Geographic variation of fig wasp communities in Ficus racemosa between Xishuangbanna (China) and Mandalay (Myanmar)
Khin Me Me AUNG, DONG Yiyi, WANG Bo, PENG Yanqiong*
(1.CAS Key Laboratory of Tropical Forest Ecology, Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences, Mengla, Yunnan 666303, China; 2.University of Chinese Academy of Sciences, Beijing 100049, China; 3.Forest Research Institute, Yezin, Nay Pyi Taw 05282, Myanmar)
Abstract: The interaction between Ficus species and their pollinating wasps (Agaonidae) is a striking example of mutualism. Each monoecious fig species also harbors diverse non-pollinating chalcid wasps. Thus, multitrophic communities of fig wasps represent an interesting model for studies on community ecology. Fig wasp communities can be influenced by regional species pools, biogeographic features, and interspecific interactions, but they remain poorly researched. In this study, fig wasp communities of monoecious Ficus racemosa distributed in Xishuangbanna (China) and Mandalay (Myanmar) were selected to investigate the effects of geographic region on wasp size and coexistence networks. Results showed that the communities in F. racemosa at both sites contained the same six species of fig wasps. The body sizes of these six wasp species were all larger in Xishuangbanna than in Mandalay. Pollinating fig wasp Ceratosolen sp. was the dominant species at both sites; however, the population size was larger in Xishuangbanna and the non-pollinating fig wasps were more abundant in Mandalay. Thus, not only did community structure and species abundance differ between sites, but variation in the coexistence networks of fig wasps also existed. The networks exhibited similar number of nodes, edges, connections, and global clustering coefficients, but higher network diameters and temperatures in Xishuangbanna than in Mandalay. These results suggest less compactness in interspecific interactions and higher disturbance of the fig wasp community in Xishuangbanna. This study should help clarify community variation driven by geographic distribution.
Key words: Fig trees; fig wasps; mutualism; exploitation; abundance; community structure
1 Introduction
Understanding community structure, which depends on both ecology and evolutionary history (Ricklefsetal,1993; Emersonetal,2008), can help illuminate the coexistence of species competing for similar resources. On the one hand, related species are restricted by their ability to use resources so may chose a similar habitat and use the same resources. On the other hand, interspecific competition can prevent similar species from coexisting within a community (Codyetal,1975). Fig wasp community structure is complex, typically involving the coexistence of multitrophic interactions, which are influenced by ecological and evolutionary factors (Segaretal,2013; Darwelletal,2018).
Currently, some 800 species ofFicusfrom the family Moraceae are distributed worldwide. These species are well-known for their unique urn-shaped inflorescences (fig or syconium).Ficusand species-specific pollinating fig wasps (Hymenoptera: Chalcidoidea: Agaonidae) often exhibit obligate mutualism (Cooketal,2003; Cruaudetal,2012), i.e., they depend on each other for pollination of flowers and larval development inside the fig (Janzen,1979; Wiebes,1979). In addition to pollinating wasps, figs are also exploited by multiple species of non-pollinating fig wasps belonging to 5 families (Agaonidae, Pteromalidae, Eurytomidae, Ormyridae, and Torymidae) (Weiblen,2002; Zhenetal,2005; Comptonetal,2018). Non-pollinating fig wasps use their long ovipositor to deposit eggs through the fig wall, and most species are flower gallers, inquilines, or parasitoids of other fig wasps or of each other (Eliasetal,2008; Gharaetal,2010; Comptonetal,2018). In addition, they attack the figs at different stages of fruit growth but complete their own development at the same time as pollinating fig wasps, thus forming a multitrophic fig wasp community (Kerdelhuéetal,2000; Borgesetal,2018).
Figs and fig wasps are an excellent model of insect-plant association to study the patterns of species diversity, community structure (Lewinsohnetal,2005), ecology of mutualistic and exploitive communities (Cooketal,2003; Herreetal,2008), and the evolution of ecological interactions (Borgesetal,2018). A fig wasp community associated with a single fig species can consist of more than 40 wasp species at different trophic levels (Comptonetal,2018). Most figs are pollinated by a single or limited number of wasp species and most wasps are specifically related to a single fig species (Cooketal,2003; Molboetal,2003; Cooketal,2010; Cruaudetal,2012). However, the diversity of non-pollinating fig wasps may differ depending on the reproductive systems of plants (Kerdelhuéetal,1996). Two studies on fig wasp communities in different geographic regions found that community structure and genus level composition are largely conserved (Darwelletal,2018), but with convergent multitrophic community structure (Segaretal,2013). The ecological interactions between species may play a persistent role in shaping these communities, but variation in the fig wasp community in the underlying ecological networks across biogeographic regions is rarely studied.
Ficusracemosais pollinated by 4 species ofCeratosolenwasps in the Indo-Australian region.Ceratosolenfuscipesis only distributed in India,Ceratosolensp.1 is distributed in China-Thailand,Ceratosolensp.2 is located in Borneo, andCeratosolensp.3 is distributed in northern Australia, thus suggesting different evolutionary histories of populations (Kobmooetal,2010; Bainetal,2016). In addition, several species of non-pollinating fig wasps coexist insideF.racemosafigs (Xuetal,2003). In India,F.racemosais pollinated byC.fuscipesand exploited by 6 species of non-pollinating fig wasps, includingSycophagastratheni,Sycophagatestacea,Sycophagafusca,Sycophagaagraensis,Apocryptawestwoodi, andApocryptasp. (Ranganathanetal,2010). However, only 5 species of non-pollinating fig wasps are found in China, includingS.testacea,Sycophagamayri,S.agraensis,A.westwoodi, andApocryptasp. (Xuetal,2003). Moreover, a comparative investigation onF.racemosain southern Xishuangbanna and northern Liuku of Yunnan reported a higher number of pollinators in Xishuangbanna, but the opposite result for non-pollinating fig wasps (Chenetal,2018). Myanmar is located between China and India, with naturalF.racemosadistribution. Therefore, we first checked whether fig wasp species inF.racemosawere the same in China and Myanmar, and addressed the following questions: (i) Do the morphological traits of fig wasps vary between China and Myanmar? (ii) What is the community structure and species abundance at both sites? and (iii) Do the coexistence networks of fig wasp communities differ between sites?
2 Materials and methods
2.1 Study sites and species
The 2 study sites were located at Xishuangbanna Tropical Botanical Garden, Menglun, Yunnan Province, southwest China (21°56′54.87"N, 101°15′22.19"E, 555 m above sea level) and in Patheingyi Township, Mandalay Division, central Myanmar (21°59′03.18"N, 96°12′29.34"E, 169 m above sea level). From September to December, 2018, we collected at least 20 figs per tree from two trees per site.
FicusracemosaL. is a monoecious fig species widely distributed throughout the Indo-Australasian region. The trees grow rapidly, reaching up to 25-40 m in height, and produce 2-7 fig crops per year (Zhangetal,2006; Krishnanetal,2015).F.racemosafigs, which are located on the tree trunk, reach about 55 mm in diameter at maturity, with an average fig wall thickness of 4.97 mm (4.01-5.23 mm) (Zhenetal,2005). There are 5 developmental phases inF.racemosafig phenology (Galiletal,1968; Ranganathanetal,2010): A phase (pre-female) consisting of immature flowers; B phase (receptive or female) consisting of mature flowers, with pollinators entering figs for pollination and oviposition; C phase (inter-floral) consisting of seed and gall development, with similar developmental time for figs and wasps; D phase (male), where male wasp pollinators exit the galls to mate with female wasps and tunnel through the fig wall. The female wasps escape through the tunnel, and then move to another host tree to start their new life cycle; E phase (post-floral), during which time the color of the fig changes to attract seed dispersers (Borgesetal,2008). In the current study, we collected fig wasps from D phase figs.
In Xishuangbanna, the pollinating fig wasp ofF.racemosawas initially thought to beC.fuscipes. However, recent molecular evidence has shown that there are 4 species of pollinating fig wasps from India to north Australia, withC.fuscipesonly distributed in India, andF.racemosaactively pollinated byCeratosolensp. in China-Thailand (Kobmooetal,2010; Bainetal,2016). Figs are also exploited by the other 5 species of non-pollinating fig wasps in Xishuangbanna, which visit figs and oviposit at different developmental phases. For example,S.testaceaand thenApocryptasp. first display oviposition during the A phase;S.mayrioviposits from late A, to B and early C phases; theCeratosolensp. pollinator only visits and oviposits in the B phase; andS.agraensisandA.westwoodioviposit in the C phase (Figure 1). These differences in oviposition time are beneficial for the coexistence of multiple wasp species in a single fig species.

Figure 1 Developmental stages of figs and oviposition times of fig wasps in Ficus racemosa
2.2 Collection of D-phase Ficus racemosa figs to obtain fig wasps
In total, 58 D-phase figs from twoF.racemosatrees in Xishuangbanna and 40 D-phase figs from twoF.racemosatrees in Mandalay were collected before the wasps exited. Each fig was place into a nylon bag to allow the wasps to naturally emerge. All wasps were collected and preserved in 75% ethanol. The figs were then dissected to count all flowers, seeds, and bladders. We identified all pollinating and non-pollinating fig wasps per fig and counted wasp numbers.
2.3 Measuring morphological traits of fig wasps
Thirty female wasps per species from the 2 study sites were sampled to measure 9 morphological body-size traits under the microscope with a micrometer. Traits included head width, head length, thorax length, wing width, wing length, front femur length, hind femur length, abdomen length, and ovipositor length (ovipositor sheath excluded) (Liuetal,2011).
2.4 Statistical analysis
We used principle component analysis to compare which morphological traits were the strongest indicators of wasp body size. Two-samplet-test was used to analyze the different morphological traits and to test whether the species composition and abundance of fig wasps inF.racemosadiffered between Xishuangbanna and Mandalay. The Bipartite package (Dormannetal,2009) was used to calculate network metrics. Nodes: nodes of the network, including individuals, species, population, and community; edges: observed ties or interactions in the network; connections: count of edges; global clustering coefficients: proportion of closed triangles in the network, ranging from 0 to 1; diameter: shortest distance between any two nodes in the network or the longest of the shortest paths across all pairs of nodes; matrix temperature: the deviation degree between the observed community structure and completely nested community.
All analyses were performed in R v3.5.2 using the dplyr, ggplot2, psych, and vegan (community ecology package. R package. Version) packages.
3 Results
3.1 Comparison of body sizes of six fig wasp species between Xishuangbanna and Mandalay
The same 6 species of fig wasps were identified in Xishuangbanna and Mandalay, i.e., pollinating fig waspCeratosolensp. and non-pollinating fig waspsS.testacea,S.mayri,S.agraensis,A.westwoodi, andApocryptasp.. The 9 morphological body-size traits were measured to compare the differences between sites. Results showed that almost all measured traits showed significant differences for the 6 species of fig wasps between Xishuangbanna and Mandalay, except for abdomen length between the twoApocryptaspecies (Figure S1). Principle component analysis of the 9 morphological traits showed a mean item complexity of 1, which implied that one component was enough representative of wasp sizes. The PC1 values for the 9 traits are shown in Table 1. The wing traits stably demonstrated the highest values. As such, wing length was used to represent wasp body size for the 6 fig wasp species found inF.racemosa.

Table 1 PC1 values of principle component analysis for nine morphological traits of six fig wasp species in Ficus racemosa
Wing length was regarded as representative of body size and used to compare the differences in fig wasps between Xishuangbanna and Mandalay. Results showed that the body sizes of the 6 fig wasp species inF.racemosawere all significantly larger in Xishuangbanna than in Mandalay (t-test, allP<0.001) (Figure 2).

Note: *** indicate significant difference between different sites (P<0.001).
3.2 Community composition and structure of fig wasps in F. racemosa between Xishuangbanna and Mandalay
In total, 56 547 fig wasps from 58 figs ofF.racemosawere collected in Xishuangbanna and 22 712 fig wasps from 40 figs ofF.racemosawere collected in Mandalay. The community composition of fig wasps differed between sites. The pollinatorCeratosolensp. was the dominant species and exhibited the highest abundance in the fig wasp community at both sites, accounting for 76.48% (43 245 wasps) in Xishuangbanna and 44.36% (10 076 wasps) in Mandalay. The number of non-pollinating fig wasps per species was also very different between sites. The percentages of the 5 species were all less than 7% in Xishuangbanna. Among themS.testaceashowed the largest population (6.54%), followed byA.westwoodi(6.35%), withApocryptasp. showing the lowest abundance (0.33%). In contrast, there were more non-pollinating fig wasps in Mandalay, withA.westwoodishowing the highest abundance (24.38%), followed byS.mayri(14.04%),S.agraensis(11.76%),S.testacea(3.96%), andApocryptasp. (1.5%)(Figure 3). Thus, the species composition and community structure of fig wasps inF.racemosabetween Xishuangbanna and Mandalay showed marked geographic variation.
3.3 Abundance variation of six fig wasp species in F. racemosa between Xishuangbanna and Mandalay
In the fig wasp community ofF.racemosa,Ceratosolensp. was the most abundant species, accounting for an average of 745.60±59.08 (SE,n=58) wasps in Xishuangbanna and 251.90±45.75 (SE,n=40) wasps in Mandalay, which were significantly different (t=6.61,P<0.001). Similarly, the number ofS.testaceawasps was significantly higher in Xishuangbanna (63.81±7.35) than in Mandalay (22.50±3.16) (t=5.16,P<0.001). In contrast, the other 4 species of non-pollinating fig wasps were found in greater numbers in Mandalay than in Xishuangbanna, with significant differences in abundances ofA.westwoodi(Xishuangbanna: 61.86±8.16, Mandalay: 138.45±10.47;t=-5.77,P<0.001),S.agraensis(Xishuangbanna: 41.02±3.77, Mandalay: 66.75±8.16;t=-2.86,P<0.01), andApocryptasp. (Xishuangbanna: 3.28±0.94, Mandalay: 8.48±1.53;t=-2.90,P<0.01). OnlyS.mayriabundance showed no differences between Xishuangbanna (59.38±10.11) and Mandalay (79.73±11.09) (t=-1.36,P=0.18) (Figure 4). These results suggest that species abundance in the fig wasp community ofF.racemosavaried between sites.

Figure 3 Species composition of fig wasps in F. racemosa between Xishuangbanna (58 figs and 56 547 fig wasps) and Mandalay (40 figs and 22 712 fig wasps)

Note: ns indicate the difference is not significant between different sites (P>0.05); ** indicate significant difference between different sites (P<0.01); *** indicate significant difference between different sites (P<0.001).
3.4 Coexistence networks of fig wasp community in F. racemosa between Xishuangbanna and Mandalay
InF.racemosa, six species of fig wasps coexisted and demonstrated the same species composition in Xishuangbanna and Mandalay. However, interspecific interaction was different, resulting in different coexistence networks in the fig wasp communities at both sites. The fig wasp community networks at both sites consisted of 6 nodes, 15 edges, 1 connection, 1 global clustering coefficient, and 5 node connections (Figure 5). The network diameters in Xishuangbanna and Mandalay were 57 and 40, respectively. Each fig wasp species exhibited species interaction. The matrix temperature of the fig wasp community in Xishuangbanna (2.22) was higher than that in Mandalay (0.29). In short, the larger network diameter and higher matrix temperature in Xishuangbanna suggests less compactness in interspecific interactions and higher disturbance in the fig wasp community, which could be less stable than that in Mandalay.
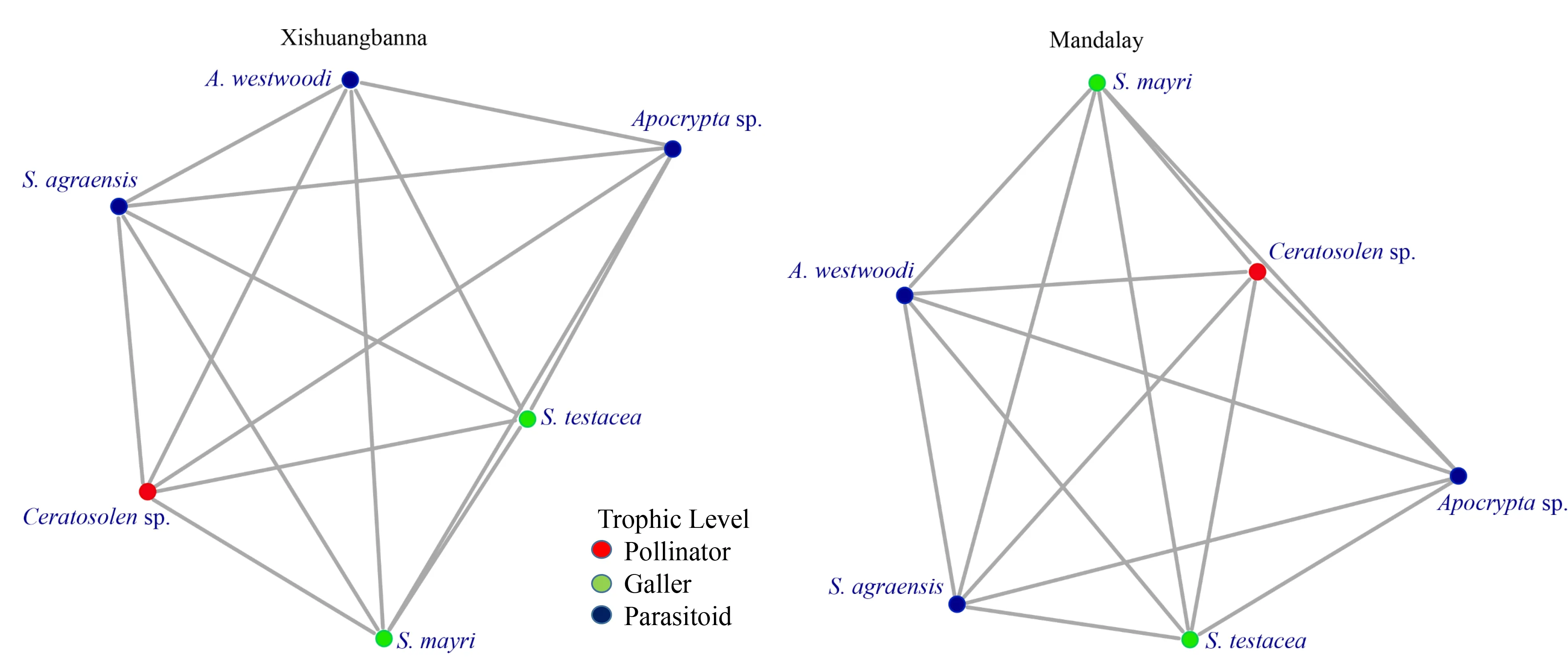
Figure 5 Species interaction networks of fig wasps in F. racemosa between Xishuangbanna and Mandalay
4 Discussion
Fig wasp community structure and genus-level composition are largely conserved over a wide geographic range but can show convergent structure of multitrophic communities (Segaretal,2013; Darwelletal,2018). However, species composition and community structure can exhibit geographic variation (Ranganathanetal,2010; Chenetal,2018). The monoeciousF.racemosais pollinated by differentCeratosolenwasps in India and China and is associated with different species of non-pollinating fig wasps. This study confirmed that theF.racemosafig wasps in Mandalay were the same to those in Xishuangbanna, but showed different community structure, species abundance, and coexistence networks. Moreover, the body sizes of all 6 wasp species were larger in Xishuangbanna than in Mandalay. Adult body size is related to larval development and their feeding period and food resources (Slanskyetal,1985). We also measured fig diameter and counted the flowers. The figs in Xishuangbanna showed a larger diameter and more flowers than those in Mandalay, indicating a richer resources pool, which should be advantageous to wasp reproduction. Recent study reported that the ovipositor ofPhilotrypesiswasps onF.hirtais longer in northern Guangdong, Fujian, and southern Jiangxi provinces than those in southern Guangdong, Hainan, and Guangxi provinces in China (Yuetal,2018), suggesting that large fig diameter and fig wall thickness may shape ovipositor length of non-pollinating fig wasps. In this study, our comparison of the ovipositor lengths of the 5 non-pollinating fig wasp species onF.racemosasupported the conclusion that non-pollinating fig wasps with long ovipositors can utilize large figs. Although there are about 100Ficusspecies in Myanmar, this study is the first report on fig wasp communities in that country, which will hopefully encourage further study on fig and fig wasp co-evolution in the region.
The ecological and evolutionary characteristics of competitors in communities are important fundamental factors of resource partitioning (Bonsalletal,2004). For fig wasps, ovipositor morphology is correlated with the thickness of the fig wall and is regarded as an important morphological trait (Gharaetal,2011; Eliasetal,2017). Fig size varies with fig development time. Most non-pollinating fig wasps lay eggs from the outside of the fig, so the length of their ovipositor can differ according to their oviposition time (Kerdelhuéetal,1996; Darwelletal,2018). Early-arriving gallers have shorter ovipositor length, and lay eggs at the initial stages of fig development, whereas late-arriving parasitoids have longer ovipositors and usually oviposit during late fig development (Westetal,1996; Kerdelhuéetal,2000). The differentiation in oviposition times allows multiple species of fig wasps to coexist in one fig species. MonoeciousFicusspecies harbor multiple species of non-pollinating fig wasps, with the general pattern to exploit multiple figs but reproduce few individuals (Kerdelhuéetal,2000; Comptonetal,2018; Darwelletal,2018). In contrast, the non-pollinating fig wasps ofF.racemosahave large population sizes and easily coexist together. In additional to temporal heterogeneity of oviposition, the pattern and network of interspecific interaction can also influence the stability of fig wasp communities.
Previous study identified a latitudinal effect on fig wasp communities ofF.racemosa, with northern communities in Liuku having fewer pollinating fig wasps and more non-pollinating fig wasps per fig than those in southern Xishuangbanna (Chenetal,2018). Xishuangbanna and Mandalay have similar latitude, but the altitude and longitude of both sites are different. Thus, geographic heterogeneity could be driving the differences in the structure and species abundance of the fig wasp communities ofF.racemosaat both sites. The pollinators were the dominant fig wasp species in both sites. However, these pollinators occupied a higher ratio and exhibited a larger population in Xishuangbanna, whereas non-pollinating fig wasps demonstrated a higher ratio and numbers in Mandalay. Pollinators and gallers compete for the same female flower resources, and the interaction between gallers and parasitoids may prevent the over-utilization of female flowers to maintain stable fig-fig wasp mutualism (Kerdelhuéetal,2000). In this study, ecological networks were used to determine differences in fig wasp communities between both sites. Results showed similar network traits at both sites, such as the same numbers of nodes, edges, connections, and global clustering coefficients, suggesting similar species numbers and interspecific interactions of fig wasps onF.racemosaat both sites. However, both network diameter and temperature were higher in Xishuangbanna than in Mandalay, implying that the interspecific interactions resulted in higher disturbance in Xishuangbanna and greater fig wasp community instability than that found in Mandalay. These results provide basic information for community stabilization and species conservation; however, the reasons for network differences require further study.
Acknowledgements:We thank H. Zhang and H. A. Ju for valuable assistance with experiments.

