a 24-hour reperfusion. An IL-4/anti-IL-4 complex (10 μg) was intraperitoneally administered 30 minutes before surgery. We found that administration of IL-4 significantly alleviated the neurological deficits, oxidative stress, cell apoptosis, and autophagy and reduced infarct volume of the mice with cerebral ischemia/reperfusion injury 24 hours after reperfusion. Simultaneously, IL-4 activated Akt/GSK-3β signaling pathway. However, an Akt inhibitor LY294002, which was injected at 15 nmol/kg via the tail vein, attenuated the protective effects of IL-4. These findings indicate that IL-4 has a protective effect on cerebral ischemia/reperfusion injury by mitigating oxidative stress, reducing apoptosis, and inhibiting excessive autophagy, and that this mechanism may be related to activation of the Akt/GSK-3β pathway. This animal study was approved by the Animal Ethics Committee of Renmin Hospital of Wuhan University, China (approval No. WDRY2017-K037) on March 9, 2017.
Key Words: Akt/glycogen synthase kinase-3β pathway; apoptosis; autophagy; cerebral ischemia/reperfusion injury; infarct volume; interleukin-4; neuroprotection; oxidative stress
Introduction
Cerebrovascular disease has a serious social and economic burden due to its high morbidity, high disability rate, high mortality, and multiple complications, for which ischemic stroke accounts for about 80% of cases (Dubuc et al., 2014; Lee et al., 2018). Restoration of blood f low after ischemia is the key to recovery of cerebral tissue exposed to ischemia; however, reperfusion of ischemic tissue can cause cerebral ischemia/reperfusion injury (CIRI), which exacerbates neuronal apoptosis and neural dysfunction in the cortex and hippocampus (Aronowski et al., 1997; Kuroda and Siesjö, 1997). While the pathogenesis of CIRI is still not entirely clear, it has been associated with calcium overload, increased oxygen radicals, the release of inflammatory cytokines, apoptosis, and autophagy activation (White et al., 2000; Eltzschig and Eckle, 2011). Autophagy plays an important role in the regulation of neuronal function in CIRI, and can lead to neuronal apoptosis by over-activation or inhibition of autophagy (Gabryel et al., 2012; Shi et al., 2012). Therefore, recent research has focused on the regulatory mechanism underlying autophagy in neurons in CIRI.
Interleukin (IL)-4 is an important anti-inf lammatory factor that can increase the secretion of other anti-inf lammatory factors, such as IL-10 and tumor necrosis factor-β (Tang et al., 2019). IL-4 can also inhibit the release of inf lammatory cytokines, such as tumor necrosis factor-α and IL-1β, by promoting the differentiation of mononuclear macrophages into alternative activated macrophages via the IL-4 receptor (Bhattacharjee et al., 2013; Francos-Quijorna et al., 2016; Tang et al., 2019). Both clinical and animal model studies have reported that IL-4 is important in the acute stage of stroke. In patients with stroke, IL-4 serum levels increase dramatically several hours after ischemia (Kim et al., 2000). In animal models of stroke, IL-4 deficiency achieved through gene-knockout exacerbates neurological deficits and brain injury 24 hours after reperfusion, and exogenous IL-4 supplement improves behavioral outcomes and long-term cognitive function; these results indicate that IL-4 confers good neuroprotection after stroke (Xiong et al., 2011, 2015; Liu et al., 2016). However, the mechanism underlying the neuroprotective effect of IL-4 is still unclear; it could be related to the reduction of inf lammatory factors, apoptosis, and/or autophagy during ischemia/reperfusion.
As a key signaling pathway that regulates cell survival, growth, and proliferation, the Akt/glycogen synthase kinase-3β (GSK-3β) pathway is widely expressed in various tissues and organs. In CIRI, the GSK-3β pathway regulates neural apoptosis and of autophagy (Qi et al., 2012; Ye et al., 2015; Zhao et al., 2017). It has been shown that IL-4 can increase the phosphorylation of Akt and regulate its downstream proteins, such as GSK-3β, Bad, and the Bcl-2 family, through the IL-4 receptor (Xiong et al., 2015). GSK-3β is expressed abundantly in the brain, and has neuroprotective effects following ischemia damage via prevention of reactive oxygen species (ROS) production and restoration of impaired mitochondrial biogenesis (Zhao et al., 2017). The neuroprotective effect of IL-4 in ischemia/reperfusion may be related to Akt/GSK-3β pathway activation.
Materials and Methods
Animal groups and drug interventions
A total of 40 adult male Balb/c mice of clean grade aged 10-12 weeks and weighing 20-25 g were obtained from Charles River Labs, Beijing, China (license No. SCXK (Jing) 2016-0006). Mice were housed in groups of f ive and had free access to food and water. The conditions were suitable for mice in terms of temperature (22 ± 2°C), humidity (55 ± 5%), and photoperiod (12-hour light/12-hour dark). All mice were acclimatized in cages for 3 days prior to the experiments. The animal handling procedures were approved by the Animal Ethics Committee of Renmin Hospital of Wuhan University, China (approved No. WDRY2017-K037) on March 9, 2017, and were in accordance with the laboratory animal management procedures of Renmin Hospital of Wuhan University, China.
The Balb/c mice were randomly divided into the following four groups of 10 mice each: sham, ischemia/reperfusion, ischemia/reperfusion + IL-4 (IR + IL-4), and ischemia/reperfusion + IL-4 + LY294002 (IR + IL-4 + LY294002) groups. LY294002 is an Akt inhibitor that is usually used to inhibit the activation of Akt. The half-life of exogenous recombinant mouse IL-4 is 30 minutes, but the half-life of IL-4/anti-IL-4 complexes (IL-4C) can be 24 hours (Milner et al., 2010), so recombinant mouse IL-4 (10 μg; PeproTech, Rocky Hill, NJ, USA) and rat anti-mouse IL-4 neutralizing antibody (rat IgG1 anti-mouse IL-4 monoclonal antibody; 50 μg; Verax, Marlborough, MA, USA) were mixed at a mass ratio of 1:5 to prepare IL-4C for a longer duration. Then, IL-4C was diluted to 50 μg/mL with physiological saline containing 1% Balb/c mice serum. A total of 0.2 mL IL-4C was administered via intraperitoneal injection 30 minutes before the application of middle cerebral artery occlusion model in the IR + IL-4 and the IR + IL-4 + LY294002 groups. For the sham and IR groups, mice were injected 0.2 mL vehicle (physiological saline containing 1% Balb/c serum). Akt inhibitor LY294002 (MedChemExpress, Monmouth Junction, NJ, USA) was also injected at 15 nmol/kg via the tail vein at the same time in the IR + IL-4 + LY294002 group, and physiological saline was given at 15 nmol/kg to the other groups.
Establishment of focal CIRI model
The mouse model of focal CIRI was established with suture to block the middle cerebral artery, as described previously (Belayev et al., 1999; Xiong et al., 2011). Brief ly, anesthesia of mice was induced with 5% isoflurane by face mask and maintained with 1-2% isof lurane during the operation. After the effective induction of anesthesia, the skin was incised in the middle of the neck, the left common carotid artery was gently isolated from the muscle and nerve fibers, and the external and internal carotid arteries were then carefully separated. To block the origin of the middle cerebral artery, a 6-0 silicon suture (Doccol Corp, Redlands, CA, USA) was inserted into the external carotid artery lumen until mild resistance was felt. After 60 minutes of ischemia, 24 hours of reperfusion was implemented by removal of the suture. The sham group received all surgical procedures except for the insert of the silicon suture. Vital signs, including heart rate, breathing rate, and rectal temperature, were constantly monitored, and the temperature of mice was maintained at 36.5 to 37.5°C using a heating pad. We excluded animals that had a neurological deficits score < 1 after ischemia and anesthesia, died during the 24-hour reperfusion, or were diagnosed with subarachnoid hemorrhage, from the final analysis.
Neurological deficits score
Twenty-four hours after reperfusion, the neurological status of mice was evaluated by an evaluator who was blind to the experimental groups. Neurological deficits were scored from 0-4 (Longa et al., 1989), whereby 0 = no neurological deficits; 1 = the contralateral forepaw cannot extend fully; 2 = circling to the contralateral side when walking; 3 = falling to the contralateral side when walking; 4 = unable to walk spontaneously or loss of consciousness.
2,3,5-Triphenyltetrazolium chloride staining
Cerebral tissue was stained by 2,3,5-triphenyltetrazolium chloride (Sigma-Aldrich, St. Louis, MO, USA) at 24 hours after reperfusion, mice were sacrif iced under anesthesia, and their brains were carefully removed. The brains were frozen at -80°C for 5 minutes, then cut into coronal sections at a thickness of 2 mm using a rodent brain slicer matrix. The sections were incubated in 2% 2,3,5-triphenyltetrazolium chloride solution at 37°C in the dark for 30 minutes, immersed in 4% paraformaldehyde overnight and then photographed. Normal tissue was stained red and the infarcted tissue was stained white. Infarction volume was analyzed and corrected for edema using the NIH Image J software (National Institutes of Health, Bethesda, MD, USA). The volume of cerebral infarct was calculated by the sum of the infarction area multiplied by slice thickness. The final results are expressed as a percentage of infarct volume to the contralateral cortical volume.
Terminal-deoxynucleotidyl transferase-mediated nick end labeling staining for cell apoptosis
To evaluate neuron apoptosis in the penumbra of the ischemic region after reperfusion, terminal-deoxynucleotidyl transferase mediated nick end labeling (TUNEL) staining was performed and the percentage of positive cells were calculated. The experiment was carried out according to the manufacturer’s guidelines (Roche, Boston, MA, USA). Paraffin sections of penumbra were dewaxed and rehydrated, incubated in 3% H2O2for 10 minutes, and digested in proteinase K for 10 minutes. Subsequently, the slides were incubated in TUNEL reaction mixture for 60 minutes and then in converter horseradish peroxidase solution for 30 minutes at 37°C. Cells with brown-stained granules in the nucleus were counted as TUNEL positive cells, and cells with blue staining in the nucleus were counted as negative cells. Five fields of view were randomly captured in each slice under a light microscope (BX51 microscope, Olympus Inc., Tokyo, Japan), and the number of positive cells per field was calculated by an evaluator who was blind to the experimental design. Apoptotic index (AI) was calculated using the following formula: AI (%) = TUNEL positive cells/total cells × 100.
Oxidative stress detection
The operation was carried out according to the instructions of superoxide dismutase (SOD), malondialdehyde (MDA), reactive oxygen species (ROS) assay kit (Jiancheng Bioengineering, Nanjing, China). The brain tissue of the cerebral ischemic penumbra area was placed on an ice pad, washed with physiological saline, and prepared to homogenate solution with a tissue content of 10%. The solution was centrifuged at 4°C for 10 minutes, then SOD activity, MDA and ROS levels were measured by colorimetry (f luorescence spectrophotometer; Hitachi, Tokyo, Japan) using the supernatant of the centrifuged solution.
Western blot analysis
Animals were re-anesthetized, brains were quickly removed and immediately frozen at -70°C, and then cut into 1-mm3coronal slices by a brain slicer. Then, the bilateral hippocampus was removed, tissues of the ischemic penumbra (tissues around the core necrotic region) were harvested under a microscope and homogenized in cold radioimmunoprecipitation assay lysis buffer, and the target proteins were separated by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis before being electro-transferred onto a polyvinylidene dif luoride membrane (Liu et al., 2016). After being blocked with skimmed milk powder for 1 hour, the membranes were washed by Tris-buffered saline-Tween 20 and incubated with diluted primary antibodies of Akt (1:1000; mouse monoclonal antibody; Cat# 4821; Cell Signaling, Beverly, MA, USA), phospho (p)-Akt (1:2000; rabbit monoclonal antibody; Cat# 4060, Cell Signaling), GSK-3β (1:1000; mouse monoclonal antibody; Cat# 9832, Cell Signaling), p-GSK-3β (1:1000; rabbit monoclonal antibody, ser9; Cat# 9323, Cell Signaling), microtubule-associated protein light chain 3II/I (LC3-II/I; 1:500; mouse monoclonal antibody; Cat# sc-271625, Santa Cruz Biotechnology, Dallas, TX, USA), and Beclin-1 (1:500; rabbit monoclonal antibody; Cat# EPR19662, Abcam, Cambridge, MA, USA) overnight at 4°C. Then, the blots were washed three times for 5 minutes using Tris-buff-ered saline-Tween 20, incubated with secondary antibodies of goat anti-mouse IgG (1:10,000; LI-COR Biosciences, Lincoln, NE, USA), which was labeled by horseradish peroxidase for 1 hour at room temperature. Subsequently, the blots were washed three times again using Tris-buffered saline-Tween 20 and incubated with chemiluminescence substrate. Immunoblots were exposed to autoradiography f ilm and analyzed using Bio-Rad software (Bio-Rad Laboratories, Hercules, CA, USA). Glyceraldehyde 3-phosphate dehydrogenase was chosen as a loading control, and levels of target proteins were expressed as the ratio of the target protein optical density compared with that of the loading control.
Transmission electron microscopy
Tissues of the ischemic penumbra were cut into a size of 1 cm3and fixed in cool 4% glutaraldehyde for more than 4 hours. After being washed with phosphate buffer saline, tissues were f ixed in 1% osmic acid, dehydrated by gradient acetone, embedded with resin, and cut into 50-70 nm-thick sections. Then, sections were stained using uranyl acetate followed by lead citrate. Five random fields of each section were photographed using transmission electron microscopy (Hitachi), and autophagosomes in neurons per 100 μm2of different groups were calculated by an investigator who was blind to the experiment.
Statistical analysis
Experimental data are expressed as the mean ± standard deviation (SD) and were analyzed using SPSS 21.0 software (IBM SPSS, Chicago, IL, USA). Differences between groups were tested using one-way analysis of variance followed by Bonferroni post-hoc tests. Differences were considered to be statistically significant at a P-level < 0.05.
Results
IL-4 ameliorates neurological deficits and reduces infarct volume in CIRI mice
As shown in Figure 1, as expected, mice in the sham group showed little neurological deficits and infarct volume as expected. Compared with the sham group, IL-4 increased infarct volume and the neurological deficit score in the IR group (P < 0.05). LY294002 attenuated the neuroprotective effect of IL-4, and the neurological deficits and infarction volume of the IR + IL-4 + LY294002 group were worse/larger than those of the IR + IL-4 group (P < 0.05).
IL-4 increases Akt and GSK-3β phosphorylation in the penumbra of CIRI mice
Akt is also called protein kinase B, and has been reported to be phosphorylated and activated at sites Thr308 and Ser473, while GSK-3β is thought to be phosphorylated and inactivated at site Ser9 (Endo et al., 2006). As shown in Figure 2A and B, compared with the sham group, p-Akt/Akt and p-GSK-3β/GSK-3β levels were significantly lower in the IR group (P < 0.05). p-Akt/Akt and p-GSK-3β/GSK-3β levels in the ischemic penumbra of the IR + IL-4 group were significantly higher than those of the IR group (P < 0.05). However, LY294002 decreased the levels of p-Akt/Akt and p-GSK-3β/GSK-3β compared with the IR + IL-4 group (P < 0.05).
IL-4 decreases neuronal apoptosis in CIRI mice
The apoptosis of neurons in the ischemic penumbra at 24 hours after reperfusion was analyzed using TUNEL staining (Figure 3A). As shown in Figure 3B, the apoptosis index in the IR, IR + IL-4, and IR + IL-4 + LY294002 groups was significantly higher than that of the sham group (P < 0.05). When IL-4 was applied as a CIRI pretreatment, the apoptosis index of neurons around the penumbra regions in the IR + IL-4 group was lower than that of the IR group (P < 0.05). This indicates that IL-4 could reduce neuronal apoptosis in CIRI. When compared with the IR + IL-4 group, the apoptosis index of neurons was increased in the IR + IL-4 + LY294002 group (P < 0.05).
IL-4 reduces oxidative stress after CIRI
As shown in Figure 4, compared with the sham group, SOD activity was lower and MDA and ROS levels were higher in the IR, IR + IL-4, and IR + IL-4 + LY294002 groups (P < 0.05). Compared with the IR group, the activity of SOD was higher and MDA and ROS levels were lower in the IR + IL-4 group (P < 0.05). In the IR + IL-4 + LY294002 group, the activity of SOD was lower and MDA and ROS levels were higher than those of the IR + IL-4 group (P < 0.05).
IL-4 inhibites LC3-II/I and Beclin-1 protein expression and autophagosome formation in CIRI mice
Compared with the sham group, Beclin-1 and LC3-II/I protein expression in the ischemic penumbra was increased in the IR, IR + IL-4, and IR + IL-4 + LY294002 groups (P < 0.05; Figure 5A-C). Furthermore, the levels of Beclin-1 and LC3-II/I protein were decreased in the IR + IL-4 group compared with the IR and IR + IL-4 + LY294002 groups (P < 0.05). Autophagosomes in neurons of the ischemic penumbra were detected under a transmission electron microscope (Figure 5D), and autophagosomes with a double-layer membrane structure were found in the cytoplasm of neurons. Similarly to Beclin-1 and LC3-II/I, there were significantly fewer autophagosomes in the IR + IL-4 group compared with the IR and IR + IL-4 + LY294002 groups (P < 0.05; Figure 5E).
Discussion
IL-4 is an important anti-inflammatory cytokine that confers a neuroprotective effect in neurodegeneration and acute ischemic diseases; however, the neuroprotection mechanisms of IL-4 in IR are still poorly understood (Gadani et al., 2012; Liu et al., 2016; Cruz et al., 2018; Lee et al., 2018). The present study found that exogenous IL-4 preconditioning decreased cerebral infarction volume, neurological deficits, oxidative stress, cell apoptosis, and autophagy. It also increased p-Akt and p-GSK-3β levels, which indicates that the Akt/GSK-3β pathway was activated during this process. Furthermore, the IL-4-induced neuroprotective effect in IR was blocked by the Akt inhibitor LY294002. Thus, these results demonstrate that IL-4 pretreatment could exert neuroprotective effects by suppressing neuronal apoptosis, oxidative stress, and autophagy through activation of the Akt/GSK-3β pathway.
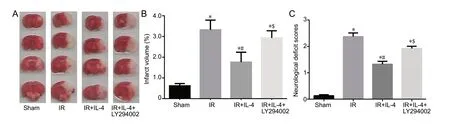
Figure 1 The effect of IL-4 on infarct volume and neurological deficit scores in mice with focal cerebral ischemia/reperfusion injury.
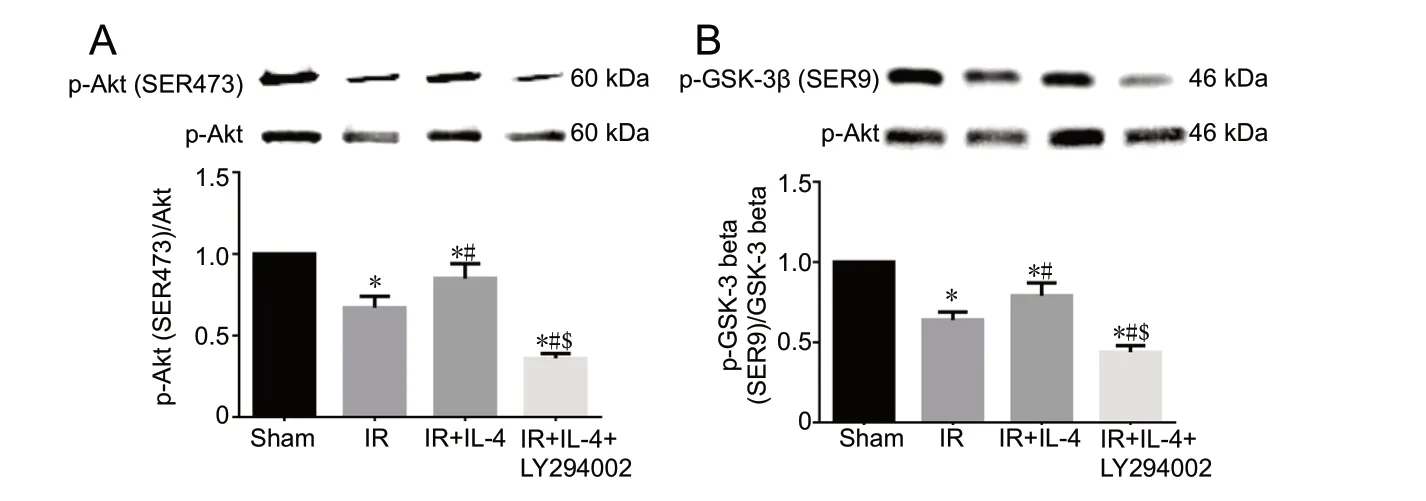
Figure 2 Western blot assay showing the effect of IL-4 on Akt (A) and GSK-3β (B) phosphorylation in the cerebral ischemic penumbra of focal CIRI mice 24 hours after reperfusion.

Figure 3 The effect of IL-4 on apoptosis in the penumbra area 24 hours after reperfusion in focal cerebral ischemia/reperfusion injury mice revealed by terminal-deoxynucleotidyl transferase mediated nick end labeling (TUNEL) staining.
Cytokines regulate the immune response in animals; IL-4 is an important anti-inf lammatory cytokine that is secreted by activated T cells, eosinophilic granulocytes, basophilic granulocytes, mast cells, and natural killer cells. IL-4 can exert anti-inflammatory effects by regulating the function of inf lammation-associated cells such as T cells, B cells, and macrophages via the IL-4 receptor (Bhattacharjee et al., 2013). IL-4 plays an important role not only in immune-related diseases, such as asthma and scleroderma, but also in nervous system diseases, such as Alzheimer’s disease, glioblastoma, spinal cord injury, multiple sclerosis, and ischemic stroke (Gadani et al., 2012; Francos-Quijorna et al., 2016). In ischemic stroke, IL-4 has been reported to have a neuroprotective effect by regulating the function of immune cells; for example, it promotes the differentiation of macrophages into alternatively activated macrophages that secrete anti-inf lammatory factors such as IL-10 and transforming growth factor-β (Bhattacharjee et al., 2013; Martinez and Gordon, 2014). Recent studies have found that IL-4 knockout mice showed increased infarct volume and neurological deficits, and that exogenous IL-4 administration significantly decreased the infarct area and improved long-term neurological function in CIRI mice (Liu et al., 2016). Under normal conditions, neurons produce and secrete IL-4 a few minutes after injury, and this IL-4 secretion can last for 2-3 days, which may contribute to the neuroprotective effect of IL-4 in acute brain injury. Interestingly, one in vitro experiment found that IL-4 in a culture medium reaches maximal levels 24 hours after functional “reperfusion” (Zhao et al., 2015). Other clinical studies have shown that the expression of IL-10, IL-4, and the IL-4 receptor can be used as markers for the prognosis of CIRI (Marousi et al., 2011). In our study, cerebral infarction volume, and neurological deficit scores increased after cerebral ischemia reperfusion in mice, as expected; when treated with exogenous IL-4, the cerebral infarction volume and neurological deficit scores significantly decreased. These results confirm that IL-4 has a protective effect against CIRI, as shown in previous studies.
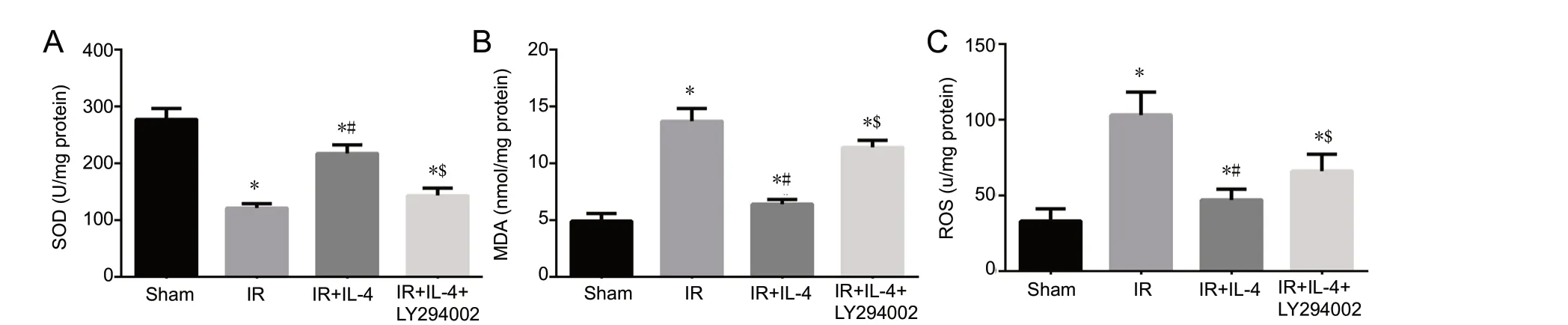
Figure 4 The effect of interleukin-4 (IL-4) on oxidative stress in the ischemic penumbra of mice with focal cerebral ischemia/reperfusion (IR) injury.

Figure 5 The effect of interleukin-4 (IL-4) on autophagy in mice with focal cerebral ischemia/reperfusion (IR) injury.
The Akt/GSK-3β pathway mediates various cellular processes, including survival, proliferation, and metastasis, and which also plays an important role in apoptosis and autophagy. Recent studies have shown that activation of the Akt/GSK-3β signaling pathway leads to a reduction in apoptosis, and further studies have shown that activation of this pathway can inhibit cell autophagy (Guo et al., 2012; Yu et al., 2016; Zhao et al., 2017). Akt can suppress apoptosis by inactivating pro-apoptotic factors such as pro-caspase-9, Bcl-2 family members, and GSK-3β (Datta et al., 1997; Cardone et al., 1998; Brunet et al., 2001; Woodgett, 2005). GSK-3β induces apoptosis by stimulating downstream pro-apoptotic factors, while its inactive form can downregulate pro-apoptotic effects and suppress cell death induction through phosphorylation at the Ser9 site (Ye et al., 2015). In our study, levels of p-Akt and p-GSK-3β increased in the IR + IL-4 group compared with those in the IR group, which suggests that IL-4 activated the Akt/GSK-3β pathway in CIRI. However, when treated with LY294002, p-Akt and p-GSK-3β levels decreased and neurological deficits simultaneously worsened, which indicates that the neuroprotective effect of IL-4 was related to activation of the Akt/GSK-3β pathway.
The pathological mechanism of CIRI involves multiple factors and pathways. The current findings suggest that the pathogenesis of CIRI mainly includes energy disorders, glutamate excitotoxicity, neuro-inf lammatory response, oxidative stress, and activation of neuronal apoptosis (White et al., 2000; Eltzschig and Eckle, 2011; Kalogeris et al., 2016). When pretreated with IL-4, we found that neural apoptosis and the content of MDA and ROS decreased, while SOD expression increased, which indicates that IL-4 decreased apoptosis and oxidative stress after ischemia reperfusion. However, the effect of IL-4 on apoptosis and oxidative stress was blocked by LY294002 administration, which indicates that IL-4 pretreatment might induce apoptosis and oxidative stress via the Akt/GSK-3β pathway.
Recent studies have shown that autophagy plays an important role in CIRI (Przyklenk et al., 2012). Autophagy is an vital metabolic process that occurs within eukaryotic cells that rely on lysosomal degradation. Autophagy involves the recycling of autologous organelles and proteins, and eliminates damaged organelles and proteins. This process has been highly conserved during biological evolution and acts to maintain the intracellular environment (Russell et al., 2014). An increasing number of studies have found that autophagy has a dual function — it exerts neuroprotective effects but can also lead to cell death (Li et al., 2015; Yao et al., 2019). For example, when ischemia reperfusion occurs, autophagy can be over-activated, which leads to increased neuronal apoptosis and aggravated brain injury (Shi et al., 2012; Zhang et al., 2013). Evidence has shown that GSK-3β can increase the level of autophagy, while a GSK-3β inhibitor can reduce autophagy and brain damage after CIRI (Zhou et al., 2011). To determine whether autophagy was up- or down-regulated by IL-4 in ischemia reperfusion, we measured the expression of autophagic markers Beclin-1 and LC3-II/I (using immunoblotting) and the number of autophagosomes of neurons in the ischemic penumbra region (using transmission electron microscopy). The level of LC3-II/I and Beclin-1 in the penumbral region and neuronal autophagy were increased after ischemia reperfusion, which confirms that cerebral reperfusion activated autophagy and aggravated neurological injury, as previously reported. Furthermore, we found that pretreatment with IL-4 significantly decreased LC3-II/I and Beclin-1 as well as autophagosomes after IR. However, when activation of the Akt/GSK-3β pathway was blocked by LY294002, the IL-4-induced inhibition of autophagy was impaired. These findings indicate that the neuroprotective effect of IL-4 is related to autophagy down-regulation, and that IL-4-induced regulation of autophagy might work via activation of the Akt/GSK-3β pathway.
Ischemic stroke is common all over the world. Thrombolytic therapy is one of the most commonly used treatments for acute ischemic stroke, but the therapeutic time window for thrombolytic therapy is only 4-5 hours, and reperfusion of the occluded artery can be due to CIRI, which aggravates the neurological injury. Alleviation of CIRI after IR is a hotspot in current medical research. While recent studies have found that IL-4 has a neuroprotective effect on CIRI in focal cerebral ischemia mice, the mechanisms underlying the neuroprotective effect of IL-4 in IR remain unclear. The present study revealed that Akt/GSK-3β pathway activation might participate in the protective effect of IL-4 on CIRI. Thus, the Akt/GSK-3β pathway might represent a therapeutic target for ischemic cerebrovascular disease, whereby regulation of this pathway could help to improve the prognosis of patients with cerebrovascular disease.
In this study, we were mainly concerned with the impact of the Akt/GSK-3β pathway on the neuroprotective effect of IL-4 at 24 hours after CIRI, i.e., in the acute phase of CIRI. However, the long-term inf luence of IL-4 and the Akt/GSK-3β pathway in CIRI still needs further investigation. For example, future work could examine behavioral scores and proteins of the Akt/GSK-3β pathway from 1 week to 4 weeks after CIRI. Other pathways, such as the protein kinase B/mammalian target of rapamycin pathway, are also likely to be involved in the neuroprotective effect of IL-4 in response to CIRI. The interactions between these signaling pathways should be investigated further in future work.
In conclusion, the present research demonstrated that IL-4 plays a protective role in CIRI by mitigating oxidative stress, reducing apoptosis, and inhibiting excessive autophagy, and that the underlying mechanism may be related to activation of the Akt/GSK-3β pathway.
Author contributions:Study conception and design: WWG and XXX; experiment implementation: ML, YFW, BZ; data analysis: YG; manuscript writing: ML, LL. All authors approved the final version of the paper. All authors approved the final version of the paper.
Conf licts of interest:The authors declare that they have no competing interests.
Financial support:This study was supported by the National Natural Science Foundation of China, Nos. 81901994 (to BZ) and 81571147 (to XXX); the Natural Science Foundation of Hubei Province, China, No. 2019CFC847 (to WWG); the Fundamental Research Funds for the Central Universities, China, No. 2042018kf0149 (to ML). The funding bodies played no role in the study design, collection, analysis and interpretation of data, in the writing of the report, or in the decision to submit the paper for publication.
Institutional review board statement:The study protocol was approved by the Animal Ethics Committee of Renmin Hospital of Wuhan University, China (approved No. WDRY2017-K037) on March 9, 2017. All experimental procedures described here were in accordance with the National Institutes of Health (NIH) guidelines for the Care and Use of Laboratory Animals (Publication No. 85-23, revised 1996).
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewers:C-Yoon Kim, Seoul National University, Republic of Korea; Sergei Fedorovich, National Academy of Sciences of Belarus, Belarus.
Additional file:Open peer review report 1.
- 中国神经再生研究(英文版)的其它文章
- Recovery of an injured ascending reticular activating system with recovery from a minimally conscious state to normal consciousness in a stroke patient: a diffusion tensor tractography study
- The role of vascularization in nerve regeneration of nerve graft
- New insights into Wnt signaling alterations in amyotrophic lateral sclerosis: a potential therapeutic target?
- Advanced diffusion magnetic resonance imaging in patients with Alzheimer’s and Parkinson’s diseases
- Modulation of autophagy for neuroprotection and functional recovery in traumatic spinal cord injury
- Decoding epigenetic codes: new frontiers in exploring recovery from spinal cord injury

