Dynamic changes of inducible nitric oxide synthase expression in rat’s retina and its role on blood-retinal barrier injury after acute high intraocular pressure
INTRODUCTION
Statistical Analysis GraphPad Prism 8 Software was used for statistical analysis of the data obtained from the Western blot and EB quantitative detection and for histogram drawing.Student’s unpaired
-test was performed on samples between Cont group and other groups,
<0.05 indicated that a difference was statistically significant.
Many diseases can cause retinal ischemia-hypoxia
, such as diabetic retinopathy, age-related macular degeneration, retinal detachment, and partial acute glaucoma. Hypoxia-ischemia damage to the BRB is one of the causes of the degeneration of retinal nerve cells
. The acute high intraocular pressure (IOP)animal model is an acute experimental glaucoma model
. The expression of hypoxia-inducible factor-1α (HIF-1α) increases in the retina after acute high IOP in rats and plays an important role in the destruction of the BRB
. Under hypoxic conditions,HIF-1α binds to HIF-1β to form a HIF-1α/β complex
.The complex binds to HIF-responsive elements (HREs)and triggers the transcription of more than 100 downstream genes
. The target genes regulated by HIF-1α mainly include erythropoietin (EPO), vascular endothelial growth factor(VEGF), heme oxygenase-1 (HO-1), adrenomedullin (ADM),glucose transporter-1 (GLUT-1), basic fibroblast growth factor (bFGF) and inducible nitric oxide synthase (iNOS)
.Among them, the cell signalling pathways of EPO
, VEGF
,and HO-1
have significant effects in mediating retinal neuroprotection. In experimental diabetic rats, EPO can protect the integrity of the BRB by inhibiting the downregulation of TJ proteins ZO-1 and occludin expression, especially in the outer BRB, thereby maintaining barrier function
. Although VEGF plays an important role in the physiological function of the retina, anti-VEGF drugs have been widely used in the treatment of BRB injury-related retinal diseases
.However, ADM
, GLUT-1
, bFGF and iNOS are rarely reported to have roles in retinal neuroprotection. At present, three NOS subtypes have been found in the central nervous system: NOS1 or neuronal NOS (nNOS), NOS2 or iNOS, and NOS3 or endothelial NOS (eNOS). The activity of iNOS is closely related to its expression level and is induced by cellular inflammation, while the activities of nNOS and eNOS depend on the level of intracellular Ca
. In summary,some downstream molecules of HIF-1α are involved in retinal neuroprotection. After acute high IOP, glial cell activation and iNOS expression are related to the severity of retinal and optic nerve damage
. Glial cells and iNOS may play an important role in the mechanism of acute high IOP ischemia-reperfusion, but the specific mechanism of action is still unclear. Subsequent experiments showed that in the rat glaucoma model, the expression of iNOS is upregulated 7d after high IOP and that glaucoma can cause redox imbalance by upregulating iNOS, thereby causing damage to the central nervous system
. However, the mechanism of action of iNOS during BRB injury in a rat model of acute high IOP is still unclear. Therefore, we designed an experiment to explore the expression of iNOS after acute high IOP in rats and to clarify its role in BRB injury.
MATERIALS AND METHODS
Dynamic Changes in Inducible Nitric Oxide Synthase and Zonula Occludens-1 Protein Expression After Acute High Intraocular Pressure in Rats After acute high IOP,the rat retinas were taken at different time points to detect the expression of iNOS and ZO-1 by Western blot. The results showed that iNOS began to increase at 3h after acute high IOP,reached a peak at 6h (
<0.05), and then iNOS was basically not expressed at 12, 24, 48, and 72h. The expression of ZO-1 at 3 and 6h had no difference compared with the normal group,and then the expression was downregulated at 12, 24, 48, and 72h (
<0.05; Figure 2).
Materials
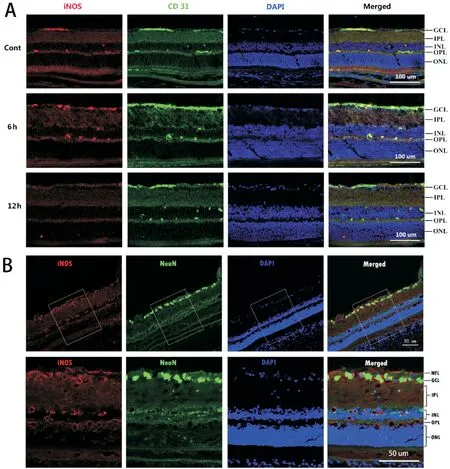
Previous studies have shown that the iNOS-induced expression of ganglion cell layer, inner plexiform layer and inner nuclear layer in the retina 5d after acute high IOP ischemiareperfusion is the most obvious
, which is consistent with the localization of iNOS expression in our experimental results, but the time course and peak value of expression were different. This may be related to the different processes used for making various animal models of acute high IOP. A previous study showed that iNOS is not expressed by retinal ganglion cells, which is consistent with our experimental results (Figure 1). Studies have shown that the regulation of iNOS subtypes occurs at the transcriptional level and is closely related to the neuroinflammatory process associated with glial cells
. Therefore, we speculate that the upregulation of iNOS expression in the early period (3h, 6h) after acute high IOP may be related to acute neuroinflammation during retinal ischemia and reperfusion in rats. Immunofluorescence staining and Western blotting results showed that iNOS expression was found in the Cont group, such expression may be induced by the stimulation of ischemia caused by saline perfusion. We found that iNOS is mainly expressed in the retinal nerve fiber layer, ganglion cell layer and inner nuclear layer after acute high IOP and is coexpressed with the vascular endothelial cell marker CD31, suggesting that iNOS is expressed in the vascular endothelial cells of the retina, which may be related to the structural integrity and functional maintenance of the BRB.
将“互联网+”理念与农产品销售相结合,有效地破解了传统模式下农产品销售的弊端和制约,扩宽了农产品的销售途径,提高了农产品的流通效率,刺激农产品消费潜力,进而增加农民的经济收入,推动相关服务业的发展。

Methods
Establishments of the acute high intraocular pressure model in rats The rats were anaesthetized by intraperitoneal injection of 10% chloral hydrate solution 3-5 mL/kg. After a few minutes, the rats were pinched to determine whether the anesthesia was good. If there was no response, the anesthesia was considered acceptable. The rat was placed on a Jiangwan Type I locator (Second Military Medical University), the upper teeth of the rat were hung with an iron block, and the limbs were tied and fixed with cotton thread with a slipknot.Chloramphenicol Eye Drops were applied to the eyes of the rats to inhibit bacteria, Proparacaine Hydrochloride Eye Drops(Alcaine Company, Imported Drug Registration Number:H20160133) were used as eye analgesics, and Compound Tropicamide Eye Drops (Univision Pharmaceutical, National Medicine Standard: H20066782) were used as a mydriatic agent. After pupil dilation was completed, the infusion device was adjusted to make the needle droplets drip out slowly.Then, we inserted the needle from the junction between the cornea and the conjunctiva into the anterior chamber of the eye. The insertion is successful if whitening of the iris and no fluid flows from the eyes
. After the needle insertion, the scalp needle was fixed. We moved the saline bottle up by 0.3 m every 2min until it reached 1.5 m, and started timing for 1h. To prevent the rats from moving, 0.1-0.2 mL of chloral and eye analgesics were given every 30min as appropriate.After 1h, the 0.3 m was moved down every 2min until it was parallel to the rat. After 2min, the needle was placed in parallel to complete the modelling. The rats were placed flat for 10min and chloramphenicol eye drops were applied to the eyes of the rats and then they were returned to their cages.In the process of making high IOP model in rats, the exclusion criteria were to exclude the animal model with leakage of normal saline at the insertion needle of the anterior chamber and the animal model with corneal ulcer or cataract after making the model.
皇上说,胡人犯境,夺占城池,成千上万的老百姓没处去,就投奔秀容元帅,秀容元帅把那些钱拿出来,盖房子,买地,买牛,买水磨,让他们都过上好日子,这是好事。
大数据跟我们每个人相关,但我们绝大多数人其实并不掌握大数据,当然也不能从中直接获益。大数据掌握在极少数的机构手里,掌握在腾讯、阿里、百度等大公司手里。我们每个身处互联网的人其实不过扮演了大数据采集节点供应器的角色,让自己的数据汇入大数据的洪流之中,但我们闹得再欢腾,却也不过仅仅如此而已。我们绝大多数的个体并不是大数据宴会的真正拥有者,我们只是大数据的贡献者甚至是牺牲者。
Tissue preparation The rat model of acute high IOP was established, and the rats were anaesthetized at each corresponding time point. The rat was infused with 200 mL saline preheated to 37°C in a water bath, rapidly perfused with 200 mL 4% paraformaldehyde, and then slowly perfused with 200 mL 4% paraformaldehyde. Then, the eyes were dissected,the cornea, lens, and vitreous were removed, and the eyeballs were made into optic cups. The optic cup was dehydrated with gradient sucrose solution and embedded with Tissue-Tek optimum cutting temperature (O.C.T.) compound (SAKURA,Japan), and sliced on an automatically frozen slicer (Thermo,USA). The slices were 10 μm thick and placed at room temperature for 30min. After the sample was firmly attached to the slide, it was placed in a refrigerator at 4°C for storage until use. The samples used for Western blot were obtained from rats that were anaesthetized immediately after making the rat model of acute intraocular hypertension and from those at the corresponding time points. The eyes were dissected, the cornea, lens and vitreous were removed, and the retina was scraped off. It was placed in a prechilled tube and frozen in
liquid nitrogen, then stored at -80℃ until use.Immunofluorescence The slices are taken out of the fridge and left at room temperature to dry. After the sections were blocked with a mixture of 5% donkey serum and 0.3%Triton X-100 prepared in 1×phosphate buffer saline (PBS),the primary antibody (Table 1) was added and incubated overnight in a refrigerator at 4°C. After washing with PBS for 3×5min, the sample and the secondary antibody (Table 1)were incubated at 37℃ under dark conditions for 1h. After washing with PBS for 3×5min, the slides were mounted with an anti-fluorescence quencher containing 4’,6-diamidino-2-phenylindole (DAPI). The slides were observed and images were taken under a laser confocal microscope (Olympus FV1000).
Western blot After extracting the retinal proteins, 60 μL of total protein was tested with a BCA protein assay kit (Thermo)to determine the protein content, and the remaining protein was stored at -80°C until use. SDS-PAGE electrophoresis was performed with 40 μg protein in each well. The voltage was set to 80 V until the loading buffer reached the boundary between the separation gel and the stacking gel (approximately 25min) and then changed to 120 V electrophoresis to separate the proteins for 60min. After the electrophoresis, the protein was transferred to a nitrocellulose membrane under a current of 330 mA for 90min. The membrane was placed in 5% skimmed milk powder prepared with TBST, blocked at room temperature for 2h, and washed with TBST for 3×10min.Then, the membrane and the corresponding specific primary antibody were diluted in TBST and incubated overnight at 4°C.The membrane was washed again with TBST for 3×10min and incubated with the diluted secondary antibody for 90min at room temperature to detect the antibody-antigen complexes,and the protein bands were visualized by chemiluminescence with an ultrasensitive ECL chemiluminescence kit (Beyotime).β-actin were used as standard proteins, and iNOS and ZO-1 were used as detection proteins (Table 1). Image J was used to calculate the ratio of iNOS or ZO-1 to the gray value of the standard protein, which represents the protein level of iNOS or ZO-1.
Drug intervention Rats in the Cont+Inh group were given 1 mg/100 g 1400W prepared with 10% dimethyl sulfoxide(DMSO) and 90% [20% sulfobutylether-β-cyclodextrin(SBE-β-CD) in saline] intraperitoneal injection
1d before sampling. Rats in the 6h+Inh group were intraperitoneally injected with 10% DMSO and 90% (20% SBE-β-CD in saline)1 mg/100 g 1400W 1d before the establishment of the acute high IOP model, while the rats in the Cont group and 6h group were intraperitoneally injected with 0.9% normal saline.
中新生代大型推(滑)覆构造前锋带波及全区,并与北北东向走滑冲断带复合,沿深断裂带为晚侏罗世-早白垩世中酸性斑岩带,属多层次大型构造叠置极其优越而独特的成矿地质环境,具有丰富的矿质来源(矿源层)、反复叠加富集的多种热流场和成矿异常复合构造,有着巨大的铜多金属矿资源潜力。
Evans blue detection of the blood-retinal barrier damage Rats in Cont group, Cont+Inh group, 6h group and 6h+Inh group were anaesthetized by 10% chloral hydrate injection in the abdominal cavity and 3% EB (4.5 mg/100 g; Sigma,E2129) injection in the great saphenous vein before death.After injection, the eyes and toes of rats turned blue. At the corresponding time point, the rats were immediately put on ice to take eyeballs, the cornea was cut off, the lens and vitreous were dugout, and then placed in 4% paraformaldehyde under the condition of light protection for 30min
. Then the retina was covered and observed by fluorescence microscope(Olympus IX51). In quantitative EB detection, 1 μL EB (2%)was diluted 1000 times with 1 mL methylphthalide to a final concentration of 20 ng/μL, followed by 7 times of half-dilution and a total of 8 standard tubes of methylphthalide blank tubes for standard curve preparation of EB in methylphthalide
.The retina was removed, dried naturally and weighed, then mixed with 160 μL formamide in an EP tube and incubated at 60 ℃for 24h. Then the extract was centrifuged at a high speed of 15 294 g at 4℃ for 30min. The supernatant of the standard tube and sample tube was measured by spectrophotometer at wavelength 620 nm. Dye concentration is calculated according to the standard curve in methylphthalamide. EB (ng) content was standardized with retinal dry weight (mg) and was expressed as ng/mg.
G laucoma is the second most common irreversible blinding eye disease in the world. In recent years,many studies have focused on the death of retinal ganglion cells
. The survival of retinal neural cells is closely related to the surrounding microenvironment. The blood-retinal barrier (BRB) is the key to maintaining the homeostasis of the retinal microenvironment
. The BRB consists of two different barriers: the outer layer BRB (oBRB), which is comprised of retinal pigment epithelial cells, regulating the transport between the choroidal capillaries and the retina;and the inner layer BRB (iBRB), which regulates transport between retinal capillaries
. The iBRB is not an absolute barrier because substances in the blood can pass through it through two different mechanisms, vesicle-mediated transport(transcellular) and paracellular transport
. Paracellular transport is strictly dependent on tight junctions (TJs), such as claudins and zonula occludens (ZO); adhesion junctions (AJs),such as vascular endothelial (VE) cadherin; and gap junctions(GJs), such as junction protein 43 (Cx43). Studies have shown that ZO-1 is a sign of BRB integrity, and the loss or decrease of ZO-1 is related to the increase in barrier permeability
.
RESULTS
Localization of Inducible Nitric Oxide Synthase in the Retina After Acute High Intraocular Pressure The positive expressions of iNOS were observed in the retina of the Cont group and the 6h group by immunofluorescence double staining, but not in the 12h group. The expressions of iNOS were mainly located in the retinal nerve fiber layer, ganglion cell layer and inner nuclear layer; it was co-expressed with the vascular endothelial cell marker CD31 and not with the retinal ganglion cell marker NeuN (Figure 1).
Ethical Approval We adhered to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Blood-retinal Barrier Leakage at Different Time Points After Acute High Intraocular Pressure in Rats After EB was injected, the retinas were taken and the EB was used to quantitatively detect the BRB damage. The results showed that the leakage of EB began to increase at 3h after the induction of acute high IOP but there was little leakage at 6h, and there was no difference compared with the Cont group (
>0.05). The amount of EB leakage was the highest at 12h and it gradually decreased thereafter, but it was still greater than that of the Cont group (
<0.05; Figure 3).
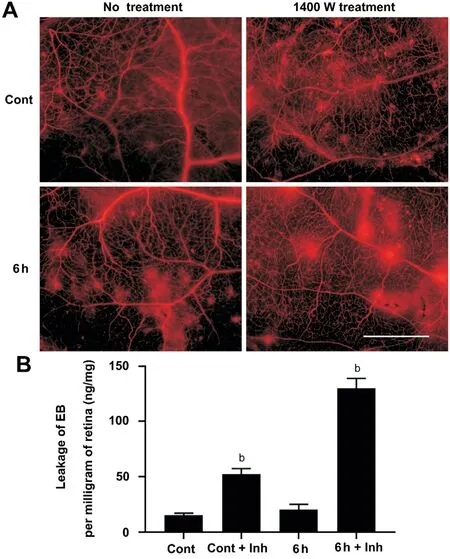
Verify the Role of Inducible Nitric Oxide Synthase in Blood-Retinal Barrier Injury Western blotting was performed to detect the expression of iNOS and ZO-1 in the rat retinas of the Cont group, Cont+Inh group, 6h group and 6h+Inh group. The results showed that the expression of iNOS was up-regulated in the 6h group, but not in the Cont+Inh group and 6h+Inh group. The expression of ZO-1 was not significantly changed in the Cont+Inh group and the 6h group, and was significantly down-regulated in the 6h+Inh group (Figure 4). The rats in the Cont group, Cont+Inh group,6h group and 6h+Inh group were injected with EB, and the rat retina was used to do the whole retina mounted and to quantitatively detect BRB leakage. The results showed that no red EB fluorescent spots were observed outside blood vessels in the Cont group, but such fluorescent spots can be observed outside blood vessels after the addition of inhibitors. A little amount of red EB fluorescent spots could be seen in the 6h group outside blood vessels, while the inhibitor treatment made obviously leakage of red EB fluorescent in the 6h+Inh group.Quantitative results showed that the amount of EB leakage increased in the Cont+Inh group and significantly increased in the 6h+Inh group (
<0.001; Figure 5).


DISCUSSION
This experiment showed that iNOS was mainly expressed in the retinal nerve fiber layer, ganglion cell layer and inner nuclear layer after acute high IOP, and it is co-expressed with the vascular endothelial cell marker CD31. In the early period (3h, 6h) after acute high IOP, the expression of iNOS was upregulated, then the down-regulation of iNOS were tested in the follow-up timing spots. ZO-1 expression showed a continuous down-regulation after 6h. The quantitative results of EB showed that there was obvious damage to the BRB after acute high IOP, but at 6h, there was very little EB leakage. To verify the role of iNOS in the early upregulation of acute high IOP in BRB injury, we used an iNOS-specific inhibitor (1400 W) and found that the expression of iNOS and ZO-1 was downregulated, and the amount of EB leakage was increased after the inhibitor treatment. These results suggest that the early upregulation of iNOS after acute high IOP may protect against BRB injury.

Experimental animals and groups The inclusion criteria for experimental animals were healthy SD rats with a bodyweight of 200-250 g and good growth and development.The animals were purchased from Hunan SJA laboratory animal Co., Ltd. and Haikou Yushi Biotechnology Co., Ltd.During the experiment, the rats were housed 2-3 in each cage under standard conditions and provided with standard food and water. Animal certificate numbers: 1107262011000875,430727211101955725 and 430727211102090613. The expression of iNOS was detected by immunofluorescence localization method, the expression of iNOS protein after high IOP was detected by Western blot, and the damage of the BRB in the retina at different periods of acute high IOP was quantitatively detected by Evans Blue (EB) quantitative detection, and then iNOS specific inhibitor 1400W was used to study the role of iNOS in BRB injury. Nine rats were divided into control (Cont) group, 6h group, and 12h group by immunofluorescence double-standard staining; 21 rats were used for Western blot, and the animals were randomly divided into Cont, 3h, 6h, 12h, 1d, 2d, and 3d groups, with 3 rats in each group; 21 rats were used for EB quantitative detection,and the grouping was the same as that of Western blot. Twelve rats were used for Western blot and 24 rats were used for EB detection after treatment with 1400W (Med Chem Express), a specific inhibitor of iNOS. The animals were randomly divided into Cont group, Cont+inhibitor (Inh) group, 6h group and 6h+Inh group, with 3 rats in each group. The antibodies used in this study were showed in Table 1.
应用SPSS 19.0统计软件进行数据分析。计量资料(均数±标准差)表示,计量资料采用t检验,计数资料比率表示,x2检验,(P<0.05)表示数据之间的差异具有统计学意义。
The BRB mainly consists of an iBRB and an oBRB. The iBRB is made up of retinal capillary endothelial cells and their TJs,supported by pericytes, Müller cells, and astrocyte effects
.Claudin is involved in the formation of the BRB, and ZO-1 participates in barrier assembly through interactions with transmembrane proteins
. It has been shown that the loss of ZO-1, the TJ to the actin cytoskeleton, leads to the destruction of the iBRB
. Therefore, the expression of the TJ protein ZO-1 is an index reflecting the degree of damage to the BRB.Our results show that the BRB is significantly damaged after acute high IOP, which is manifested as a significant increase in EB leakage and downregulation of ZO-1 expression
. This is consistent with the results of our previous studies. However,we noticed that EB leakage increased at 3h after acute high IOP, while ZO-1 expression was stable. As two indicators of BRB injury, our experimental results (Figures 2 and 3) showed that was not synchronous. EB leakage began to increase at 3h after acute high IOP, while ZO-1 was significantly downregulated at 12h after acute high IOP, with time delay in reflecting BRB injury. The reasons for this phenomenon still need to be further studied and determined. At 6h after acute high IOP, EB leakage was not obvious, while iNOS was the most expressed at this time. We speculate that iNOS works at this time to protect BRB from damage. This was confirmed by our experiments (Figures 4 and 5). Among the current iNOS inhibitors, the small molecular 1400W has high specificity and effectiveness, because it does not affect the biological activity of eNOS and nNOS, and can penetrate the blood-brain barrier(BBB)
. Inhibition of iNOS activity by a specific iNOS inhibitor 1400W resulted in more severe EB leakage and more obvious down-regulation of ZO-1 expression, suggesting that the up-regulation of iNOS in the early stage after acute high IOP may play a protective role in BRB injury. We must admit that this study lacks data on the localization and expression of iNOS in other cells of the retina (such as retinal pericytes,Müller cells, and astrocytes), and there is no data on the localization and expression of the three subtypes of NOS.Second, there are many TJ proteins related to the BRB, such as claudins, occludins, VE-cadherin and Cx43. They may also play an important role in the process of acute high IOP causing BRB damage, but there is a lack of data on other TJ proteins in this study. Studies have shown
that in the BRB model
, high glucose induced BRB damage and increased permeability, accompanied by a decrease in connexins such as ZO-1 and VE-cadherin, and an increase in iNOS levels. The results were basically consistent with our study, but the study did not clarify the role of iNOS in BRB destruction. Previous studies
believed that iNOS is one of the pro-inflammatory mediators, and iNOS mediated NO generation is related to the induction of early vascular changes
, which may play a proinflammatory role in the process of BRB injury and promote or aggravate the damage of BRB. However, our study showed that in the early stage of BRB injury induced by high IOP,the up-regulation of iNOS induced expression could protect BRB from damage. In addition, the expression of iNOS was downregulated at 24h and 72h after acute high IOP, while the BRB was still damaged, showing an increase in EB leakage(peaked at 12h) and a downregulation of ZO-1 expression,indicating that iNOS did not play a major role in BRB injury in the later stage of acute ocular hypertension. We speculate that it may be that other downstream molecules of HIF-1α, such as GLUT-1
, act on BRB damage or those other molecules are involved in this process. Therefore, we still need to identify the specific molecular mechanism of BRB damage in the late stage of acute high IOP.
河西学院开展扎实有效的职前专题训练活动,强化学生教学技能培养。援疆实习支教前,学校聘请当地、新疆中小学优秀教师开展专题性质的中学教学法和民族语言、民俗民风等环节的实践培训工作;组织实地走进中小学课堂观摩各学科教学见习活动。通过观模名师课堂、微格教学和小组研讨等形式,要求学生分析教材、撰写教案、教学设计、试讲演练,实行职前训练不过关不达标不准参加实习支教工作的硬性指标。针对师范生专业技能要点,通过职前训练,使学生有备而战,帮助实习支教学生尽快适应岗位角色[11],大大缩短心理准备期和工作适应期,确保实习支教工作的有序开展。
In summary, our study showed that iNOS is expressed in retinal vascular endothelial cells after acute high IOP and that its early upregulation may have a protective effect on BRB injury. This reveals a possible molecular mechanism of early BRB protection after acute high IOP in rats. These results are of great significance for understanding the pathogenesis of ischemic-hypoxic retinal diseases.
ACKNOWLEDGEMENTS
Authors’ contributions: Li M: Writing original draft, investigation,methodology; Huang JF: Supervision, resources; Li Y: Investigation,methodology; Shen J: Resources, conceptualization; Yang LJ: Investigation; Chen Q: Investigation; Zhang QP: Review& editing, supervision, project administration, funding acquisition, conceptualization, validation; Yi XN: Review &editing, supervision, conceptualization.
Supported by the National Natural Science Foundation Project (No.81660217); National College Students Innovation and Entrepreneurship Training Program Project(No.201911810004).
Conflicts of Interest: Li M, None; Huang JF, None; Li Y,None; Shen J, None; Yang LJ, None; Chen Q, None; Zhang QP, None; Yi XN, None.
1 Russo R, Varano GP, Adornetto A, Nucci C, Corasaniti MT, Bagetta G,Morrone LA. Retinal ganglion cell death in glaucoma: exploring the role of neuroinflammation.
2016;787:134-142.
2 Campbell M, Humphries P. The blood-retina barrier: tight junctions and barrier modulation.
2012;763:70-84.
3 Díaz-Coránguez M, Ramos C, Antonetti DA. The inner blood-retinal barrier: cellular basis and development.
2017;139:123-137.
4 Muthusamy A, Lin CM, Shanmugam S, Lindner HM, Abcouwer SF, Antonetti DA. Ischemia-reperfusion injury induces occludin phosphorylation/ubiquitination and retinal vascular permeability in a VEGFR-2-dependent manner.
2014;34(3):522-531.
5 Kaur C, Foulds WS, Ling EG. Hypoxia-ischemia and retinal ganglion cell damage.
2008;2(4):879-889.
6 Kerr NM, Johnson CS, Zhang J, Eady EK, Green CR, Danesh-Meyer HV. High pressure-induced retinal ischaemia reperfusion causes upregulation of gap junction protein connexin43 prior to retinal ganglion cell loss.
2012;234(1):144-152.
7 Huang JF, Zhang QP, Tong JB,
. Upregulation of hypoxia-inducible factor-lα in rat retina following acute highintraocular pressure promotes the blood-retinal barrier damage.
2013;36(1):61-64.
8 Cheng L, Yu HH, Yan NH, Lai KB, Xiang MQ. Hypoxia-inducible factor-1α target genes contribute to retinal neuroprotection.
2017;11:20.
9 Resende AP, Rosolen SG, Nunes T, São Braz B, Delgado E. Functional and structural effects of erythropoietin subconjunctival administration in glaucomatous animals.
2018;3(2):1-11.
10 Foxton RH, Finkelstein A, Vijay S, Dahlmann-Noor A, Khaw PT,Morgan JE, Shima DT, Ng YS. VEGF-A is necessary and sufficient for retinal neuroprotection in models of experimental glaucoma.
2013;182(4):1379-1390.
11 Lu LL, Seidel CP, Iwase T, Stevens RK, Gong YY, Wang XY, Hackett SF, Campochiaro PA. Suppression of GLUT1; a new strategy to prevent diabetic complications.
2013;228(2):251-257.
12 Wang X, Yuan ZL. Activation of Nrf2/HO-1 pathway protects retinal ganglion cells from a rat chronic ocular hypertension model of glaucoma.
2019;39(10):2303-2312.
13 Zhang CY, Xie H, Yang Q, Yang YT, Li WY, Tian HB, Lu LX,Wang F, Xu JY, Gao FR, Wang J, Jin CX, Xu GX, Xu GT, Zhang JF.Erythropoietin protects outer blood-retinal barrier in experimental diabetic retinopathy by up-regulating ZO-1 and occludin.
2019;47(9):1182-1197.
14 Le YZ. VEGF production and signaling in Müller glia are critical to modulating vascular function and neuronal integrity in diabetic retinopathy and hypoxic retinal vascular diseases.
2017;139:108-114.
15 Blom J, Giove TJ, Pong WW, Blute TA, Eldred WD. Evidence for a functional adrenomedullin signaling pathway in the mouse retina.
2012;18:1339-1353.
16 Toft-Kehler AK, Skytt DM, Poulsen KA, Brændstrup CT, Gegelashvili G, Waagepetersen H, Kolko M. Limited energy supply in Müller cells alters glutamate uptake.
2014;39(5):941-949.
17 Yuste JE, Tarragon E, Campuzano CM, Ros-Bernal F. Implications of glial nitric oxide in neurodegenerative diseases.
2015;9:322.
18 Cho KJ, Kim JH, Park HY, Park CK. Glial cell response and iNOS expression in the optic nerve head and retina of the rat following acute high IOP ischemia-reperfusion.
2011;1403:67-77.
19 Hvozda Arana AG, Lasagni Vitar RM, Reides CG, Lerner SF, Ferreira SM. Glaucoma causes redox imbalance in the primary visual cortex by modulating NADPH oxidase-4, iNOS, and Nrf2 pathway in a rat experimental model.
2020;200:108225.
20 Yan H, Peng YL, Huang W, Gong LY, Li L. The protective effects of αB-crystallin on ischemia-reperfusion injury in the rat retina.
2017;2017:7205408.
21 Du R, Wang X, He SG. BDNF improves axon transportation and rescues visual function in a rodent model of acute elevation of intraocular pressure.
2020;63(9):1337-1346.
22 Sedaghat Z, Kadkhodaee M, Seifi B, Salehi E. Inducible and endothelial nitric oxide synthase distribution and expression with hind limb per-conditioning of the rat kidney.
2019;15(4):1081-1091.
23 Shi KP, Li YT, Huang CX, Cai CS, Zhu YJ, Wang L, Zhu XB.Evans blue staining to detect deep blood vessels in peripheral retina for observing retinal pathology in early-stage diabetic rats.
2021;14(10):1501-1507.
24 Fang MY, Wan WC, Li QM, Wan WW, Long Y, Liu HZ, Yang X.Asiatic acid attenuates diabetic retinopathy through TLR4/MyD88/NF-κB p65 mediated modulation of microglia polarization.
2021;277:119567.
25 Leal EC, Martins J, Voabil P, Liberal J, Chiavaroli C, Bauer J, Cunha-Vaz J, Ambrósio AF. Calcium dobesilate inhibits the alterations in tight junction proteins and leukocyte adhesion to retinal endothelial cells induced by diabetes.
2010;59(10):2637-2645.
26 Shi QH, Liu X, Wang N, Zheng XC, Ran JH, Liu ZX, Fu JF, Zheng J.1400W ameliorates acute hypobaric hypoxia/reoxygenation-induced cognitive deficits by suppressing the induction of inducible nitric oxide synthase in rat cerebral cortex microglia.
2017;319:188-199.
27 Fresta CG, Fidilio A, Caruso G, Caraci F, Giblin FJ, Leggio GM,Salomone S, Drago F, Bucolo C. A new human blood-retinal barrier model based on endothelial cells, pericytes, and astrocytes.
2020;21(5):1636.
28 Eo HJ, Jang JH, Park GH. Anti-inflammatory effects of berchemia floribunda in LPS-stimulated RAW264.7 cells through regulation of NF-κB and MAPKs signaling pathway.
(
) 2021;10(3):586.
29 Leal EC, Manivannan A, Hosoya K, Terasaki T, Cunha-Vaz J,Ambrósio AF, Forrester JV. Inducible nitric oxide synthase isoform is a key mediator of leukostasis and blood-retinal barrier breakdown in diabetic retinopathy.
2007;48(11):5257-5265.
30 Zhang TY, Ouyang H, Mei XY, Lu B, Yu ZY, Chen KX, Wang ZT, Ji LL. Erianin alleviates diabetic retinopathy by reducing retinal inflammation initiated by microglial cells
inhibiting hyperglycemia-mediated ERK1/2-NF-κB signaling pathway.
2019;33(11):11776-11790.
 International Journal of Ophthalmology2022年7期
International Journal of Ophthalmology2022年7期
- International Journal of Ophthalmology的其它文章
- Impact of OCT scan-patterns in identifying morphological features of lamellar macular holes and macular pseudoholes
- Virtual reality training improves accommodative facility and accommodative range
- Short-term effect of 0.01% atropine sulphate eye gel on myopia progression in children
- Reduced choroidal peripapillary capillaries in thyroidassociated ophthalmopathy with early stage of dysthyroid optic neuropathy
- Incidence of ocular manifestations in patients with graft versus host disease after allogeneic stem cell transplant in Riyadh, Saudi Arabia
- Clinical features, surgical outcomes and genetic analysis of ectodermal dysplasia with ocular diseases
