Research Progress in Surface Passivation of Colloidal Quantum Dots Solar Cells
(Jingdezhen Ceramic Institute, Jingdezhen 333403, Jiangxi, China)
Abstract: As one of the third-generation solar cells, colloidal quantum dot solar cells have attracted huge attentions owing to their simple preparation process and device flexibility. Notably, the surface passivation of the colloidal quantum dots for building the active layer has a critical impact on the device performances. This paper was aimed to briefly describe the recent research progress in surface passivation of the device-graded colloidal quantum dots and the effects of various ligands on device performances, with prospects of the future development trend.
Key words: colloidal quantum dots; ligand exchange; passivation; perovskite
1 Introduction
As a group of nanomaterials, colloidal quantum dots (CQDs) include II-VI and II-V compounds, with particle sizes in the range of 1-10 nm. Due to their small sizes, CQDs have various unique physic and chemical properties, such as size effect, surface effect, quantum tunneling effect and dielectric confinement effect[1-4]. Because their bandgap,electron state energy level and surface chemistry can be adjusted over a wide range, with the presence of multi-exciton generation (MEG) effect, CQDs have been used as active layers (light absorption layers) of solar cells[5].
Because of the relatively large specific surface area, the surface of CQDs usually contains numerous dangling bonds, which easily trigger the “selfquenching” of the photogenerated carriers. Also, the transport of carriers in between the CQDs possibly induces trapping effect. The carriers could even be captured by the barren defects as the recombination-centers. All these effects could be dynamically competitive with the radiation recombination, so that the latter will be weakened,thus resulting in reduced luminescent efficiency of the CQDs films[6,7]. To prevent the occurrence of self-quenching, CQDs are passivated by using surface ligands, so as to reduce the density of defects at the surface. Generally, the effects of surface ligands on physical and chemical properties of CQDs include (i) influencing their nucleation and growth in the synthesis process, (ii) offering static stability to the CQDs, (iii) altering photoelectric properties of the CQDs through the modification of energy level and carrier mobility and (iv) minimizing the negative effect of environment[7-11].
Because long-chain macromolecules have special groups at the ends, they serve to control the reactivity of the precursors of CQDs. As a result, the nucleation and crystal growth of CQDs can be controlled to take place at different stages, so that the CQDs will have desired crystal size, size distribution and morphology. To this end, long-chain macromolecules, such as oleic acid and oleylamines,are used for the synthesis of CQDs[12]. However,due to their insulating characteristics, these macromolecules could limit the electrical performances when CQDs are used to fabricate photoelectronic devices, such as photodetectors and solar cells. In this case, it is necessary to use other ligands to passivate the surface of CQDs. This article is aimed to overview the progress in the effect of ligands on CQDs-based photovoltaic devices, by focusing on surface ligand exchange and passivation.
2 Ligand Exchange
To develop high performance devices, the macromolecules at the surface of CQDs should be replaced by ligands with short-chain molecules or atomic scale items. Currently, there are two types of exchange methods, i.e., solid-state ligand exchange(SSLE) and liquid phase transfer exchange (LPTE),both of which have advantages and disadvantages.
In SSLE process, CQDs are dispersed in nonpolar solvents (such as toluene, hexane or octane)with concentrations of 30-80 mg/ml, which were then coated on transparent substrates as thin layers.The CQDs layers were treated with short-chain molecules or atomic level ligands, in order to induce exchange reaction. After that, the samples are cleaned with methanol or acetonitrile to remove the residual ligands[13-16]. To develop high performance of photoelectronic devices, this process can be repeated multiple times to make the CQDs layers reach the desired thickness. In this regard,layer-by-layer (LBL) method has been widely employed to deposit CQDs layers. With the combination of LBL and SSLE, the inter-particle distance of CQDs can be readily controlled, while the CQDs films usually have high quality surface,which are beneficial to improving the carrier mobility. However, the multiple steps of deposition of CQDs, SSLE and cleaning result in low utilization efficiency of the CQDS, high consumption of solvents and long time requirement, as shown schematically in Fig. 1 (a).
In comparison, in LPTE, CQDs particles are wrapped with nonpolar long-chain alkyl molecules that are already dispersed in the solvents, which are mixed with ligands that are dissolved in polar solvents, so as to trigger the desired exchange,followed spin-coated into a film in one step. LPTE method has high utilization efficiencies for the precursors and solvents, but the thick CQDs film tend to crack due to the large lateral stress, as illustrated in Fig. 1 (b).
Currently, CQDs active layers are usually fabricated by using the two strategies. SSLE process is simple and cost-effective, but the proton solvent(e.g., methanol) often leads to loss of the ligand and induces defects in the films[18]. LPTE has no such problems, while the key requirement is that interaction between the long-chain molecules and the CQDs should be sufficiently weak and nonoxidative polar solvents should be used for the exchange reactions[19]. Therefore, in practice, a trade-off must be considered in selecting reasonable exchange processes.
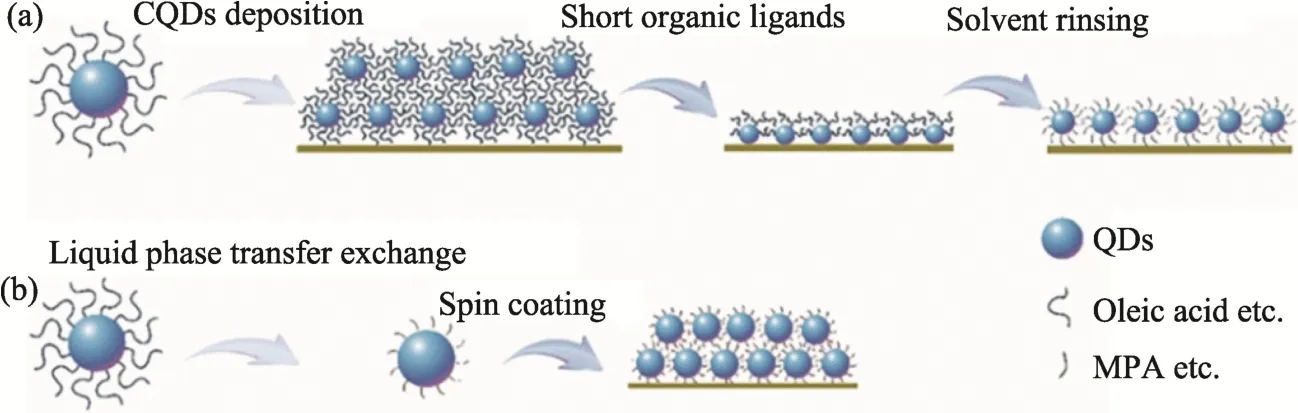
Fig.1 (a) Schematic diagram of solid state ligand exchange and film fabrication, (b) Schematic diagram of liquid phase transfer exchange and film fabrication, taking MPA as an example [16]
3 Passivation with Organic Ligands
When the functional and specific groups of the organic ligands are anchored onto the surface of the CQDs, their interaction with environment can be adjusted and they will be stabilized in the solutions.It has been found that the carrier mobility of the CQDs films is exponentially increased with decreasing length of the ligand molecules. Therefore, it is desired to use short-chain ligands to replace the long-chain molecules in the CQDs films. The commonly used short-chain organic ligands include Aromatic mercaptans[20,21], mercapto carboxylic acids[22],alkylamines[23]and organometallic ligands[24].
Due to the strong interaction with surface cations of CQDs, thiol groups are usually adopted for the exchange reactions. For instance, Chuang et al[25]used 1, 2-ethanedithiol (EDT) as the ligand to treat PbS CQDs, which served as electron barrier layer. The resultant solar cells exhibited a power conversion efficiency (PCE) of 8.55%, which was much higher than that of the PbS-based device without passivation. Owing to the encapsulation effect of EDT, the solar cells showed no change in performance for 150 days. The exchange process is schematically demonstrated in Fig.2.
Kitada et al[26]employed ethylenediamine to exchange with oleic acid encapsulated CQDs,confirming that the short-chain exchange could shorten the inter-particle distance of the CQDs. As a result, the carrier mobility was significantly improved, thus leading enhancement in the efficiency of solar cells, further proving the determining effect of the chain length of the ligand molecules on the electrical performances of the CQDs-based devices.
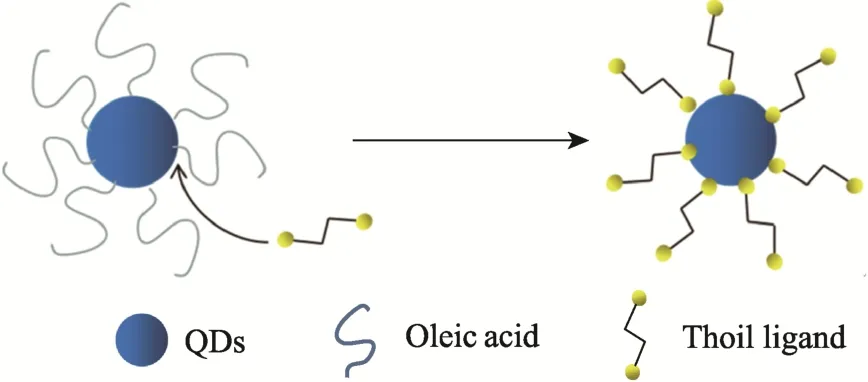
Fig.2 Schematic diagram of thiol ligand exchange
Ming et al[27]studied the effect of chain length of ligands on the exchange reaction for AgBiS2CQDs. It is observed that the chain property of the ligand had a straightforward effect on energy level of the CQDs, thus influencing the electrical performance of the photoelectronic devices. Brown et al[28]adopted different ligands to modify the surface of CQDs, whereas the results further confirmed the effect of chain length of the ligands by using UPS to measure the variation in the energy level of the CQDs after exchange with different ligands. According to energy level matching principle[28-30], the output of a photovoltaic device can be controlled by adjusting the energy levels of the different layers, which can be used as reference for the design of high performance photoelectronic devices.
4 Passivation with Inorganic Ligands
In 2009, Kovalenko et al[31]for the first time proposed to use inorganic ligands for the exchange reactions of CQDs. Since then, various inorganic ligands have been reported, such as metal chalcogenide complexes (MCCs) and metal halides.The use of inorganic ligands not only improved the stability of the CQDs and passivated their surface defects, but also enhanced the coupling of electron wave functions and promoted the carrier transportation[32].
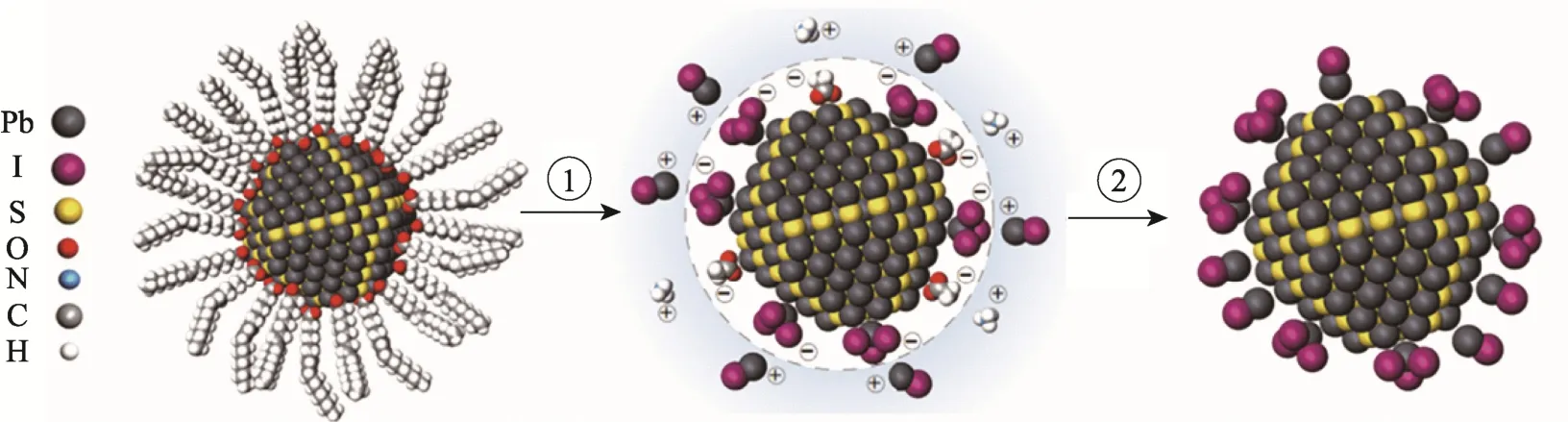
Fig.3 Liquid phase ligand exchange process between metal halide precursor and ammonium acetate(process 1: ligand exchange, process 2: CQDs precipitation) [34]
4.1 Metal chalcogenides
According to the hard-soft acid-base (HSAB)theory, Kovalenko et al[31]developed a dual-phase liquid ligand exchange reaction to prepare MCCs-modified CQDs in polar organic system.Generally, the exchange occurs between the acid-base couple with comparable hardness metrics.The sulfur aions in chalcogenides (e.g., Na4SnS6and Na3AsS3) tend to react with the cations at the surface of CQDs, thus replacing the long-chain molecules.With the realization of exchange with MCCs, the inter-particle interactions of the CQDs are enhanced,thus leading to high carrier mobility. Although the report on this kind of ligands in the applications of photoelectronic devices (especially solar cells) is still not very popular, it is highly expected they will have great potential for such applications.
4.2 Metal halides
Currently, PbS is the most widely candidate for highly efficient CQDs-based heterojunction solar cells. As atomic scale ligands, metal halides can be used to replace the long-chain molecules to improve the carrier transport efficiency of PbS CQDs. The strong interaction between the halides and the cations at the surface of the CQDs helps to guarantee the perfect surface passivation. As a consequence,the carrier mobility is increased, while the concentration of defects in the CQDs films is decreased.
In 2016, Liu et al[34]reported an exchange process with metal halides. With the aid of weak acidic ammonia ions, the oleic acid molecules of PbS CQDs can be completely replaced by [PbX3]-. After the exchange process, toluene was introduced into the N, N-dimethylformamide (DMF) to crush out the CQDs from the solvent system, which can help to remove the ammonia acetate and excessive lead halide, as shown schematically in Fig. 3. The as-obtained product was then re-dispersed in N-butylamine to prepare the spin-ink. PbS QCDs films spin-coated with the ink had a smooth surface and soft texture, resulting solar cells with a high PCE of 11.28%.
In one word, the inter-particle distance of the CQDs films can be effectively shortened after the exchange reactions with metal chalcogenides and halides, thus leading an enhancement in carrier mobility. In addition, the CQDs after exchange with MCCs or metal halides should be dispersed in polar solvent with relatively high dielectric constant, e.g.,DMF. This is because the CQDs passivated with the smaller ligands require strong ions shielding effect conditions to form diffusion double layers, so that they can be well dispersed through electrostatic interactions[19,35], as schematically shown in Fig. 4.
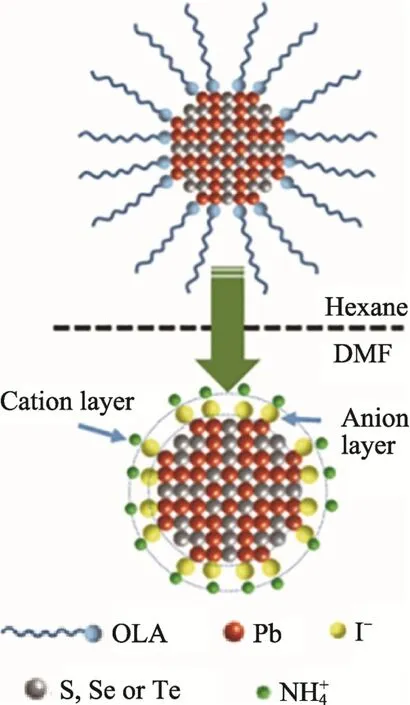
Fig.4 Schematic diagram of ligand exchange process(taking NH4I ligand as an example) [36]
5 Passivation with Hybrid Ligands
Hybrid ligands, consisting of both organic and inorganic ligands, have combined advantages of the two components[16,36]. In 2012, Alexander et al[37]proposed such an organic-inorganic hybrid passivation strategy. They mixed oleic acid encapsulated PbS CQDs and halide solutions at a relatively low temperature. After the exchange reaction, the passivated CQDs were dispersed in octane, followed by SSLE process with mercaptopropionic acid (MPA) in methanol. Finally,the passivated CQDs were made into solar cells,achieving a record PCE of 7.0%, which was much higher than those of the devices from the CQDs passivated with only organic[38]or inorganic[39]ligands, as illustrated in Table 1.
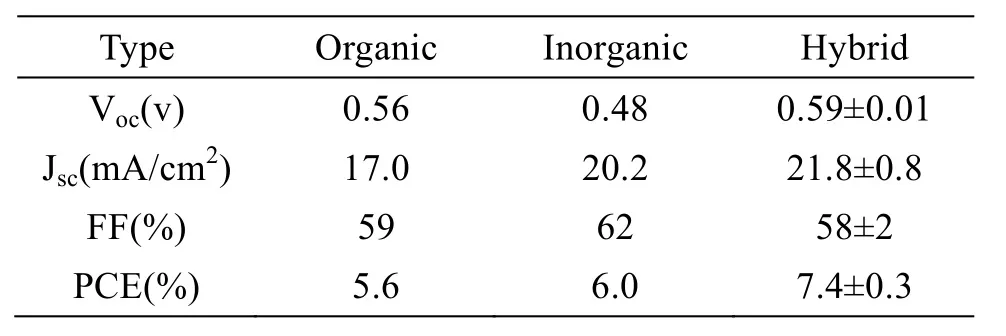
Tab.1 Performances of different ligand passivated devices [37]
Distance between oleic-acid-capped CQDs measured by GISAXS was 4.4 nm. In the SSLE process, after passivation with MPA, the average inter-particle distance of the CQDs films was reduced to 3.4 nm, while it was kept unchanged after the exchange with the inorganic ligand. As compared with the passivation with only inorganic ligand, the CQDs films were further densified after exchange with MPA due to its dual-functional groups. From the steric effect point of view, it is more difficult for organic ligands to passivate the CQDs due to their large molecules. In comparison, inorganic ligands have much smaller molecules, so that they can easily approach the locations with smaller spaces.Therefore, hybrid ligands would ensure that film densification and CQDs surface passivation are simultaneously maximized, as shown schematically in Fig. 5.
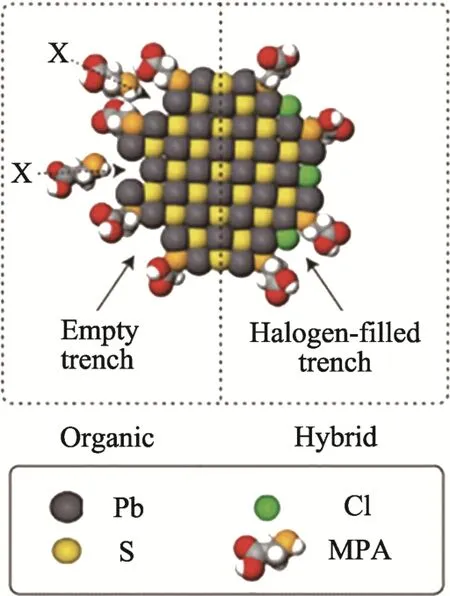
Fig.5 Schematic cross-section of organic passivation (left)and mixed passivation (right) for PbS CQDs [37]
The lower content of defects at the surface of the CQDs, the higher the voltage and PCE performances that can be achieved. In this aspect,hybrid ligand passivation has been proved to be an effective way to improve the voltage and PCE properties of CQDs based solar cells. The combination of inorganic ligand and MPA led to CQDs films with shorter inter-particle distance,higher film density, higher carrier mobility and thus higher PCE.
6 Passivation with Perovskite Ligands
Because of their large carrier diffusion length,adjustable bandgaps, high light absorption coefficient and high defect tolerance, perovskites have been widely used to passivate the surfaces of CQDs[40-46]. Perovskite passivation can be applied in two way, i.e., mixing and epitaxial method. In the first process, perovskite nanocrystals are well-mixed with the oleic acid-capped CQDs treated by chlorines in the same solvent system to trigger the halide ion exchange reaction. After that, an excess amount of ethanol is added to dissolve the excessive perovskite nanocrystals. The passivated CQDs were collected through centrifugation and dispersed in nonpolar solvent (e.g., hexane) for device fabrication[47].For the second process, perovskite precursor solutions in polar solvents are mixed with CQDs suspensions in nonpolar solvents. As a result,perovskite phase is epitaxially grown the surface of the CQDs, thus realizing surface passivation. The passivated CQDs are collected and dispersed in butylamine for film deposition[48].
6.1 Mixed perovskite
Considering the high migration rate of the halide ions in the halide-based perovskite nanocrystals, Zhang et al[47]proposed a new passivation technique, according to the HSAB and DFT theories. They studied the halide ion exchange reaction between CsPbX3(X = Br, I) nanocrystals and chlorine-passivated PbSe CQDs, as demonstrated in Fig. 6. The solar cells made of the perovskite passivated PbSe CQDs exhibited a significant improvement in both the open-circuit voltage (VOC) and fill factor (FF). In addition,the mixed-perovskite passivated CQDs had stronger anti-oxidation capability, the PCE of the devices was retained by 90% after 57 days.In 2008, Hu et al[49]similarly demonstrated that the PCE performance can be further enhanced, due to the widened depletion layer and the reduced carrier recombination.
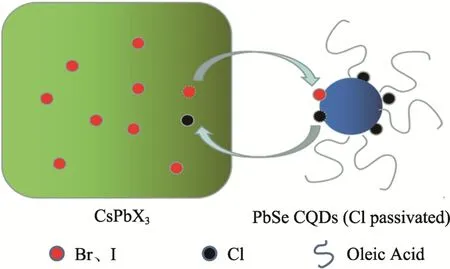
Fig.6 Schematic diagram of ion exchange between chlorinated PbSe CQDs and CsPbX3 (X=Br, I)
6.2 Epitaxial perovskite
It has been reported that there is only a 5%mismatching in lattice between methylammonium lead triiodide (MAPbI3) perovskites and PbS CQDs[50]. Therefore, MAPbI3could be a promising candidate for the passivation of PbS CQDs. With density functional theory (DFT), Ning et al[51]calculated the interface formation energy between PbS (100) and MAPbI3(110) planes. They found that it was less than 10 meV/Å2, suggesting that the growth of MAPbI3on PbS is as easy as the growth of PbS on PbS and MAPbI3on MAPbI3. Moreover,the lattices of MAPbI3and PbS CQDs are well matching in the both 3D and 2D configurations, as seen in Fig. 7 (a) and (b), respectively. Therefore,well-developed core-shell structures can be obtained through the epitaxial growth of MAPbI3on PbS CQDs. In addition, the DFT calculation also predicted that the epitaxial growth is not accompanied by interface defects, as illustrated in Fig. 7 (c) and (d)[51].
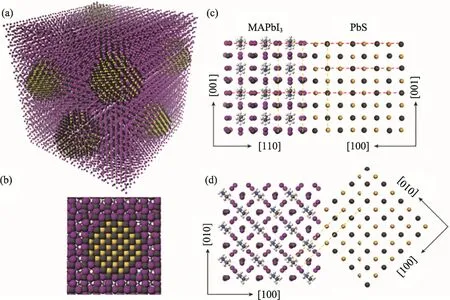
Fig.7 Theoretical model of the perovskite epitaxially grown on CQDs. (a) Three-dimensional atomistic model of CQDs in a perovskite matrix, (b) Two-dimensional view of a single CQD in perovskite. The modeling of the crystal structure and interface of PbS and MAPbI3 showed that the MAPbI3 matched well with the PbS in the X-Z plane (c) and the X-Y plane (d).(The colors in the figure are as follows: grey-lead; yellow-sulfur; purple-iodine. The red dashes show the unit cell size and the yellow dashes are guides to the eye for matching planes) [51]
In 2015, Yang et al[52]grow MAPbI3to passivate PbS CQDs, resulting in devices exhibiting a world record PCE of 8.95%, due to the reduced surface defect density and increased diffusion length.Together with the long diffusion length and high carrier mobility, the large bandgap of the perovskite ensured the generation of excitons in the CQDs with an efficiency of 80% from the photons and holes in the perovskite phase. The fact that the diffusion length of hybrid materials consisted of CQDs and perovskite grow in inverse proportion to CQDs content, suggesting that the perovskite could assist the charge transport in between the CQDs[16].Additionally, the CQDs are perfectly embedded in the matrix of perovskite so as to realize well-passivation for the CQDs’ surface, further preventing the photogenerated carriers from selfquenching effect[50]. Recently, Liu et al[53]boosted the PCE record to 12.6% by using halide-perovskite hybrid passivation technique. Meanwhile, the stability of the devices is large increased due to the suppression of the phase transition of the perovskite and the anti-oxidation capability of the CQDs was enhanced.
Due to their unique properties, perovskites have been shown strong passivation capability to improve the photoelectrical performances of CQDs-based solar cells, no matter which method(i.e., mixing or epitaxial) is applied. Although the passivation of CQDs with perovskite has not been extensively studied, it strongly deserves for further exploration in the near future.
7 Conclusions and Perspectives
In recent years, more and more attentions have been paid to the synthesis and passivation of CQDs, while the related photovoltaic devices are still under development. It is necessary to further the output performance and stability of CQDs for large-scale practical applications. In this regard, we would like to propose two areas in the future studies.
(1) Based on the flexibility of molecular engineering, it is suggested to high efficiency straight-chain planar organic ligands, with specific functional groups, which not only are able to passivate the CQDs and reduce the self-quenching problem, but also decrease the barrier of photon transport among the CQDs.
(2) According to the composition and optical properties of CQDs, more high quality perovskites with lattice matching properties should be developed. By fully utilizing the high defect tolerance, high carrier mobility and long diffusion length of perovskites, both the photogenerated electron properties and the stabilities of the CQDs are expectedly improved for high performance solar cell applications.

