PCI后损伤血管再内皮化的机制与治疗研究进展
刘玉莹 杨斌
【摘要】晚期支架内血栓形成是经皮冠状动脉介入治疗(PCI)后主要的并发症,严重影响了冠状动脉粥样硬化性心脏病患者的预后,带来不可逆转的危害。PCI后损伤血管再内皮化可减少晚期支架内血栓的形成,再内皮化的机制主要包括内皮细胞黏附和增殖的调控、平滑肌细胞黏附和增殖的调控、血小板黏附聚集和活化以及纤维蛋白原的吸附。对冠状动脉粥样硬化性心脏病患者血管损伤再内皮化机制的研究,可为损伤后血管重塑提供新的思路和治疗靶点,有利于提升PCI患者的预后。现就PCI后损伤血管再内皮化的相关机制和治疗进行综述。
【关键词】经皮冠状动脉介入治疗;晚期支架内血栓形成;血管内皮生长因子;RNA结合蛋白;药物洗脱支架
【DOI】10.16806/j.cnki.issn.1004-3934.2024.03.013
Mechanism and Therapy of Reendothelialization of Injured Vessels After Percutaneous Coronary Intervention
LIU Yuying,YANG Bin
(The Affiliated Hospital of Qingdao University,Qingdao 266000,Shandong,China)
【Abstract】Late stent thrombosis is a major complication of percutaneous coronary intervention (PCI) therapy,which seriously affects the prognosis of patients with atherosclerotic cardiovascular disease and causes irreversible harm.Reendothelialization of damaged vessels after PCI therapy is available to reduce late stent thrombosis.The mechanisms of reendothelialization mainly include the adhesion and proliferation of endothelial cell,the adhesion and proliferation of smooth muscle cell,the adhesive aggregation and activation of platelet,and the absorption of fibrinogen.The study on the mechanism of reendothelialization of vascular injury in patients with atherosclerotic cardiovascular disease provides new views and therapeutic targets for vascular remodeling after injury,which is conducive to improving the prognosis of patients undergoing PCI.This article reviews the relevant mechanism and therapy of reendothelialization of injured vessels after PCI.
【Keywords】Percutaneous coronary intervention;Late stent thrombosis;Vascular endothelial growth factor;RNA binding protein;Drug eluting stent
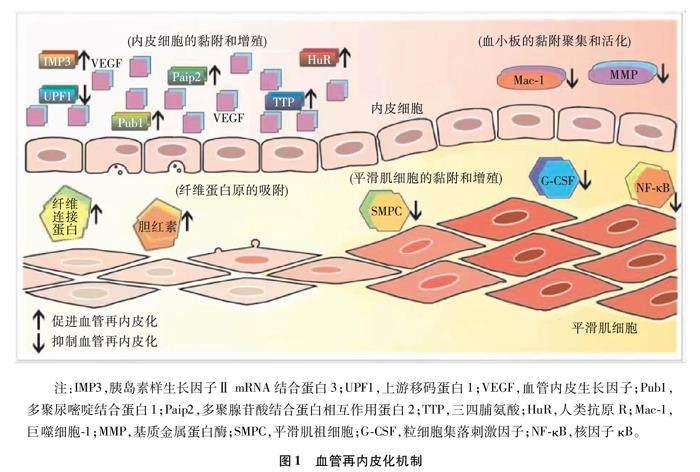
經皮冠状动脉介入治疗(percutaneous coronary intervention,PCI)是一种重建狭窄或闭塞冠状动脉血管的方法,因其简单有效的手术方式经常被用于冠状动脉粥样硬化性心脏病的治疗,显著降低了冠状动脉粥样硬化性心脏病的致病率和死亡率[1]。然而,PCI后出现的一系列并发症严重影响了患者的预后,其中最主要的并发症是支架植入30 d后发生的晚期支架内血栓形成(late stent thrombosis,LST)[2-3]。LST的主要病理机制是以不完全再内皮化和纤维蛋白持续存在为特征的动脉延迟愈合,支架缺乏内膜覆盖和洗脱支架的不均匀愈合都会增加血栓形成的风险[4-5]。支架本身的性质如支架贴壁不良、支架的钢梁过粗和支架的持久耐用性差会导致损伤血管再内皮化延迟,影响患者的预后[6-7]。除了这些外源性因素,一些内源性机制也影响支架植入患者的血管损伤再内皮化进程。如图1所示,血管再内皮化的4种内源性调控机制包括内皮细胞(endothelial cell,EC) 黏附和增殖的调控、平滑肌细胞(smooth muscle cell,SMC) 黏附和增殖的调控、血小板的黏附聚集和活化以及纤维蛋白原的吸附[8-9]。基于损伤血管再内皮化的机制,一些新型药物洗脱支架(drug eluting stents,DES)被发展和改进,极大地改善了PCI患者的预后。
1 损伤血管的再内皮化机制
1.1 EC的黏附和增殖
EC的完整性和功能活动在PCI后预防血栓中发挥重要作用,支架植入引起EC严重抑制,导致损伤血管的再内皮化延迟。这一过程主要由血管内皮生长因子(vascular endothelial growth factor,VEGF)介导[10-11],而RNA结合蛋白(RNA binding protein,RBP)在调节VEGF的生物合成、转运和翻译中发挥关键作用。
1.1.1 人类抗原R
人类抗原R(human antigen R,HuR)能通过结合位于3非翻译区(3untranslated region,3UTR)调控VEGF的表达,刺激血管生成[12]。HuR能抑制内皮型一氧化氮合酶的表达并稳定细胞间黏附分子-1和血管细胞黏附分子-1来促进内皮活化,进而实现血管再内皮化[13]。
1.1.2 胰岛素样生长因子ⅡmRNA结合蛋白3
胰岛素样生长因子ⅡmRNA结合蛋白(insulin like growth factor Ⅱ mRNA binding protein,IMP)3是IMP家族的一种新型RBP,在EC的生长和迁移中发挥关键作用[14]。IMP3可通过调节胰岛素样生长因子结合蛋白激活下游PI3K/MAPK信号通路来促进VEGF的表达,有利于损伤血管的再内皮化[15]。
1.1.3 上游移码蛋白1
上游移码蛋白1(up-frameshift protein 1,UPF1)作为一种VEGF的RBP,UPF1是无义介导的mRNA降解途径的关键蛋白,拮抗VEGF转录本的翻译[16]。UPF1与SMG-1-UPF1-eRF1-eRF3复合体结合,导致UPF1磷酸化,驱动VEGF下调,导致血管损伤的再内皮化进程的抑制[17]。
1.1.4 多聚尿嘧啶结合蛋白1
多聚尿嘧啶结合蛋白1[poly(U)-binding protein 1,Pub1]是VEGF的一种细胞聚腺苷酸化RBP,在血管损伤的再内皮化中发挥重要作用。Pub1通过与EC转录本的5非翻译区中稳定作用元件结合,并阻止了含有上游开放阅读框的VEGF降解,实现了VEGF的稳定和更新,促进了血管损伤的再内皮化。
1.1.5 三四脯氨酸
三四脯氨酸( tristetraprolin,TTP) 是一种早期反应蛋白,在VEGF的mRNA降解中起到关键作用,主要通过招募外泌体PM-Scl75和Rrp4到VEGF的mRNA来诱导VEGF衰变,抑制损伤血管的再内皮化[18]。此外,TTP通过TZF结构域与VEGF mRNA中的3UTR实现最佳结合,阻止VEGF mRNA的翻译并降低其稳定性,减少VEGF的生成,阻碍再内皮化進程[19]。
1.1.6 多聚腺苷酸结合蛋白相互作用蛋白2
多聚腺苷酸结合蛋白相互作用蛋白2[poly (A)-binding protein-interacting protein 2,Paip2]可结合VEGF中3UTR的特定区域,延长了VEGF中3UTR的半衰期,进而稳定VEGF,导致VEGF表达增加。此外,Paip2作为一种VEGF mRNA表达的调控因子,还能与HuR相互作用,协同稳定VEGF mRNA。总之,Paip2可通过多种通道稳定VEGF的表达,促进损伤血管的再内皮化。
1.2 SMC的黏附和增殖
SMC的增殖和表型改变是新生内膜增生最重要的病理机制。支架植入后,内膜被损坏,许多细胞因子和生长因子合成和释放。PCI后损伤血管的细胞内环境也发生了改变,影响了细胞内的信号转导,这些信号会控制血管SMC的增殖、迁移,最终会导致新生内膜增生。
1.2.1 平滑肌祖细胞
平滑肌祖细胞(smooth muscle progenitor cell,SMPC)一般由血管祖细胞分化而来,TGF-β1/TGF-β受体Ⅱ-ALK5/Smad2信号通路介导血管祖细胞向SMPC表型转变[20]。随后,SMPC特异性标志物的上调会导致细胞骨架重排和收缩表型的出现,大量积累的SMPC在PCI后血管机械性损伤的LST中起着关键作用。而血管损伤后炎症的发生可刺激SMPC发生动员,向SMC分化。SMC参与新生内膜形成,引起血管再内皮化延迟。
1.2.2 粒细胞集落刺激因子
粒细胞集落刺激因子(granulocyte colony-stimulating factor,G-CSF) 可促进细胞活力,通过影响血管生成调控PCI后损伤血管的再内皮化[21]。由于G-CSF能在蛋白激酶p44/42 MAPK、Akt和S6激酶信号转导下促进SMC的激活和迁移,所以G-CSF在治疗PCI后LST中并未起到很好的疗效[22]。因此,G-CSF抑制了损伤血管的再内皮化,阻碍了损伤血管的修复。
1.2.3 核因子κB
核因子κB(nuclear factor-κB,NF-κB)作为一种蛋白质家族,能上调内皮素-1,促进细胞外基质(extracellular matrix,ECM)合成,加速SMC增殖[23]。
NF-κB家族,尤其是p65和p50在血管增殖性疾病的发病机制中发挥着重要作用,EC和巨噬细胞中的p65和p50可在血管损伤时被诱导。此外,NF-κB可促进SMC表型转变,不利于损伤血管的修复[24]。
1.3 血小板的黏附聚集和活化
血小板在血管损伤后可被激活,释放趋化因子,诱导和招募单核细胞迁移,导致巨噬细胞的浸润,引起支架植入后炎症和LST的发生。随后,活化的血小板刺激了SMC的增殖和ECM的分泌,诱发LST出现[25]。总之,血小板通过调控其黏附、聚集和活化,在PCI患者内皮损伤后再内皮化和血管重塑中发挥重要作用。
1.3.1 CD11b/CD18
冠状动脉支架植入可引起血小板和中性粒细胞表面的糖蛋白CD11b/CD18 (巨噬细胞分化抗原-1)的激活和上调,通过介导白细胞黏附于纤维蛋白原引起LST的发生。此外,CD11b/CD18的上调会引起中性粒细胞活化与可溶性细胞间黏附分子-1表达增加,引起新生内膜增厚,进而导致再内皮化延迟。
1.3.2 基质金属蛋白酶
基质金属蛋白酶(matrix metalloproteinase,MMP) 释放可溶的Kit-配体刺激内皮细胞增生,通过产生的生物活性ECM片段和调节基质与非基質的降解,对血管损伤的再内皮化起着关键作用[26]。MMP-9可介导G-CSF与趋化因子的协同动员,抑制损伤血管的再内皮化[27]。此外,MMP-7还能通过裂解血小板反应蛋白-1抑制血管损伤后EC的恢复,引起再内皮化延迟[28]。
1.4 纤维蛋白原的吸附
纤维蛋白原是一种参与血液凝固的蛋白质,非常容易被氧化,导致完整性和稳定性受到破坏。纤维蛋白原的吸附受到胆红素和纤维连接蛋白的调控,影响血小板的活化,进而影响损伤血管的再内皮化[29]。
1.4.1 胆红素
胆红素对纤维蛋白原具有抗氧化作用,可防止其羰基化和聚集[30]。纤维蛋白原的结合位点对胆红素不是立体特异性的,并且能容纳两个胆红素构象体,提高了抗氧化性能[31]。总之,纤维蛋白原和胆红素在生理浓度下相互作用,保证了纤维蛋白原的完整性,促进损伤血管的再内皮化。
1.4.2 纤维连接蛋白
纤维连接蛋白是ECM中的一种主要成分,由两个单体在其C端由二硫键连接而成。它能吸附或连接到生物材料的表面,促进EC的附着、扩散和分化。此外,纤维连接蛋白的生成会受到血浆聚合物膜等物质的调节,影响纤维蛋白原的吸附,进而实现对血管内皮化的调控[32]。
2 新型药物洗脱支架治疗
为了减少PCI后LST的发生,更好地改善预后,近年来一些新型DES被发展起来。根据再内皮化延迟的机制,通过覆盖在支架表面的药物来促进EC黏附和增殖,抑制SMC黏附和增殖,防止血小板黏附聚集和减少纤维蛋白原的吸附,从而加速损伤血管的再内皮化[33-35]。
没食子酸功能化作为一种增强再内皮化的策略,涂覆在支架表面不仅能增强EC黏附、增殖和迁移,还能抑制SMC黏附和增殖[36-37]。肝素是一种由糖醛酸和葡萄糖胺组成的高度硫酸化的糖胺聚糖,常作为抗凝药物用于防止金属支架上血栓形成。肝素的抗凝作用机制是通过与抗凝血酶Ⅲ反应而发生,抗凝血酶Ⅲ可使凝血酶及其他与血栓有关的蛋白酶失活[38-39]。一些研究[40]表明,肝素修饰的底物可增强EC的黏附、增殖和迁移,抑制SMC的生长和增殖,还能降低纤维蛋白原吸附和血小板黏附。岩藻多糖是从褐藻中提取的硫酸多糖,具有与肝素相似的抗凝作用。低分子量岩藻多糖可减少SMC的增殖,从而防止新生内膜增生,促进内皮修复[41]。
透明质酸是一种带负电荷的非硫酸化多糖,通过与细胞表面受体相互作用,在细胞附着和信号转导中发挥作用[42-43]。此外,在不锈钢支架上涂覆透明质酸可减少血小板的黏附和聚集。多巴胺偶联透明质酸涂覆支架也能抑制血小板的黏附和活化,维持内皮细胞的活力和增殖,表现出最低的纤维蛋白原吸附。NO可舒张血管,抑制血小板的聚集和SMC的增殖,在维持血管内环境稳态中发挥重要作用。许多研究[44-45]已证明,支架周围缺乏NO是血栓形成和再内皮化延迟的重要原因。因此,一些NO释放支架被发展起来用于改善临床上的LST。
为了获得有利于促进内皮细胞黏附和增殖的微环境,促进损伤血管的再内皮化,多种具有不同性质的生物分子共同涂覆在支架表面,形成了多功能涂层[46]。如肝素和纤维连接蛋白复合物共同固定在钛底物上形成的涂层,能减少血小板的黏附聚集,降低纤维蛋白原的吸附[47-48]。此外,在多功能微环境中使用肝素/多聚赖氨酸、VEGF对钛底物进行功能化,已被证明能抑制血栓形成,促进损伤血管的再内皮化[49]。
3 小结
血管损伤再内皮化的机制主要包括VEGF对EC黏附和增殖的调控、SMC黏附和增殖的调控、血小板的黏附聚集和活化以及纤维蛋白原的吸附。改善动脉粥样硬化患者再内皮化的新型DES为促进血管生成提供了新的治疗策略。未来应致力于具体的治疗措施来促进损伤血管的再内皮化和修复,抑制不良的内膜增生,减少甚至消除PCI后LST的发生,进一步提高支架治疗的安全性,改善患者的预后。
参考文献
[1]Ahadi F,Azadi M,Biglari M,et al.Evaluation of coronary stents:a review of types,materials,processing techniques,design,and problems[J].Heliyon,2023,9(2):e13575.
[2]Otto S,Díaz VAJ,Weilenmann D,et al.Crystalline sirolimus-coated balloon (cSCB) angioplasty in an all-comers,patient population with stable and unstable coronary artery disease including chronic total occlusions:rationale,methodology and design of the SCORE trial[J].BMC Cardiovasc Disord,2023,23(1):176.
[3]Butala NM,Yeh RW.Improvements in coronary stent design translate to better real-world outcomes[J].JACC Asia,2021,1(3):357-359.
[4]Park DS,Jeong MH,Jin YJ,et al.Preclinical evaluation of an everolimus-eluting bioresorbable vascular scaffold via a long-term rabbit iliac artery model[J].Tissue Eng Regener Med,2023,20(2):239-249.
[5]Vidovich MI.Three decades of SVG PCI:a short history of nearly everything[J].JACC Case Rep,2023,10:101744.
[6]Bedair TM,Cho Y,Joung YK,et al.Biodegradable polymer brush as nanocoupled interface for improving the durability of polymer coating on metal surface[J].Colloids Surf B Biointerfaces,2014,122:808-817.
[7]Zhang Y,Wu Z,Wang S,et al.Clinical outcome of paclitaxel-coated balloon angioplasty versus drug-eluting stent implantation for the treatment of coronary drug-eluting stent in-stent chronic total occlusion[J].Cardiovasc Drugs Ther,2023,37(6):1155-1166.
[8]Kawagoe Y,Otsuka F,Onozuka D,et al.Early vascular responses to abluminal biodegradable polymer-coated versus circumferential durable polymer-coated newer-generation drug-eluting stents in humans:a pathological study[J].EuroIntervention,2023,18(15):1284-1294.
[9]Bedair TM,Elnaggar MA,Joung YK,et al.Recent advances to accelerate re-endothelialization for vascular stents[J].J Tissue Eng,2017,8:2041731417731546.
[10]Kawarada O,Otsuka F,Miki K,et al.Heterogeneous vascular response after implantation of bare nitinol self-expanding stents in the swine femoropopliteal artery[J].Cardiovasc Interv Ther,2023,38(2):210-222.
[11]Zhu YX,Liang L,Parasa R,et al.Early vascular healing after neXt-generation drug-eluting stent implantation in Patients with non-ST Elevation acute Coronary syndrome based on optical coherence Tomography guidance and evaluation (EXPECT):study protocol for a randomized controlled trial[J].Front Cardiovasc Med,2023,10:1003546.
[12]Chang SH,Hla T.Post-transcriptional gene regulation by HuR and microRNAs in angiogenesis[J].Curr Opin Hematol,2014,21(3):235-240.
[13]Uren PJ,Burns SC,Ruan J,et al.Genomic analyses of the RNA-binding protein Hu antigen R (HuR) identify a complex network of target genes and novel characteristics of its binding sites[J].Biol Chem,2011,286(43):37063-37066.
[14]Zhu K,Gao T,Wang Z,et al.RNA N6-methyladenosine reader IGF2BP3 interacts with MYCN and facilitates neuroblastoma cell proliferation[J].Cell Death Discov,2023,9(1):151.
[15]Zheng X,Li S,Yu J,et al.N6-methyladenosine reader IGF2BP3 as a prognostic Biomarker contribute to malignant progression of glioma[J].Transl Cancer Res,2023,12(4):992-1005.
[16]He J,Ma X.Interaction between LncRNA and UPF1 in tumors[J].Front Genet,2021,12:624905.
[17]Palo A,Patel SA,Sahoo B,et al.FRG1 is a direct transcriptional regulator of nonsense-mediated mRNA decay genes[J].Genomics,2023,115(1):110539.
[18]Banerjee R,van Tubergen EA,Scanlon CS,et al.The G protein-coupled receptor GALR2 promotes angiogenesis in head and neck cancer[J].Mol Cancer Ther,2014,13(5):1323-1333.
[19]Zhang D,Zhou Z,Yang R,et al.Tristetraprolin,a potential safeguard against carcinoma:role in the tumor microenvironment[J].Front Oncol,2021,11:632189.
[20]Merkulova-Rainon T,Broquères-You D,Kubis N,et al.Towards the therapeutic use of vascular smooth muscle progenitor cells[J].Cardiovasc Res,2012,95(2):205-214.
[21]Liu Z,Zhang G,Chen J,et al.G-CSF promotes the viability and angiogenesis of injured liver via direct effects on the liver cells[J].Mol Biol Rep,2022,49(9):8715-8725.
[22]Ziauddin SM,Nakashima M,Watanabe H,et al.Biological characteristics and pulp regeneration potential of stem cells from canine deciduous teeth compared with those of permanent teeth[J].Stem Cell Res Ther,2022,13(1):439.
[23]Aggarwal V,Tuli HS,Varol A,et al.Role of reactive oxygen species in cancer progression:molecular mechanisms and recent advancements[J].Biomolecules,2019,9(11):735.
[24]Yoshida T,Yamashita M,Horimai C,et al.Smooth muscle-selective inhibition of nuclear factor-κB attenuates smooth muscle phenotypic switching and neointima formation following vascular injury[J].J Am Heart Assoc,2013,2(3):e000230.
[25]Rohani MG,Parks WC.Matrix remodeling by MMPs during wound repair[J].Matrix Biol,2015,44-46:113-121.
[26]Quintero-Fabin S,Arreola R,Becerril-Villanueva E,et al.Role of matrix metalloproteinases in angiogenesis and cancer[J].Front Oncol,2019,9:1370.
[27]Scheau C,Badarau IA,Costache R,et al.The role of matrix metalloproteinases in the epithelial-mesenchymal transition of hepatocellular carcinoma[J].Anal Cell Pathol(Amst),2019,2019:9423907.
[28]Kessler T,Zhang L,Liu Z,et al.ADAMTS-7 inhibits re-endothelialization of injured arteries and promotes vascular remodeling through cleavage of thrombospondin-1[J].Circulation,2015,131(13):1191.
[29]Gligorijevic′ N,ukalovic′ V,Penezic′ A,et al.Characterisation of the binding of dihydro-alpha-lipoic acid to fibrinogen and the effects on fibrinogen oxidation and fibrin formation[J].Int J Biol Macromol,2020,147:319-325.
[30]Longhi G,Ghidinelli S,Abbate S,et al.Insights into the structures of bilirubin and biliverdin from vibrational and electronic circular dichroism:history and perspectives[J].Molecules,2023,28(6):2564.
[31]Gligorijevic′ N,Minic′ S,Robajac′ D,et al.Characterisation and the effects of bilirubin binding to human fibrinogen[J].Int J Biol Macromol,2019,128:74-79.
[32]Buddhadasa M,Lerouge S,Girard P.Plasma polymer films to regulate fibrinogen adsorption:effect of pressure and competition with human serum albumin[J].Plasma Process Polym,2018,15(9):e1800040.
[33]Ariyaratne TV,Ademi Z,Huq M,et al.The real-world cost-effectiveness of coronary artery bypass surgery versus stenting in high-risk patients:propensity score-matched analysis of a single-centre experience[J].Appl Health Econ Health Policy,2018,16(5):661-674.
[34]Lee P,Brennan AL,Stub D,et al.Estimating the economic impacts of percutaneous coronary intervention in Australia:a registry-based cost burden study[J].BMJ Open,2021,11(12):e053305.
[35]Rykowska I,Nowak I,Nowak R.Drug-eluting stents and balloons—Materials,structure designs,and coating techniques:a review[J].Molecules,2020,25(20):4624.
[36]Jensen LO,Christiansen EH.Are drug-eluting stents safer than bare-metal stents?[J].Lancet,2019,393(10190):2472-2474.
[37]Piccolo R,Bonaa KH,Efthimiou O,et al.Drug-eluting or bare-metal stents for percutaneous coronary intervention:a systematic review and individual patient data meta-analysis of randomised clinical trials[J].Lancet,2019,393(10190):2503-2510.
[38]Yamamoto K,Sato T,Salem H,et al.Mechanisms and treatment outcomes of ostial right coronary artery in-stent restenosis[J].EuroIntervention,2023,19(5):e383-e393.
[39]Kim BY,Moon H,Kim SS,et al.Outcomes of percutaneous coronary intervention in elderly patients with rheumatoid arthritis:a nationwide population-based cohort study[J].Healthcare(Basel),2023,11(10):1381.
[40]Gherasie FA,Valentin C,Busnatu SS.Is there an advantage of ultrathin-strut drug-eluting stents over second- and third-generation drug-eluting stents?[J].J Pers Med,2023,13(5):753.
[41]Rigatelli G,Zuin M,Vassilev D,et al.Risk of dislodgement of ultrathin drug eluting stents versus thick drug eluting stents[J].Am J Cardiol,2020,125(11):1619-1623.
[42]Wang G,Zhao Q,Chen Q,et al.Comparison of drug-eluting balloon with repeat drug-eluting stent for recurrent drug-eluting stent in-stent restenosis[J].Coron Artery Dis,2019,30(7):473-480.
[43]Kaul U,Bhatia V.The trade-off of a long drug-eluting stent[J].EuroIntervention,2021,16(16):1297-1298.
[44]Zhang B,Qin Y,Wang Y.A nitric oxide-eluting and REDV peptide-conjugated coating promotes vascular healing[J].Biomaterials,2022,284:121478.
[45]Binder RK,Lüscher TF.Duration of dual antiplatelet therapy after coronary artery stenting:where is the sweet spot between ischaemia and bleeding?[J].Eur Heart J,2015,36(20):1207-1211.
[46]Yang L,Li L,Wu H,et al.Catechol-mediated and copper-incorporated multilayer coating:an endothelium-mimetic approach for blood-contacting devices[J].J Control Release,2020,321:59-70.
[47]Krger N,Kopp A,Staudt M,et al.Hemocompatibility of plasma electrolytic oxidation (PEO) coated Mg-RE and Mg-Zn-Ca alloys for vascular scaffold applications[J].Mater Sci Eng C Mater Biol Appl,2018,92:819-826.
[48]Hong Q,Zhou H,Cheng Y,et al.Synthesis of star 6-arm polyethylene glycol-heparin copolymer to construct anticorrosive and biocompatible coating on magnesium alloy surface[J].Front Bioeng Biotechnol,2022,10:853487.
[49]Liu Y,Zhang J,Wang J,et al.Tailoring of the dopamine coated surface with VEGF loaded heparin/poly-L-lysine particles for anticoagulation and accelerate in situ endothelialization[J].J Biomed Mater Res A,2015,103(6):2024-2034.
收稿日期:2023-08-02
通信作者:楊斌,E-mail:yangbin200612736@163.com

