Pretreating Co/SiO2 to generate highly active Fischer-Tropsch synthesis catalyst with low CH4 selectivity
MU Shi-fang,SHANG Ru-jing,CHEN Jian-gang,ZHANG Jian-li
(1.School of Safety Science and Engineering, Henan Polytechnic University, Jiaozuo 454000, China;2.State Key Laboratory of Coal Conversion, Institute of Coal Chemistry of Chinese Academy Sciences, Taiyuan 030001, China;3.School of Physics &Electronic Information Engineering, Henan Polytechnic University, Jiaozuo 454000, China;4.State Key Laboratory of High-efficiency Utilization of Coal and Green Chemical Engineering,Ningxia University, Yinchuan 750021, China)
Abstract:The effects of pretreatment methods (DR,R,ROR) on the microstructure of Co/SiO2 catalysts and the activity for Fischer-Tropsch synthesis (FTS) were investigated.The pretreated catalysts were characterized by TEM,HRTEM,XRD,XPS,H2-TPD,TG and TPR.The results showed that after the pretreatments,specific morphological of the Co species changed,forming new Co active surface species.The Co particles redispersed and the Co species was facile to be re-reduced.The Co/SiO2 catalysts pretreated by different method showed different catalytic performance.The catalyst treated by the reduction-passivation had higher activity and C5+ selectivity for FTS.
Key words:pretreatment methods;Co/SiO2 catalyst;Fischer-Tropsch synthesis
Fischer-Tropsch synthesis (FTS) is an important route for ultra-clean liquid fuel synthesis from coal-based or natural-gas-based syngas[1,2].Cobalt containing catalysts are known to be effective in FTS[3].The catalytic performance of cobalt-based systems in FTS depends directly on the number and availability of active sites[4,5].The active species of cobalt-based catalysts is the metallic phase,which could be obtained by the pretreatment of the precursors in a reduction atmosphere before reaction[6].With fixed preparation(impregnation from aqueous solution) and constant metal loading,the catalyst performance is particularly affected by the extent of reduction and dispersion of metallic cobalt species, i.e.the pretreatment of the catalysts[7].
It was well known that the oxidation-reduction pretreatment of transition metals resulted in a strong enhancement of their activity in hydrogenation reaction[8 − 14].For instance, the enhancement of the activity of Co in the hydrogenation of CO2was observed after repeated reduction/oxidation cycles[13].In the literature,such an effect was considered as a result of cleaning[12],promoting role of oxygen remaining in the near surface region[14]and the surface modification[10,11,13].Kobylinski et al[15,16]reported that the reduction-oxidation (redox) pretreatment of supported cobalt catalysts could increase the catalytic activity for FTS.In the case of Co/ZrO2[17],the redox pretreatment was also found to enhance the reducibility of the catalyst,and then the cobalt oxides were facile to be re-reduced at 523 K.But for a 2% Ru-promoted 15% Co/Al2O3catalyst, the oxidation-reduction treatments were observed to assist the sintering of the metallic clusters to form a larger size.The larger crystallites in closer proximity to the Ru promoter might lead to a more easy reduction of the cobalt crystallites.As a result,the catalyst exposed to two oxidation-reduction cycles resulted in slightly higher conversion, lower methane selectivity, and better stability compared with a reduced freshly calcined catalyst[18].However,less attention had been paid to the effect of redox pretreatment methods on the physciochemical properties and the catalytic performance of Co/SiO2catalyst for FTS.The influence of reductionoxidation-reduction (ROR) pretreatment on 20%Co/SiO2had been investigated using different oxidizing gases including water vapor and oxygen in the oxidation step.It was demonstrated that ROR treatment using both oxygen and water vapor decreased the average cobalt particle size.ROR treatment in oxygen resulted in an increase in catalytic activity.In contrast, the water vapor applied in oxidation step obviously deactivated the cobalt catalyst and enhanced the selectivity of methane[19].Thus,the present work focused on the effects of pretreatment procedure on the size and structure of cobalt particles and subsequently the FTS activity of the Co/SiO2catalysts.Herein,three redox pretreatment methods, direct reduction (DR),reduction-passivation (R) and reduction-oxidationreduction (ROR) were carried out for the purpose.
1 Experimental
1.1 Catalysts preparation and pretreatments
Co/SiO2catalysts containing 15% of Co were prepared by incipient wetness impregnation of SiO2support (Degussa A200,specific surface area:177 m2/g,pore volume:0.49 cm3/g,average pore diameter:11.1 nm) with aqueous solution of Co(NO3)2·6H2O (AR,Shanghai Hengxin Chemical Reagent Co.Ltd.).Then,the impregnated support was dried at 353 K for 12 h and calcinated in air at 673 K for 5 h which was denoted as Co3O4/SiO2.The other catalysts were referenced using Co/SiO2followed by a symbol relative to the treatment.The symbols are as follows:
DR, Direct hydrogen reduction of the nitrate precursor−673 K (6 h, 1 K/min), cooled to room temperature under hydrogen flow,then passivated by 5%O2/N2(12 h).
R,hydrogen reduction of Co3O4/SiO2−673 K (6 h,1.5 K/min), cooled to room temperature under hydrogen flow,then passivated by 5%O2/N2(12 h).
ROR,hydrogen reduction of Co3O4/SiO2−673 K(6 h,1.5 K/min),cooled to room temperature under hydrogen flow, oxidized by 5%O2/N2-573 K (2 h,0.5 K/min), cooled to room temperature under 5%O2/N2flow,reduced with hydrogen-673 K (6 h,2 K/min),cooled to room temperature under hydrogen flow,then passivated by 5%O2/N2(12 h).
1.2 Characterization methods
Specific surface area,total pore volume (TPV)and average pore diameter were measured by nitrogen physisorption using a Micromeritics ASAP-2000 automatic system at 77 K.The specific surface area was estimated by the BET method.The average pore diameter and total pore volume were determined by the BJH method[20].XRD studies were performed in a Bruker D8 Advance X-ray spectrometer at 40 kV and 100 mA using monochromatic Cu-Kα radiation.TEM and HRTEM images were obtained in a JEOL 2010 apparatus.
H2-TPD was carried out in a U-tube quartz reactor with the Zeton Altamira AMI-200 unit.0.200 g of the catalyst was reduced at 673 K for 6 h using a flow of high purity hydrogen and then cooled to 373 K under hydrogen stream.The sample was kept at 373 K for 1 h under flowing argon to remove the weakly bound physisorbed species prior to increasing the temperature slowly to 673 K.Then,the catalyst was exposed to flowing argon to desorb the chemisorbed hydrogen and the TCD began to record the signal till the signal returned to the baseline.The TPD profile was integrated and the amount of desorbed hydrogen was determined by comparing to the mean areas of calibrated hydrogen pulses.Dispersion calculations were based on the assumption of a 1∶1 H∶Co stoichiometric ratio and a spherical cobalt cluster morphology.Temperature-programmed thermal analyses were performed on a TG-DTA92 analyzer.In general,samples of 0.1 g were used.
XPS was recorded on a XSAM-800 spectrometer using an Al-Kα X-ray source.The base pressure of the chamber was less than 2×10−8Pa.TPR experiments were carried out in a quartz microreactor heated by an electrical furnace.25 mg of catalyst was loaded and heated at a rate of 5 K/min to 1173 K with a gas consisting of 5% H2/N2.The gas flow rate was 50 mL/min.The H2consumption was measured by analysis of the effluent gas with a thermal conductivity detector (TCD) and calibrated by reduction of CuO powder in the usual way.
1.3 Catalytic measurements
The evaluation of catalysts was carried out in a pressured fixed-bed reactor with a catalyst loading of 2 mL.Prior to experiments,the sample Co3O4/SiO2were reduced in a flow of hydrogen at 673 K for 6 h.The other samples werein-situactivated as described in section 2.1 and then reduced in a flow of hydrogen at 673 K for 3 h.The reaction was carried out atp=2.0 MPa,GHSV=1000 h−1,H2/CO=2.0 andT=483 K.The products were collected with a hot trap and a cold trap in sequence.The tail gas was analyzed with online chromatography.The liquid and wax were analyzed with a GC-920 chromatograph equipped with OV-101 capillary columns.Both the carbon balance and mass balance of reaction were kept among (100 ±5)%.
2 Results and discussion
2.1 Physical-chemical properties of the catalysts
Specific surface area (BET),total pore volume(TPV) and average pore diameters of the catalysts are listed in Table 1.The impregnation of silica with cobalt nitrate led to the decrease in specific surface area and increase in TPV.The changes of three pretreated catalysts in the specific surface area,TPV and mean pore diameters indicated the texture changed during the pretreatment.It was primarily attributed to clogging of the pores of the support by cobalt species.After pretreatment, some Co3O4became metallic Co and cobalt species redispersed (shown in TEM,HRTEM and XRD results).

Table 1 Physico-chemical properties of the catalysts
The influence of pretreatments on the microstructure of the Co/SiO2catalyst is shown in Figure 1.TEM analysis showed that the cobalt phases did not dispersed homogeneously in the silica support but aggregated (Figure 1(a),(c)).Figure 1(c) showed a close-up of the interface between an aggregate of cobalt crystallites and the support.It could also be noted that the reduction-passivation (RP) pretreatment led to the redispersion of Co particles and increment in dispersion degree.This result was consistent with that of D.Potoczna-Petru[21].In Figure 1(b), The lattice fringes of 0.46 nm could correspond to the interplanar spacing of Co3O4.In Figure 1(d),the lattice fringes of 0.20 nm might be ascribed to the (111) planes of facecentered cubic (fcc) Co[21].The outer areas with differently oriented parallel fringes could be assigned to polycrystalline CoO.In this image, the epitaxial growth of the CoO phase on Co was visible with (111)CoO parallel to (111) Co.
XRD patterns of catalysts are shown in Figure 2.Diffraction peaks of 2θat 31.3°,36.9°,45.1°,59.4° and 65.4° indicated that after calcination,cobalt existed in the form of crystalline Co3O4spinel on the Co3O4/SiO2catalyst.After pretreatment,the diffraction peaks of Co3O4at 2θof 31.3°, 45.1°, 59.4° and 65.4°disappeared and only weak broadened and diffused peak at 36.9° was observed.Diffraction peak at 2θof 44.4° indicated the presence of dispersed metallic cobalt with fcc structure.The strongest characteristic peak of CoO at 42.6° was visible.For the pretreated catalysts,the half widths of the peaks and Co crystallite sizes was hard to be measured due to the low intensity of the diffraction peak.
Table 1 displays the Co dispersion and the estimated sizes of Co crystallites.Average crystallites sizes (nm) assuming spherical uniform crystallites with site density 14.6 atoms/nm2could be calculated from cobalt dispersion (D,%),by the use of formula:d=96/D[22,23].The resulting conversion factor for the diameter (d, nm) of a given Co3O4particle being reduced to metallic cobalt was:d(Co0) = 0.75×d(Co3O4)[22].A reasonable agreement between Co metal dispersion and crystallites sizes calculated from XRD and H2-TPD on cobalt supported silica catalysts was previously reported in the literatures[5,23].As can be seen form Table 1,the pretreatments affected metallic cobalt dispersion and crystallite size.With reductionpassivation pretreatment, cobalt dispersion increased from 5.9% to 10.6% while the crystallite size decreased from 16.3 to 9.1 nm.
To determine the effect of pretreatment methods on the cobalt species and dispersion in more detail,the results of XPS are presented in Figure 3 and Table 2.For Co/SiO2catalysts with different pretreatment,the shape of the XPS spectra were identical to that of the bulk Co3O4.The binding energy of the Co 2p3/2was 780 eV and the low intense shake-up satellite structure was observed on the high binding energy sides.Moreover,the difference in binding energy between Co3O4and CoO was very small.The concomitant difference of △E(15 eV for Co3O4,15.7 for CoO) and lineshape made it possible to distinguish between the two phases (shown in Table 2).Thus,Co3O4was likely to be the dominant phase existed in the outer layer of catalyst grains.Compared with XRD results for pretreated catalyst,it could be deduced that the metallic Co oxidized to Co3O4at the outer surface of catalyst grains during passivation.

Figure 1 TEM ((a),(c)) and HRTEM ((b),(d)) images of the catalysts:(a),(b):Co3 O4/SiO2;(c),(d):Co/SiO2-R

Figure 2 XRD patterns of the catalysts
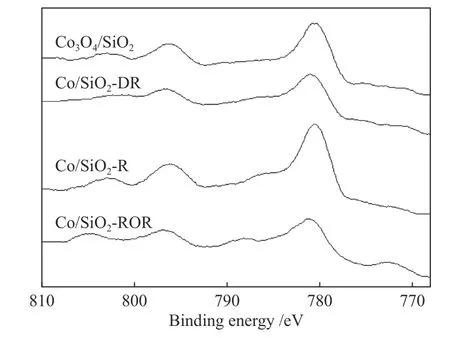
Figure 3 XPS spectra of the Co 2p level for catalysts
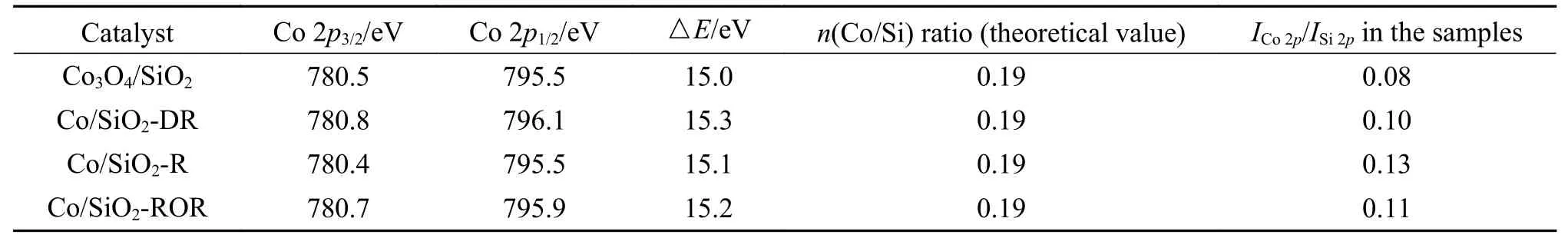
Table 2 Data from XPS characterization of the catalysts
The atomic ratios of the surface species (ICo 2p/ISi 2p)calculated from the relation proposed by Jongsomjit et al[24]are shown in Table 2.ICo 2p/ISi 2pcould provide information about relative amount of cobalt species on the outer surface.The Co/Si ratio of different catalysts were in the sequence of Co/SiO2-R >Co/SiO2-ROR >Co/SiO2-DR > Co3O4/SiO2.ICo 2p/ISi 2pis 0.08 for Co3O4/SiO2.Given the theoretical Co/Si ratio in the bulk (nCo/nSi) of 0.19,it could be inferred that silica migrated to the surface during calcination.HigherICo 2p/ISi 2pwere the characteristic of higher cobalt dispersion in the pretreated catalysts, which was consistent with the result of H2-TPD.Cobalt species tended to spread on the support surface during pretreatment,and new Co active surface species that could improve the FTS activity were formed.
TPR profiles of all samples are shown in Figure 4.By comparing the profiles of Co3O4/SiO2and the pretreated catalysts, it could be noted that H2consumption decrease drastically for the pretreated catalysts due to the presence of metallic Co (shown in Figure 1).According to previous report[25], for Co3O4/SiO2,low temperature peaks at ca.573 K could be attributed to partial reduction of Co3O4to CoO,whereas peaks at 573−673 K were assigned to the reduction of CoO to the Co metallic phase.The peak at 473 K in the TPR profile of Co3O4/SiO2were likely ascribed either to the reductive decomposition of residual cobalt nitrate or the reduction of CoO(OH)species,as suggested by Berge et al[26,27].

Figure 4 TPR profiles of the catalysts
For the pretreated catalyst,the reduction of Co3O4to Co occurred at ca.463 K,which was about 100 K lower than that of Co3O4/SiO2.On the one hand,the decrease in reduction temperature might be the result of hydrogen spillover.Matsuzaki et al[28]reported that the noble metals improved the reduction of cobalt oxide to metal effectively attributed to the spillover effect of hydrogen from noble metal.Similarly,it was inferred that the reduction of cobalt oxide species could be greatly promoted by spillover hydrogen activated on the cobalt metal from the pretreated catalysts.In addition to hydrogen spillover,it was more likely that the new structure of cobalt oxide on the surface of metallic cobalt formed by pretreatment was easier to be reduced than that of Co3O4grain.The second and third peaks (very broad and made up of multiple overlapping peaks) of the catalysts Co/SiO2-DR, R and ROR occurred between 673 and 1023 K were due to the reduction of Co species strongly interacting with the support[25].The TPR profiles of Co/SiO2-DR catalyst showed an obvious broad peak at 773 K which was ascribed to the reduction of Co2SiO4.The silicate might be produced from the CoO intermediate formed during the reduction according to the scheme of: CoO +SiO2→Co2SiO4.This reaction might start at temperatures as low as 623 K.
The reducibility of the catalysts from TG results is listed in Table 1.It could be seen that the pretreatments affected the cobalt reducibility.With reductionpassivation technique, catalyst reducibility increased from 78.2% to 85.6%.
2.2 Fischer-Tropsch synthesis activity
The catalytic performances of different catalysts for the F-T reaction are investigated and the results are listed in Table 3.Under the same reaction conditions,the FTS catalytic activity of the pretreated catalysts significantly increased from 20% to more than 40%,and the C5+selectivity also increased obviously.Especially for the catalyst Co/SiO2-R, a high C5+selectivity of up to 92% could be obtained with the lowest CH4selectivity (6.0%).The results revealed that the reconstruction of Co particles and the formation of new Co active species during pretreatment obviously promoted the catalytic activity of Co/SiO2for FTS.It had been reported that the activity in the FTS of the cobalt-based catalysts depended strongly on the amount of the cobalt atoms exposed on the surface[6].Kogelbauer et al[29]found that Ru enhanced the reducibility of Co/Al2O3by increasing the metallic cobalt sites,thus increased the overall catalytic activity.Similarly,in this case the increase of activity could be primarily due both to the more cobalt surface atoms as determined by XPS, H2-TPD and the increased reducibility (TG results).Khodakov et al[30]found that larger diameter of catalyst pores led to significantly higher C5+selectivity.The catalytic perfromance were interpreted of different cobalt particle size and reducibility in the wide pore and narrow pore silicas.Lower reducibility was likely to be one of the reasons responsible for the lower FTS activity and higher CH4selectivity.For this study, pretreatments led to an increase in pore size and reducibility (Table 1).The tendency for C5+and CH4selectivity could be explained by the effect of pore diameter and reducibility.

Table 3 Catalytic performance of the catalysts
Our study confirmed that redox treatment could improve the activity of the catalyst, which was consistent with the relevant literature reports.It was also found that the selectivity of CH4could be greatly reduced by optimizing the pretreatment procedure of the catalyst.Compared with DR and ROR,the yield of C5+product of the catalyst after reduction-passivation treatment was greatly increased,and the selectivity of C12+was significantly improved.
3 Conclusions
In this paper, three pretreated methods(DR, R,ROR) were employed and their influences on Co/SiO2microstructure and catalytic performance for FTS were investigated.The pretreatments significantly improved the FTS catalytic activity, C5+selectivity and depressed CH4selectivity,especially for the reduction-passivation technique.A restructuring and redispersion model was proposed to correlate the observed catalysts’ structure and catalytic activity.

