普洱茶中没食子酸及其改善饮食诱导的糖脂代谢紊乱研究进展
王绍梅,李晓君,宋文明,潘联云
普洱茶中没食子酸及其改善饮食诱导的糖脂代谢紊乱研究进展
王绍梅1,2,李晓君1,2,宋文明3,潘联云4*
1. 滇西科技师范学院,云南省红茶工程技术研究中心,云南 临沧 677000;2. 滇西科技师范学院,生物技术与工程学院,云南 临沧 677000;3. 临沧高级技工学校,云南 临沧 677000;4. 云南省农业科学院茶叶研究所,云南省茶学重点实验室,云南 勐海 666200
糖脂代谢紊乱是引发心血管疾病、糖尿病和脂肪肝的重要原因之一。普洱茶中的没食子酸能够通过调节能量代谢和脂肪细胞分化、促进葡萄糖吸收与利用、提高胰岛素敏感性,进而改善饮食诱导引起的葡萄糖和脂质代谢紊乱。没食子酸通过AMPK途径、IR-Akt途径以及PPAR-受体调节线粒体能量代谢、胰岛素敏感性和葡萄糖吸收,从而维持糖脂代谢稳态。本文综述了普洱茶中的没食子酸及其改善饮食诱导的糖脂代谢紊乱作用和作用机制的研究进展。
普洱茶;没食子酸;饮食诱导;糖脂代谢
葡萄糖和脂质代谢紊乱与心血管疾病、糖尿病和脂肪肝等密切相关[1]。2014年,全世界有19亿人超重,其中6亿人存在肥胖问题,这主要是由于当下全球饮食结构变化引起的[2]。本文阐述了普洱茶中没食子酸调节饮食诱导的糖脂代谢相关作用及其作用机制,为普洱茶调节糖脂代谢提供理论依据。
1 普洱茶没食子酸概述
没食子酸(Gallic acid,GA)又称五倍子酸,化学名称为3,4,5-三羟基苯甲酸,主要以酯的形式连接在儿茶素的3位羟基上,形成酯型儿茶素衍生物[3-4]。GA是普洱茶的特征性简单酚类化合物,其含量在渥堆发酵过程中呈现先增加后下降的趋势,总含量表现显著增加[3-6]。
普洱茶中GA主要来源于晒青毛茶中的植物单宁化合物和儿茶素[7-9](图1)。在普洱茶渥堆发酵过程中,GA的含量与菌群种类和发酵条件密切相关[3]。曲霉菌等微生物分泌的单宁酶可使晒青毛茶中的小木麻黄素等多种没食子单宁化合物分解形成GA[10-12];表没食子儿茶素没食子酸酯(EGCG)和表儿茶素没食子酸酯(ECG)等少部分酯型儿茶素在湿热作用下水解生成GA[13-15];简单儿茶素(C)也能转化形成GA,且GA含量变化与C呈现一定相关性,其相关系数分别为–0.869 3和–0.849 4[4]。在普洱茶渥堆发酵后期,GA在某些酶或微生物的次生代谢物作用下转化成相应的KMU-3等没食子酸衍生物[16],或与其他多酚发生氧化聚合[17],均导致其含量降低。动物生理试验表明,GA具有降糖降脂、抗菌、抗炎、抗肿瘤、抗突变等多种生物学作用[18-19]。
2 没食子酸改善糖脂代谢作用的研究现状
流行病学研究表明,普洱茶具有调节新陈代谢的作用[20-23],长期饮用普洱茶能够调节肥胖人群的内脏脂肪含量、平均腰围和身体质量指数(Body mass index,BMI),有助于控制体重和预防代谢综合征的发生[24]。普洱茶中GA可以通过降低血浆中甘油三酯、胆固醇和葡萄糖水平,调节脂肪细胞对葡萄糖的吸收,促进线粒体能量代谢,提高胰岛素敏感性和葡萄糖耐受性,改善饮食引起的糖脂代谢紊乱(图2)。
2.1 没食子酸调节血糖血脂水平
高糖高脂饮食会引起大鼠和小鼠代谢紊乱,引发高血糖和高血脂,降低胰岛素敏感性和发生胰岛素抵抗等现象[25-27]。GA能够通过抑制肠道对脂质的消化吸收、降低脂质合成和积累、增加脂肪细胞分化和促进葡萄糖吸收来调节糖脂代谢,改善葡萄糖和脂质内稳态,降低葡萄糖毒性和脂毒性作用。

图1 普洱茶后发酵过程中没食子酸生成过程

注:↑表示升高,↓表示降低
2.1.1 抑制肠道对脂肪的消化和吸收
GA能够通过抑制胰脂肪酶(Pancreatic lipase,LP)的活性来降低膳食脂肪的水解,减少肠道对甘油三酯的消化和吸收,从而降低餐后血液中甘油三酯水平。动物试验研究发现,GA可以显著抑制饮食玉米油乳剂后雄性ddY小鼠甘油三酯水平的升高[28]。体外试验表明,GA对LP活性的抑制呈剂量依赖性,且对LP的抑制活性为普洱茶提取物的11倍[29]。在GA抑制LP活性的过程中,普洱茶中儿茶素和多聚类黄酮等生物活性成分与GA具有协同作用,能够提高GA的吸收率和稳定性[28]。此外,GA能够通过疏水作用或分子间氢键与胆酸盐等血脂水平相关的生物分子结合,从而起到降血脂作用[30]。
2.1.2 降低脂质合成和积累
在高脂饮食条件下,GA能够抑制体重、肝脏器官重量、腹膜和附睾脂肪组织重量的增加[31-34]。Bak等[35]通过小鼠试验发现,GA能够减小高脂饮食小鼠的附睾白色脂肪组织中脂肪细胞的大小。Hsu等[36]通过细胞试验发现,GA对3T3-L1前脂肪细胞群体增长也具有显著抑制作用。Hsu[32]等研究表明,GA能够降低高脂饮食诱导的雄性Wistar大鼠甘油三酯、磷脂、总胆固醇和低密度脂蛋白胆固醇水平,对其血脂异常具有显著的抑制作用。同时,GA还能够显著降低饮食诱导的肥胖小鼠[35]和db/db小鼠[37]血糖浓度和血清甘油三酯水平。
Zeng等[29]研究发现,GA能够剂量依赖性抑制3-羟基-3-甲基戊二酸单酰辅酶A还原酶(3-hydroxy-3-methyl glutaryl coenzyme A reductase,HMGR)的表达和提高卵磷脂胆固醇脂酰转移酶(Lecithin-cholesterol acyltransferase,LCAT)的活性,从而抑制胆固醇合成和促进胆固醇运输,降低血浆总胆固醇水平。此外,大量细胞试验表明,GA能够降低3T3-L1脂肪细胞的胆固醇水平[37],并能通过抑制HepG2细胞株脂肪酸和胆固醇合成相关酶的活性,降低其脂质和胆固醇积累[38-39]。通过动物试验发现,GA具有抑制高脂饮食诱导的大鼠磷脂和胆固醇酯合成的作用[32,40]。
2.1.3 促进脂肪细胞对葡萄糖的吸收
脂肪组织在糖代谢中起着关键的作用[41-42]。在高糖饮食条件下,GA能够促进脂肪形成,增加脂肪组织对葡萄糖吸收,降低糖尿病大鼠空腹血糖水平,保护胰岛细胞免受葡萄糖毒性损伤[31]。GA还能够恢复高糖引起的葡萄糖和胆固醇水平异常,预防糖尿病发生[37]。体外试验表明,GA可以通过激活CCAA增强子结合蛋白(CCAAT/enhancer binding protein,C/EBP)和过氧化物酶体增殖物激活受体(Peroxisome proliferator-activated receptor-gamma,PPAR-)来刺激3T3-L1脂肪细胞分化,且GA呈浓度依赖性促进3T3-L1脂肪细胞对葡萄糖的吸收,改善葡萄糖稳态[37,43-45]。
2.1.4 调节糖异生和糖酵解作用
肝脏维持葡萄糖稳态主要通过糖原生成和糖异生作用[46]。GA能够增强肝脏糖酵解和糖原生成途径,提高肝脏对葡萄糖的利用,改善高脂饮食诱导的糖尿病大鼠肝脏葡萄糖代谢异常。正常饮食条件下,胰岛素通过信号转导级联促进糖原合成酶(Glycogen synthase,GS)的表达,提高肝脏以糖原和脂质形式储存葡萄糖的能力,从而降低血糖水平[47-48]。高脂饮食会使大鼠血糖浓度升高,诱导糖尿病发生。GA可以通过下调糖尿病大鼠肝糖异生相关蛋白(如,果糖-1,6-二磷酸酶)和上调肝脏GS和糖酵解相关蛋白(如,己糖激酶、磷酸果糖激酶和醛糖酶)的表达调控葡萄糖代谢,促进葡萄糖向糖原转化,降低血糖水平[33]。
2.2 没食子酸促进线粒体能量代谢
GA能够通过促进线粒体能量代谢来提高葡萄糖耐量。通过研究啮齿类动物脂肪组织发现,上调线粒体膜内解偶联蛋白-1(Uncoupling protein 1,UCP1)能够促进能量消耗[49-51]。在不改变食物摄入量的情况下,GA通过提高UCP1表达来促进线粒体能量代谢,提高产热和新陈代谢,增加糖脂消耗率,从而降低体重并维持代谢平衡[34,52]。此外,GA还能够激活腺苷酸活化蛋白激酶[Adenosine 5'-monophosphate (AMP)-activated protein kinase,AMPK]途径,调控下游因子表达,通过直接刺激unc-15等自噬激酶或抑制雷帕霉素mTOR信号转导来促进自噬发生,从而清除受损线粒体,增强线粒体功能,提高能量代谢[53-57]。
2.3 没食子酸改善胰岛素抵抗和提高胰岛素敏感性
高脂饮食诱导胰岛素抵抗,从而导致葡萄糖摄取和利用率降低,血浆胰岛素和血糖水平升高,最终引起糖尿病的发生[58-59]。提高胰岛素敏感性和降低内源性胰岛素水平是治疗糖尿病和代谢紊乱相关疾病的有效方法[60]。Variya等[37]研究表明,GA能够提高3T3-L1脂肪细胞和db/db小鼠的胰岛素敏感性;Hsu等[32]发现GA具有降低雄性Wistar大鼠血清胰岛素和瘦素水平的作用。同时,GA还可以通过上调胰岛素受体(Insulin receptor,IR),胰岛素受体底物-1(Insulin receptor substrate-1,IRS-1),磷脂酰肌醇-3激酶(Phosphatidylinositol-3 kinase,PI3K)和Akt/蛋白激酶B(Akt/protein kinase B,Akt/PKB)等糖尿病大鼠肝脏胰岛素信号转导相关蛋白的表达,改善其胰岛素抵抗,从而降低糖尿病大鼠空腹血糖和血浆胰岛素水平[31,33]。
此外,脂肪组织对调节胰岛素同样具有重要作用。GA通过激活转录因子PPAR-和C/EBP刺激脂肪细胞分化,促进脂联素的表达和分泌,从而提高胰岛素敏感性[61]。
3 没食子酸调节糖脂代谢的机制研究现状
在调节糖脂代谢过程中,GA通过激活AMPK来调控线粒体能量代谢和脂质合成与积累,通过IR-Akt途径调节胰岛素敏感性与葡萄糖吸收,通过促进PPAR-表达来改善胰岛素抵抗、提高胰岛素敏感性与葡萄糖耐受性(图3)。
3.1 没食子酸通过AMPK途径调节线粒体能量代谢
AMPK作为细胞能量状态传感器,在细胞能量代谢、葡萄糖代谢和脂质代谢中起着重要作用[62]。研究表明,GA能磷酸化小鼠肝脏、肌肉和肩胛间褐色脂肪组织中的AMPK,且效果高于二甲双胍[34,52]。活化的AMPK能直接激活下游因子PGC1-来增强线粒体功能[53,63-64],也能够通过激活下游靶基因Sirt1来去乙酰化调控PCG1-[65-66]。
Sirt1是哺乳动物能量稳态的调节因子[67-68]。在白色脂肪组织和胰腺B细胞中,Sirt1缺失会影响胰岛素分泌和葡萄糖摄取,同时降低能量消耗;提高Sirt1表达能够增加胰岛素分泌,增强葡萄糖耐量[69-70]。PGC1-是Sirt1下游基因,与线粒体氧化代谢相关。研究发现,GA通过上调线粒体基因PCG1-及其下游靶基因Tfam(线粒体转录因子)、Nrf-1(核呼吸因子-1)和Nrf-2来提高能量消耗,促进代谢循环,而敲除Sirt1能够抑制GA对PGC1的激活[52]。Sirt1可以激活磷酸烯醇丙酮酸羧化激酶(Phosphoenolpyruvate carboxykinase,PEPCK)和葡萄糖-6-磷酸酶(Glucose-6-phosphatase,G6Pase)等糖异生酶的基因表达来促进葡萄糖生成[71],但GA上调Sirt1不能促进PEPCK和G6Pase的表达,这可能是由于活化的AMPK多向性作用导致的[52]。AMPK活化促进小异二聚体伴侣(Small heterodimer partner,SHP)表达,而SHP对PEPCK和G6Pase表达有抑制作用[72]。
另有研究表明,GA能够通过磷酸化HepG2细胞的AMPK来抑制乙酰辅酶A羧化酶(Acetyl-CoA carboxylase,ACC)及其下游脂质合成因子的表达,从而减少脂质的合成和积累[52-53,73]。此外,GA通过磷酸化AMPK促进自噬清除受损线粒体,增强线粒体功能[56-57]。
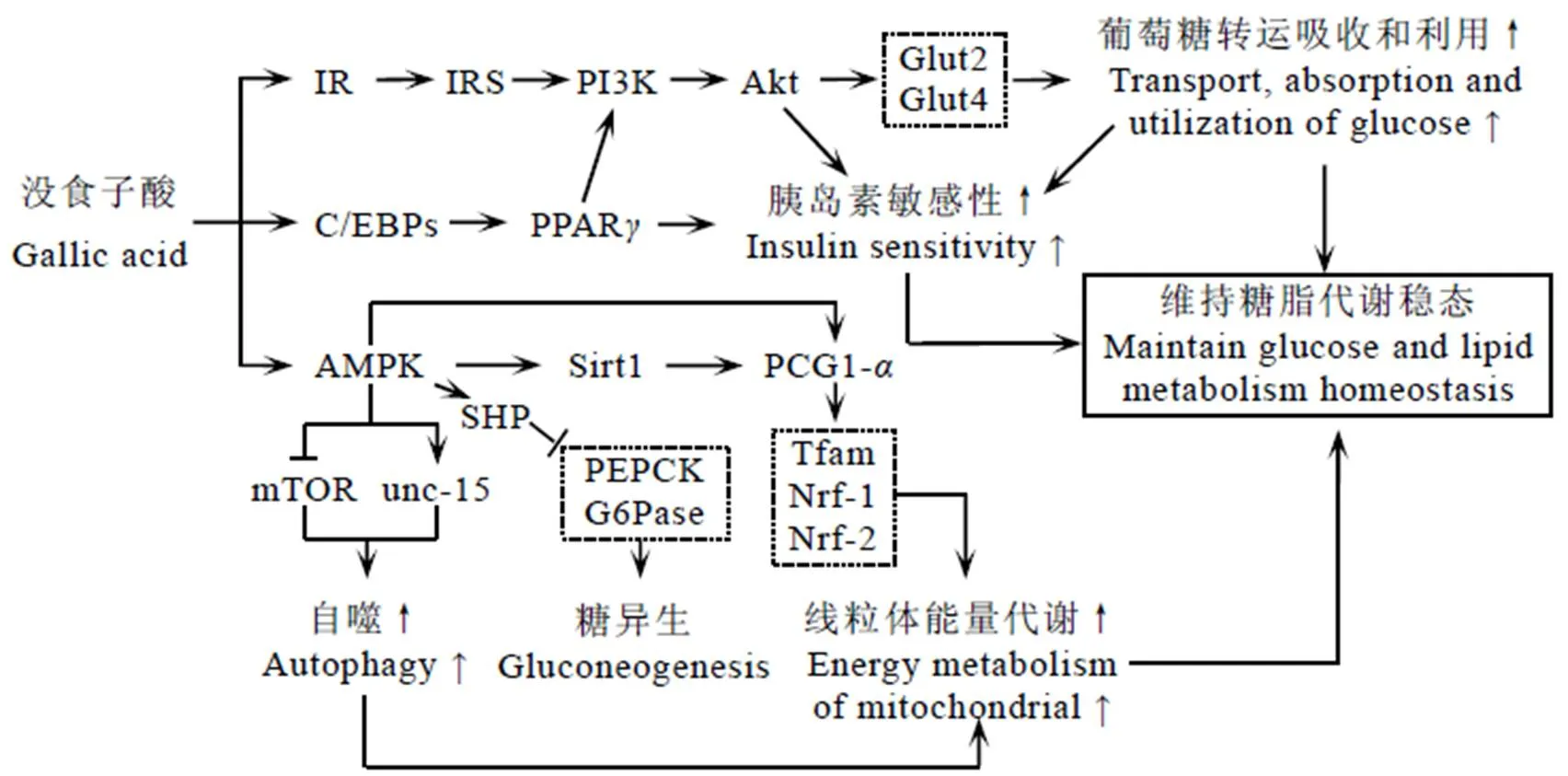
注:→:激活、促进或正调控;:抑制或负调控
3.2 没食子酸通过IR-Akt途径调节胰岛素敏感性和葡萄糖吸收
Akt属于丝氨酸/苏氨酸蛋白激酶家族,受胰岛素调节[74]。在正常条件下,胰岛素与IR结合发生酪氨酸磷酸化[75],磷酸化的IR能够进一步促进下游信号分子IRS的表达和磷酸化,从而激活PI3K[47],活化的PI3K通过激活Akt来调节胰岛素敏感性和葡萄糖吸收[76]。Akt信号通路受阻会抑制4型葡萄糖转运蛋白(Glucose transporter type 4,Glut4)膜位移,减少葡萄糖转运和吸收,从而导致血糖升高和糖尿病发生[77-79]。
大量研究表明,GA能够通过激活IR-Akt途径增加胰岛素敏感性以及葡萄糖的吸收和转运,维持血糖水平[37,80-81]。高脂饮食能够诱导糖尿病大鼠肌肉组织中IR、IRS-1、PI3K和Akt的表达降低[82],而GA可以改善并提高IR、IRS、PI3K和Akt表达,促进Glut4和Glut2膜移位增强,维持正常的葡萄糖稳态和胰岛素敏感性[33]。此外,GA还能够通过活化Akt来阻碍叉头转录因子(Forkhead box O,FOXO)介导的PEPCK和G6Pase等糖异生酶的转录,从而抑制糖异生,减少葡萄糖生成[31,80]。
3.3 没食子酸通过调节PPAR-γ维持糖代谢平衡
PPAR-属于核受体超家族,在脂肪组织中高度表达,具有脂肪组织特异性[83]。PPAR-能够促进脂肪细胞分化,增加脂肪细胞数量,调节胰岛素敏感性和糖脂质代谢,维持脂质和葡萄糖稳态[84]。在高糖饮食条件下,GA能够活化3T3-L1脂肪细胞的C/EBPs来上调PPAR-的表达[37]。PPAR-的上调表达可以促进脂肪细胞分化,增加脂肪细胞对葡萄糖的吸收,降低葡萄糖毒性并提高葡萄糖耐受性[61]。病理学研究表明,GA能够通过提高糖尿病大鼠和小鼠附睾脂肪组织的PPAR-表达来增加葡萄糖吸收,降低葡萄糖毒性[31, 36]。同时,PPAR-对PI3K也具有一定激活作用,PI3K活性的升高能够促进Glut4表达,从而提高葡萄糖转运和吸收,起到调节葡萄糖代谢的作用[85]。
4 总结与展望
GA是普洱茶特征性的生物活性成分,具有调控线粒体能量代谢,调节胰岛素敏感性和葡萄糖吸收,维持脂质和葡萄糖稳态的作用。病理学研究和生物试验均表明,GA能够通过抑制肠道对脂肪的消化和吸收、降低脂质合成和积累、促进脂肪细胞对葡萄糖的吸收、以及调节糖异生和糖酵解作用来调节血糖血脂水平。同时,GA还能够促进线粒体能量代谢、改善胰岛素抵抗和提高胰岛素敏感性,从而调节饮食诱导引起的糖脂代谢紊乱。本文综述了普洱茶GA的代谢途径及其生物学作用,为进一步研究提高普洱茶中GA的积累和利用提供理论依据。
目前对GA的生物学作用研究已较为深入,但对其利用的研究还较少。因此提高普洱茶中GA的合成和积累,探讨GA的利用方式以及进一步深入研究GA在代谢过程中的作用是未来研究普洱茶功能成分的课题之一。
[1] Chen L, Chen X W, Huang X, et al. Regulation of glucose and lipid metabolism in health and disease [J]. Science China Life Sciences, 2019, 62: 1420-1458.
[2] Palermo A, Tuccinardi D, Defeudis G, et al. BMI and BMD: The potential interplay between obesity and bone fragility [J]. International Journal of Environmental Research and Public Health, 2016, 13(6): 544. doi: 10.3390/ijerph13060544.
[3] 折改梅, 张香兰, 陈可可, 等. 茶氨酸和没食子酸在普洱茶中的含量变化[J]. 云南植物研究, 2005, 27(5): 572-576. She G M, Zhang X L, Chen K K, et al. Content variation of theanine and gallic acid in Pu-er tea [J]. Acta Botanica Yunnanica, 2005, 27(5): 572-576.
[4] 吴桢. 普洱茶渥堆发酵过程中主要生化成分的变化[D]. 重庆: 西南大学, 2008. Wu Z. The variation of chemical component during the fermentation procedure of Pu'er tea [D]. Chongqing: Southwest University, 2008.
[5] Pedan V, Rohn S, Holinger M, et al. Bioactive compound fingerprint analysis of aged raw Pu'er tea and young ripened Pu'er tea [J]. Molecules, 2018, 23(8): 1931. doi: 10.3390/molecules23081931.
[6] Shao W, Powell C, Clifford M N. The analysis by HPLC of green, black and Pu'er teas produced in Yunnan [J]. Journal of the Science of Food and Agriculture, 1995, 69(4): 535-540.
[7] Lv H P, Zhang Y J, Lin Z, et al. Processing and chemical constituents of Pu-erh tea: A review [J]. Food Research International, 2013, 53(2): 608-618.
[8] 周志宏, 杨崇仁. 云南普洱茶原料晒青毛茶的化学成分[J]. 云南植物研究, 2000(3): 343-350. Zhou Z H, Yang C R. Chemical constituents of crude green tea, the material of Pu-er tea in Yunnan [J]. Acta Botanica Yunnanica, 2000(3): 343-350.
[9] 张雯洁, 刘玉清, 李兴从, 等. 云南“生态茶”的化学成分[J]. 云南植物研究, 1995(2): 204-208. Zhang W J, Liu Y Q, Li X C, et al. Chemical constituents of “Ecolocical tea” from Yunnan [J]. Acta Botanica Yunnanica. 1995(2): 204-208.
[10] Diepeningen A D V, Debets A J M, Varga J, et al. Efficient degradation of tannic acid by blackspecies [J]. Fungal Biology, 2004, 108(8): 919-925.
[11] Mukherjee G, Banerjee R. Biosynthesis of tannase and gallic acid from tannin rich substrates byand[J]. Journal of Basic Microbiology, 2004, 44(1): 42-48.
[12] 郭鲁宏, 杨顺楷. 利用固定化黑曲霉单宁酶制备没食子酸的研究[J]. 生物工程学报, 2000(5): 614-617. Guo L H, Yan S K. Study on gallic acid preparation by using immobilized tannase from[J]. Chinese Journal of Biotechnology, 2000(5): 614-617.
[13] Anaingsih V K, Sharma A, Zhou W. Green tea catechins during food processing and storage: A review on stability and detection [J]. Food Research International, 2013, 50(2): 469-479.
[14] Macedo J A, Ferreira L R, Camara L E, et al. Chemopreventive potential of the tannase-mediated biotransformation of green tea [J]. Food Chemistry, 2012, 133(2): 358-365.
[15] Tanaka T, Umeki H, Nagai S, et al. Transformation of tea catechins and flavonoid glycosides by treatment with Japanese post-fermented tea acetone powder [J]. Food Chemistry, 2012, 134(1): 276-281.
[16] Park Y, Lee J, Hong V S, et al. Identification of KMU-3, a novel derivative of gallic acid, as an inhibitor of adipogenesis [J]. Plos One, 2014, 9(10): e109344. doi: 10.1371/journal.pone.0109344.
[17] 吕海鹏, 林智, 谷记平, 等. 普洱茶中的没食子酸研究[J]. 茶叶科学, 2007, 27(2): 104-110. Lv H P, Lin Z, Gu J P, et al. Study on the gallic acid in Pu-erh tea [J]. Journal of Tea Science, 2007, 27(2): 104-110.
[18] 李肖玲, 崔岚, 祝德秋. 没食子酸生物学作用的研究进展[J]. 中国药师, 2004(10): 767-769. Li X L, Cui L, Zhu D Q. Research progress on the biological effects of gallic acid [J]. China Pharmacist, 2004(10): 767-769.
[19] 张冬英, 邵宛芳, 刘仲华, 等. 普洱茶中没食子酸对过氧化物酶体增殖激活受体作用研究[J]. 营养学报, 2009, 31(1): 47-50. Zhang D Y, Shao W F, Liu Z H, et al. Study of gallic acid in Pu-erh tea on the peroxisome proliferators activated receptors function [J]. Acta Nutrimenta Sinica, 2009, 31(1): 47-50.
[20] Gao X, Xie Q, Kong P, et al. Polyphenol- and caffeine-rich postfermented Pu-erh tea improves diet-induced metabolic syndrome by remodeling intestinal homeostasis in mice [J]. Infection and Immunity, 2017, 86(1): e00601-17. doi: 10.1128/IAI.00601-17.
[21] Huang H, Lin J. Pu-erh tea, green tea, and black tea suppresses hyperlipidemia, hyperleptinemia and fatty acid synthase through activating AMPK in rats fed a high-fructose diet [J]. Food & Function, 2012, 3(2): 170-177.
[22] Gong J, Peng C, Chen T, et al. Effects of theabrownin from Pu-erh Tea on the metabolism of serum lipids in rats: mechanism of action [J]. Journal of Food Science, 2010, 75(6): 182-189.
[23] Du W, Peng S, Liu Z, et al. Hypoglycemic effect of the water extract of Pu-erh tea [J]. Journal of Agricultural and Food Chemistry, 2012, 60(40): 10126-10132.
[24] Kubota K, Sumi S, Tojo H, et al. Improvements of mean body mass index and body weight in preobese and overweight Japanese adults with black Chinese tea (Pu-Erh) water extract [J]. Nutrition Research, 2011, 31(6): 421-428.
[25] Silva G, Ferraresi C, De Almeida R T, et al. Insulin resistance is improved in high-fat fed mice by photobiomodulation therapy at 630 nm [J]. Journal of Biophotonics, 2020, 13(3): e201960140. doi: 10.1002/jbio.201960140.
[26] Collison K S, Saleh S M, Bakheet R H, et al. Diabetes of the liver: the link between nonalcoholic fatty liver disease and HFCS-55 [J]. Obesity (Silver Spring, Md), 2009, 17(11): 2003-2013.
[27] Samuel V T. Fructose induced lipogenesis: from sugar to fat to insulin resistance [J]. Trends in endocrinology and metabolism: TEM, 2011, 22(2): 60-65.
[28] Oi Y, Hou I, Fujita H, et al. Antiobesity effects of Chinese black tea (Pu-erh tea) extract and gallic acid [J]. Phytotherapyresearch: PTR, 2012, 26(4): 475-481.
[29] Zeng L, Yan J, Luo L, et al. Effects of Pu-erh tea aqueous extract (PTAE) on blood lipid metabolism enzymes [J]. Food & Function, 2015, 6(6): 2008-2016.
[30] Zeng X, Sheng Z, Li X, et al.studies on the interactions of blood lipid level-related biological molecules with gallic acid and tannic acid [J]. Journal of the Science of Food and Agriculture, 2019, 99(15): 6882-6892.
[31] Gandhi G R, Jothi G, Antony P J, et al. Gallic acid attenuates high-fat diet fed-streptozotocin-induced insulin resistance via partial agonism of PPARγ in experimental type 2 diabetic rats and enhances glucose uptake through translocation and activation of GLUT4 in PI3K/p-Akt signaling pathway [J]. European Journal of Pharmacology, 2014, 745(15): 201-216.
[32] Hsu C, Yen G. Effect of gallic acid on high fat diet-induced dyslipidaemia, hepatosteatosis and oxidative stress in rats [J]. British Journal of Nutrition, 2007, 98(4): 727-735.
[33] Huang D W, Chang W C, Wu J S, et al. Gallic acid ameliorates hyperglycemia and improves hepatic carbohydrate metabolism in rats fed a high-fructose diet [J]. Nutrition Research, 2016, 36(2): 150-160.
[34] Paraíso A F, Sousa J N, Andrade J M, et al. Oral gallic acid improves metabolic profile by modulating SIRT1 expression in obese mice brown adipose tissue: A molecular and bioinformatic approach [J]. Life sciences, 2019, 237(11): 116914. doi: 10.1016/j.lfs.2019.116914.
[35] Bak E J, Kim J, Jang S, et al. Gallic acid improves glucose tolerance and triglyceride concentration in diet-induced obesity mice [J]. Scandinavian Journal of Clinical & Laboratory Investigation, 2013, 73(8): 607-614.
[36] Hsu C, Huang S, Yen G. Inhibitory effect of phenolic acids on the proliferation of 3T3-L1 Preadipocytes in Relation to their antioxidant activity [J]. Journal of Agricultural and Food Chemistry, 2006, 54(12): 4191-4197.
[37] Variya B C, Bakrania A K, Patel S S. Antidiabetic potential of gallic acid from: Improved glucose transporters and insulin sensitivity through PPAR-and Akt signaling [J]. Phytomedicine, 2019, 73: 152906. doi: 10.1016/j.phymed.2019.152906.
[38] 吕季桦, 孙璐西. 普洱茶抑制HepG2细胞株生合成胆固醇之有效成分探讨[C]//中国茶叶学会. 第四届海峡两岸茶业学术研讨会论文集, 2006. Lv J H, Sun L X. Investigation of Pu-erh tea active principles to inhibitthe cholesterol synthesis in Hep G2cell line [C]// China Tea Scienc Society. The Fourth Cross-Straits Tea Industry Proceedings, 2006.
[39] Way T, Lin H, Kuo D, et al. Pu-erh tea attenuates hyperlipogenesis and induces hepatoma cells growth arrest through activating AMP-activated protein kinase (AMPK) in human HepG2 cells [J]. Journal of Agricultural and Food Chemistry, 2009, 57(12): 5257-5264.
[40] Elrokh E M, Yassin N A Z, Elshenawy S M, et al. Antihypercholesterolaemic effect of ginger rhizome () in rats [J]. Inflammopharmacology, 2010, 18(6): 309-315.
[41] Okuno A, Tamemoto H, Tobe K, et al. Troglitazone increases the number of small adipocytes without the change of white adipose tissue mass in obese Zucker rats [J]. Journal of Clinical Investigation, 1998, 101(6): 1354-1361.
[42] Chao L C, Marcussamuels B, Mason M, et al. Adipose tissue is required for the antidiabetic, but not for the hypolipidemic, effect of thiazolidinediones [J]. Journal of Clinical Investigation, 2000, 106(10): 1221-1228.
[43] Cao Z, Umek R M, Mcknight S L. Regulated expression of three C/EBP isoforms during adipose conversion of 3T3-L1 cells [J]. Genes & Development, 1991, 5(9): 1538-1552.
[44] Farmer S R. Transcriptional control of adipocyte formation [J]. Cell Metabolism, 2006, 4(4): 263-273.
[45] Furuyashiki T, Nagayasu H, Aoki Y, et al. Tea catechin suppresses adipocyte differentiation accompanied by down-regulation of PPARγ2 and C/EBPα in 3T3-L1 cells [J]. Bioscience, Biotechnology, and Biochemistry, 2004, 68(11): 2353-2359.
[46] Huang D W, Shen S C. Caffeic acid and cinnamic acid ameliorate glucose metabolism via modulating glycogenesis and gluconeogenesis in insulin-resistant mouse hepatocytes [J]. Journal of Functional Foods, 2012, 4(1): 358-366.
[47] Saltiel A R, Kahn C R. Insulin signalling and the regulation of glucose and lipid metabolism [J]. Nature, 2001, 414(6865): 799-806.
[48] Ferrer J C, Favre C, Gomis R R, et al. Control of glycogen deposition [J]. FEBS Letters, 2003, 546(1): 127-132.
[49] Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance [J]. Physiological Reviews, 2004, 84(1): 277-359.
[50] Oelkrug R, Polymeropoulos E T, Jastroch M. Brown adipose tissue: physiological function and evolutionary significance [J]. Journal of Comparative Physiology B-biochemical Systemic and Environmental Physiology, 2015, 185(6): 587-606.
[51] Bartelt A, Heeren J. Adipose tissue browning and metabolic health [J]. Nature Reviews Endocrinology, 2014, 10(1): 24-36.
[52] Doan K V, Ko C M, Kinyua A W, et al. Gallic acid regulates body weight and glucose homeostasis through AMPK activation [J]. Endocrinology, 2015, 156(1): 157-168.
[53] Oneill H M, Holloway G P, Steinberg G R. AMPK regulation of fatty acid metabolism and mitochondrial biogenesis: implications for obesity [J]. Molecular and Cellular Endocrinology, 2013, 366(2): 135-151.
[54] Hardie D G. AMPK: a target for drugs and natural products with effects on both diabetes and cancer [J]. Diabetes, 2013, 62(7): 2164-2172.
[55] Liesa M, Shirihai O S. Mitochondrial dynamics in the regulation of nutrient utilization and energy expenditure [J]. Cell Metabolism, 2013, 17(4): 491-506.
[56] Kim J, Kundu M, Viollet B, et al. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1 [J]. Nature Cell Biology, 2011, 13(2): 132-141.
[57] Zhao M, Klionsky D J. AMPK-dependent phosphorylation of ULK1 induces autophagy [J]. Cell Metabolism, 2011, 13(2): 119-120.
[58] Jermendy G. PPARγ agonists: Antidiabetic drugs with a potential role in the treatment of diseases other than diabetes [J]. Diabetes Research and Clinical Practice, 2007, 78(3): 29-39.
[59] Latha R C R, Daisy P. Insulin-secretagogue, antihyperlipidemic and other protective effects of gallic acid isolated fromRoxb. in streptozotocin-induced diabetic rats [J]. Chemico-Biological Interactions, 2011, 189(1): 112-118.
[60] Goldstein B J. Insulin resistance as the core defect in type 2 diabetes mellitus [J]. American Journal of Cardiology, 2002, 90(5): 3-10.
[61] Makihara H, Koike Y, Ohta M, et al. Gallic acid, the active ingredient of terminalia bellirica, enhances adipocyte differentiation and adiponectin secretion [J]. Biological & Pharmaceutical Bulletin, 2016, 39(7): 1137-1143.
[62] Hardie D G. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy [J]. Nature Reviews Molecular Cell Biology, 2007, 8(10): 774-785.
[63] Jager S, Handschin C, Stpierre J, et al. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1[J]. Proceedings of the National Academy of Sciences of the United States of America, 2007, 104(29): 12017-12022.
[64] Lagouge M, Argmann C A, Gerharthines Z, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1[J]. Cell, 2006, 127(6): 1109-1122.
[65] Canto C, Auwerx J. PGC-1α, SIRT1 and AMPK, an energy sensing network that controls energy expenditure [J]. Current Opinion in Lipidology, 2009, 20(2): 98-105.
[66] Fulco M, Cen Y, Zhao P, et al. Glucose restriction inhibits skeletal myoblast differentiation by activating SIRT1 through AMPK-mediated regulation of nampt [J]. Developmental Cell, 2008, 14(5): 661-673.
[67] Pfluger P T, Herranz D, Velascomiguel S, et al. Sirt1 protects against high-fat diet-induced metabolic damage [J]. Proceedings of the National Academy of Sciences of the United States of America, 2008, 105(28): 9793-9798.
[68] Michan S, Sinclair D C. Sirtuins in mammals: insights into their biological function [J]. Biochemical Journal, 2007, 404(1): 1-13.
[69] Kelly G. A Review of the sirtuin system, its clinical implications, and the potential role of dietary activators like resveratrol: part 1 [J]. Alternative Medicine Review: A Journal of Clinical Therapeutic, 2010, 15(3): 245-263.
[70] Ramadori G, Fujikawa T, Fukuda M, et al. SIRT1 deacetylase in POMC neurons is required for homeostatic defenses against diet-induced obesity [J]. Cell Metabolism, 2010, 12(1): 78-87.
[71] Erion D M, Yonemitsu S, Nie Y, et al. SirT1 knockdown in liver decreases basal hepatic glucose production and increases hepatic insulin responsiveness in diabetic rats [J]. Proceedings of the National Academy of Sciences of the United States of America, 2009, 106(27): 11288-11293.
[72] Kim Y D, Park K G, Lee Y S, et al. Metformin inhibits hepatic gluconeogenesis through AMP-activated protein kinase-dependent regulation of the orphan nuclear receptor SHP [J]. Diabetes, 2008, 57(2): 306-314.
[73] Fullerton M D, Galic S, Marcinko K, et al. Single phosphorylation sites in Acc1 and Acc2 regulate lipid homeostasis and the insulin-sensitizing effects of metformin [J]. Nature Medicine, 2013, 19(12): 1649-1654.
[74] Kido Y, Nakae J, Accili D. The insulin receptor and its cellular targets [J]. The Journal of Clinical Endocrinology and Metabolism, 2001, 86(3): 972-979.
[75] White M F. Insulin signaling in health and disease [J]. Science, 2003, 302(5651): 1710-1711.
[76] Lietzke S E, Bose S, Cronin T C, et al. Structural basis of 3-phosphoinositide recognition by pleckstrin homology domains [J]. Molecular Cell, 2000, 6(2): 385-394.
[77] Kim Y B, Peroni O D, Franke T F, et al. Divergent regulation of Akt1 and Akt2 isoforms in insulin target tissues of obese Zucker rats [J]. Diabetes, 2000, 49(5): 847-856.
[78] Cho H, Mu J, Kim J K, et al. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKBβ) [J]. Science, 2001, 292(5522): 1728-1731.
[79] Katome T, Obata T, Matsushima R, et al. Use of RNA Interference-mediated gene silencing and adenoviral overexpression to elucidate the roles of AKT/Protein kinase B isoforms in insulin actions [J]. Journal of Biological Chemistry, 2003, 278(30): 28312-28323.
[80] Tzatsos A, Kandror K V. Nutrients suppress phosphatidylinositol 3-Kinase/Akt signaling via raptor-dependent mTOR-mediated insulin receptor substrate 1 phosphorylation [J]. Molecular and Cellular Biology, 2006, 26(1): 63-76.
[81] Ma X, Tsuda S, Yang X, et al. Pu-erh tea hot-water extract activates Akt and induces insulin-independent glucose transport in rat skeletal muscle [J]. Journal of Medicinal Food, 2013, 16(3): 259-262.
[82] Tzeng T, Liou S, Liu I. Myricetin ameliorates defective post-receptor insulin signaling via-endorphin signaling in the skeletal muscles of fructose-fed rats [J]. Evidence-based Complementary and Alternative Medicine, 2011: 150752. doi: 10.1093/ecam/neq017.
[83] Soccio R E, Chen E R, Lazar M A. Thiazolidinediones and the promise of insulin sensitization in type 2 diabetes [J]. Cell Metabolism, 2014, 20(4): 573-591.
[84] Plutzky J. PPARs as Therapeutic targets: reverse cardiology? [J]. Science, 2003, 302(5644): 406-407.
[85] Sharma B R, Kim H J, Rhyu D Y.extract ameliorates insulin resistance and regulates glucose metabolism in C57BL/KsJ-db/db mice via PI3K/AKT signaling pathway in myocytes [J]. Journal of Translational Medicine, 2015, 13(1): 62. doi: 10.1186/s12967-015-0412-5.
Research Progress of Gallic Acid in Puer Tea and Its Improvement of Diet Induced Glucose and Lipid Metabolism Disorder
WANG Shaomei1,2, LI Xiaojun1,2, SONG Wenming3, PAN Lianyun4*
1. Black Tea Engineering Research Center of Yunnan, West Yunnan university, Lincang 677000, China; 2. School of Biotechnology and Engineering, West Yunnan University, Lincang 677000, China; 3. Senior Technical School of Lincang, Lincang 677000, China; 4. Tea Research Institute, Yunnan Academy of Agricultural Sciences, Yunnan Provincial Key Laboratory of Tea Science, Menghai 666200, China
The disorder of glucose and lipid metabolism is one of the important causes of cardiovascular diseases, diabetes and fatty liver. Gallic acid of Puer tea can ameliorate diet induced glucose and lipid metabolism disorder through regulating energy metabolism and adipocyte differentiation, promoting glucose absorption and utilization, and increasing insulin sensitivity. Gallic acid regulates mitochondrial energy metabolism, insulin sensitivity and glucose absorption to maintain glucose and lipid metabolism homeostasis through AMPK and IR-Akt pathway and PPAR gamma receptor. In this review, we summarized gallic acid in Puer tea and its mechanism to improve the disorder of glucose and lipid metabolism induced by diet.
Puer tea, gallic acid, diet induced, glucose and lipid metabolism
S571.1
A
1000-369X(2020)04-431-10
2019-11-19
2019-12-05
王绍梅,女,教授,主要从事茶叶加工、审评和茶文化方面的研究,lc-wangshaomei@163.com。
Panly11@lzu.edu.cn
投稿平台:http://cykk.cbpt.cnki.net

