Comparative study on effects of dexmedetomidine and dexamethasone on the incidence of postoperative nausea and vomiting in patients undergoing laparoscopic surgery
Manpreet Singh, Awadh Bihari Tiwari, Priya Taank, Shalendra Singh, Amrinder Kaur, Munish Sood, Rahul Yadav
1Department of Anaesthesiology & Critical Care, Air Force Hospital, Kanpur 208004, India
2Department of Ophthalmology, Command Hospital (SC), Pune 411040, India
3Department of Anaesthesiology & Critical Care, Armed forces Medical College, Pune 411040, India
4Department of Periodontics, 304 Field Hospital, Kanpur 208004, India
5Department of Orthopaedics, Indian Naval Hospital Ship Asvini, Mumbai 400005, India
6Department of Anaesthesiology & Critical Care, Indian Naval Hospital Ship Asvini, Mumbai 400005, India
ABSTRACT Objective: To compare the safety and efficacy of dexmedetomidine and dexamethasone for the prevention of postoperative nausea and vomiting (PONV) in patients scheduled for laparoscopic surgery.Methods: A total of 86 female patients were prospectively administered dexmedetomidine 1 µg/kg i.v.(the group A, n=43), and dexamethasone 8 mg i.v.(the group B, n=43). The two groups were compared in treatment response, hemodynamic changes, and Numerical Analog Scale (NAS).Besides, the relation of PONV with patient baseline characteristics in the perioperative period was determined as well.Results: Patients in group A had lower PONV scores (t=3.1, P<0.002), less needs for rescue anti-emetics (χ2=0.47, P<0.001), and decreased intraoperative heart rate (t=9.72, P<0.001) and mean arterial pressure (t=7.58, P<0.001) compared to that of group B.Group A reported lower NAS than group B (t=2.66, P<0.001).In addition, we found no relationship between PONV score and rescue anti-emetic requirement, age, or body mass index (P=0.96, P=0.60, P=0.28, respectively).Conclusion: Dexmedetomidine could be used as an effective antiemetic in laparoscopic surgeries, with better efficacy than dexamethasone.Dexmedetomidine not only can reduce PONV but also is effective in postoperative analgesia.
KEYWORDS: Dexmedetomidine; Postoperative nausea and vomiting; Dexamethasone; Laparoscopic surgery; Anti-emetic
1.Introduction

Postoperative nausea and vomiting (PONV) is one of the most common distresses, with incidence ranging 12%-38% and sometimes as high as 70% after in patients who underwent laparoscopic cholecystectomy (LC) but did not receive preventive anti-emetic treatment[1-3].The possible reasons for increased incidence are excessive bowel handling during surgery, bowel wall edema secondary to inflammation, as well as the release of 5-HT secondary to gut ischemia.Gastric inflation during mask ventilation, use of opioids and nitrous oxide, sex are other risk factors[4,5].Type of surgery also affects the incidence of PONV.Gynaecologic, cholecystectomy, and laparoscopic procedures are linked with increased risk of PONV compared with other general surgical procedures[4,5].Apart from decreasing patients’ satisfaction, it can also complicate recovery by causing oesophageal rupture, wound dehiscence, increased intracranial pressure, aspiration, dehydration, and unanticipated hospital admission.
Various common anesthetic drugs are involved in the induction of or protection against PONV[6-9].Dexamethasone is an economical and efficacious antiemetic drug with negligible side effects that have been reported for preventing PONV along with other drugs.Dexmedetomidine is a sedative-hypnotic drug having a highly selective α2-adrenoceptor agonist property[10].A recent meta-analysis revealed that dexmedetomidine bolus infusion at 0.5-1.0 µg/kg could adequately reduce the incidence of PONV[11].
The primary objective of this study was to compare the preventive effects of prophylactic dexmedetomidine and dexamethasone administration against PONV in patients undergoing laparoscopic abdominal surgeries.The study also aimed to find the effect of these two drugs on intraoperative hemodynamic parameters, postoperative Numerical Analogue Scale (NAS), and to determine the relation of PONV with physical baseline characteristics.
2.Patients and methods
2.1.Study design
This prospective study was carried out at a LevelⅠtertiary hospital from June 2018 to January 2019, including inpatients who were scheduled for elective laparoscopic surgery under general anesthesia (GA).
2.2.Sample size
The sample size was calculated keeping the true mean response rate for dexmedetomidine (θ1) 50% (0.5), the true mean response rate for dexamethasone (θ2) 22% (0.22), the marginal error was set at P<0.05, and the power of the study at 80% (0.8).A sample size of 43 was obtained.Considering observational bias, errors of sampling, withdrawn bias, and prevalence of PONV, the sample size was doubled.
2.3.Patients selection
Diagnosis of PONV was based on the American Society of Anaesthesiologists (ASA) classⅠorⅡ[12].Patients were between 18 to 60 years of age.As the incidence of PONV is higher in females, and the fact that a majority of laparoscopic surgeries were conducted on female patients at our center, only female patients undergoing laparoscopic surgery under GA were included.Patients with a known history of PONV, systemic disease with nausea and vomiting, body mass index≥36 kg/m2, and those requiring opioids in the postoperative period were excluded from the study.
2.4.Ethical consideration
This study was approved by the Institutional Ethical Committee (IEC committee no.1/2018, No.7 Air force hospital Kanpur, India dt 01 May 2018), and written informed consent was secured from the patients.
2.5.Treatment and follow-up
All patients were given glycopyrrolate 0.2 mg intravenously (i.v.), midazolam 0.035 mg/kg i.v., and fentanyl 1 µg/kg i.v.as part of premedication.During the study, 43 patients received dexmedetomidine 1 µg/kg i.v.as the group A, and another 43 patients received dexamethasone 8 mg i.v.as the group B.Patients were given with propofol 2 mg/kg i.v.and vecuronium 0.1 mg/kg i.v.for neuromuscular blockade.Anesthesia was maintained under controlled ventilation in volume control mode with positive end-expiratory pressure of 5 cm water, nitrous oxide, oxygen, and sevoflurane (FiO2of 0.4, MAC of 1.0).A tidal volume of 6-8 mL/kg body weight, respiratory rate of 12-14 breaths/min, and inspiratory to the expiratory ratio of 1:2 were kept intraoperatively.Ventilator parameters were tuned to maintain an end-tidal carbon dioxide between 32-36 mm Hg.Neuromuscular blockade was maintained with intermittent vecuronium 0.01 mg/kg i.v..An orogastric tube was inserted after intubation to deflate the stomach, and it was taken out before extubation.
2.6.Data collection
Patients were monitored using pulse oximetry (SpO2), non-invasive blood pressure (NIBP), electrocardiography (ECG), and end-tidal carbon dioxide.Heart rate (HR) and mean arterial pressure (MAP) were recorded every 15 min.All patients were put in a supine position with 30 degrees head higher than pelvis with a left lateral tilt of 15 degrees.Pneumoperitoneum was established with carbon dioxide, and intra-abdominal pressure was kept around 10-12 mmHg.During anesthesia, all patients were given Ringer’s lactate solution i.v.at a rate of 10-15 mL/kg/h.After surgery, reversal of neuromuscular blockade was achieved with neostigmine 0.05 mg/kg i.v., and glycopyrrolate 10 µg/kg i.v..Paracetamol infusion 15 mg/kg i.v.every 8 hours was given for postoperative analgesia.Postoperatively, patients were monitored for nausea, vomiting, and requirement of rescue anti-emetics.All scores were estimated 8 h after operation.
Nausea was defined as an unpleasant sensation with the urge to vomit without expulsion of gastric contents.Vomiting was defined as the vigorous ejection of gastric contents through the mouth.Ondansetron 4 mg i.v.was used as a rescue anti-emetic wherever required.For assessment of nausea postoperatively, patients were subjectively assessed on a 4-point grading scale (0=does not affect activities of daily living, 1=sometimes affects, 2=often or mostly affects, 3=unable to carry out activities of daily living[13,14]).Vomiting was recorded as per a 4-point of vomiting scale (0=no episode, 1=once, 2=twice, 3= thrice or more[14]).PONV scores were measured as nausea scores + vomiting scores.Postoperative pain was assessed using NAS, where 0 indicates no pain and 10 indicates the worst possible pain[15].
2.7.Statistical analysis
Statistical Package for Social Science version 16.0 (SPSS Inc.; Chicago, IL, USA) was used to analyze the data.Categorical data were expressed as frequency and percentage.Quantitative data were expressed as mean±SD.A two-tailed t-test for two independent samples and Chi-square test was used for statistical analysis of PONV, MAP, HR, and NAS.Pearson’s correlation coefficient was used to study an association between PONV scores and the requirement of additional anti-emetics in the postoperative period.
3.Results
The prophylactic efficacy of dexmedetomidine and dexamethasone against PONV was studied in 86 patients who underwent elective laparoscopic abdominal surgeries under GA.Out of a total of 98 patients scheduled for surgery in the course of the study period, 6 patients did not fall in inclusion criteria, and 6 patients were excluded due to refusal or unattainability.Finally, 86 patients were divided into the group A (n=43) and the group B (n=43) (Figure 1).Clinical profile and demographic characteristics were comparable between the two groups.Laparoscopic cholecystectomy was the most frequent surgery conducted during the study period, followed by laparoscopic cystectomy for adnexal mass (Table 1).

Figure 1.The study flowchart.

Table 1.Patient characteristics.

Table 2.Hemodynamic changes and pain and vomiting scores.
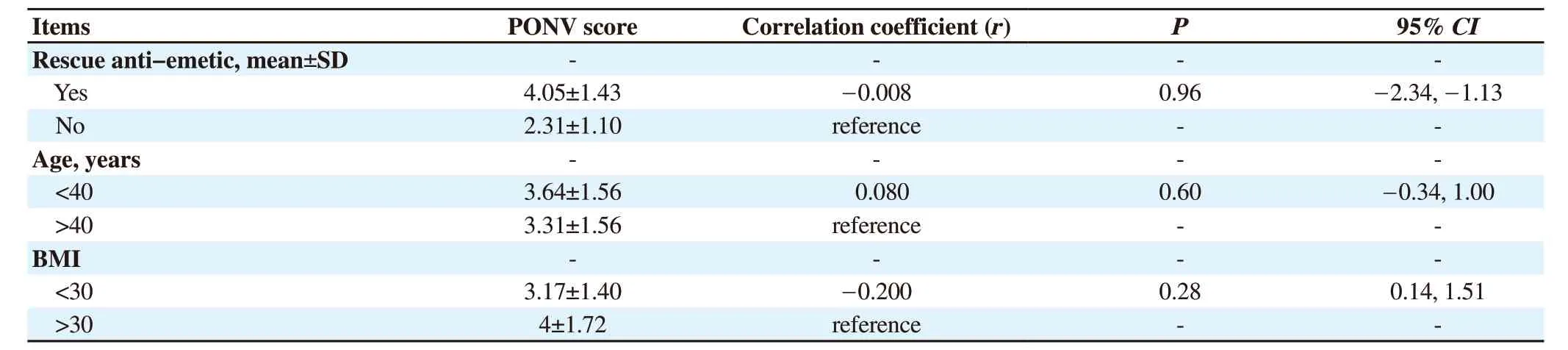
Table 3.The relation between PONV and rescue anti-emetic and age, BMI.
Patients in the group A had a significantly lower mean intraoperative HR and MAP (P<0.001) compared to the group B.NAS Score of the group A was significantly lower than that of the group B (P<0.01).Besides, the group A showed lower PONV scores and less rescue anti-emetic requests (P=0.002 and P<0.001, respectively) (Table 2).
Pearson’s correlation test was used to estimate the association between PONV score and requirement of rescue anti-emetic, and no significant correlation was found between the two variables (r=-0.008, P=0.96).Besides, no relation was found between PONV scores and age or BMI (r=0.080, P=0.60; r=-0.200, P=0.28) (Table 3).
4.Discussion
Despite advances in pharmacological and anesthetic management used in its prevention, PONV continues to be a significant problem.Even though laparoscopic surgeries aid in faster recovery, they also cause an increased incidence of PONV than other types of surgeries due to excessive gut handling and inflammation, carbon dioxide insufflation, and pneumoperitoneum, etc.
Our findings of reduction in the occurrence of PONV with dexmedetomidine were consistent with most studies available in the literature[16].In a meta-analysis consisting of 82 trials and 6 480 patients conducted by Liang et al., it was culminating that dexmedetomidine lessens postoperative nausea [relative risk (RR) 0.61] and vomiting (RR 0.48) when compared with placebo[11].It was also revealed that a higher dose of dexmedetomidine 1 µg/kg was more beneficial than a dose of 0.5 µg/kg (RR: 0.29 and 0.46, respectively). Jin et al.in their meta-analysis of randomized control trials concluded the superiority of dexmedetomidine over placebo in reducing PONV in patients scheduled for surgery under GA (RR: 0.56)[17].This meta-analysis also conclusively proved a reduction in perioperative opioid consumption (opioid-sparing effect), thus implying that a reduction in pain scores in the postoperative time in the dexmedetomidine group may be the contributing factor.This reduced requirement of opioids could also have contributed to less PONV in this group of patients.Kim et al., in their study, revealed a lower occurrence of PONV, and more importantly a significantly reduced severity of PONV in the course of the first 24 h after surgery with the use of intra-operative dexmedetomidine[18].Bakri et al.compared the effects of dexmedetomidine and dexamethasone in the prevention of PONV, wherein they found that although the occurrence and severity of PONV in the dexmedetomidine group were lessened than the dexamethasone group, this difference did not attain statistical significance[19].However, their study showed a significant reduction in the perioperative opioid requirement in patients exposed to dexmedetomidine, which could be attributed to the lower Visual Analog Scale score which they used for pain assessment.
The present study also revealed better postoperative analgesia and thus a reduction in perioperative opioid requirement in the dexmedetomidine group of patients.Possible explanations for lesser PONV in the dexmedetomidine group could also be lesser consumption of perioperative opioids and intraoperative inhaled anesthetics.
It was found that mean MAP and HR in the dexmedetomidine group were significantly lower than that of the dexamethasone group.Khare et al.in their study on patients undergoing laparoscopic cholecystectomy noted that dexmedetomidine achieved significantly lower MAP and HR values at multiple instances during the surgery[20].A study presented by Masoori et al.showed a significant attenuation of hemodynamic stress response in patients undergoing laparoscopic cholecystectomy[21].It may be hypothesized that stable intraoperative hemodynamic parameters independently contribute to a reduction in PONV.However, further studies are required to conclusively prove this association.
A statistically significant lower pain scores was detected in patients of the dexmedetomidine group, implying this drug’s favorable role in postoperative analgesia as well.Sharma et al.found a statistically significant lower Visual Analog Scale pain score as well as a reduction in postoperative analgesic requirement when dexmedetomidine infusion was used intraoperatively over paracetamol infusion in patients scheduled for laparoscopic cholecystectomy[22].Panchgar et al.studied the effects of dexmedetomidine infusion intraoperatively in laparoscopic surgeries and observed similar favorable effects on efficacy and duration of postoperative analgesia when compared with placebo[23].Furthermore, the role of dexamethasone in prolonging postoperative analgesia and opioid-sparing effect has also been documented.Waldron et al.in their meta-analysis of 45 articles revealed lower pain scores and diminished requirement of opioids up to 24 h postoperatively in patients taken for surgery under GA[24].Similar results have been obtained by various studies[25,26].Some studies compared the effects of dexmedetomidine and dexamethasone on postoperative analgesia and found better outcomes with the former[19,27].In the present study as well, patients in the dexmedetomidine group reported statistically significant lower pain scores.
Limitations of the present study include the restriction of the sample population to female patients only.Secondly, the scale used by us for the measurement of PONV is the most commonly used PONV measurement tool referred to in literature.But it may not be optimal for assessing PONV occurring in association with laparoscopic surgeries under GA.Thirdly, this study has not examined the efficacy in non-fasting patients or those with a full stomach, should the need of operating on such patients arise.Preoperative volume status may affect intraoperative hypotension and PONV, so patients should be evaluated for their intravascular volume before surgery using measurement techniques like inferior vena cava diameter.Finally, the sample is comparatively small, data from a substantially larger cohort in daily practice is required for its safe use.
To sum up, dexmedetomidine has the potential to be used as an effective antiemetic in laparoscopic surgeries, with better efficacy than dexamethasone.Dexmedetomidine not only reduced the PONV but was also helpful as postoperative analgesia.However, further studies require to be conducted to endorse these findings.
Conflict of interest statement
The authors report no conflict of interest.
Authors’ contributions
S.M.(Manpreet Singh) and K.A.collected and curated the data; T.A.B.guided and concept of Study; S.S., T.P., Y.R., and S.M.(Munish Sood) contributed to writing the manuscript; All authors read and approved the final manuscript.
 Journal of Acute Disease2022年2期
Journal of Acute Disease2022年2期
- Journal of Acute Disease的其它文章
- Electrocardiographic abnormalities in prevalent infections in tropical regions: A scoping review
- Goal-directed fluid therapy in gastrointestinal cancer surgery: A prospective randomized study
- Effect of cold weather on carotid artery stenosis and occlusion: A retrospective observational study
- Body mass index and COVID-19 outcomes: A retrospective crosssectional study at a tertiary care center in India
- Mortality characteristics during the two waves of COVID-19 in India: A retrospective observational study
- An alveolate kidney: A case report of emphysema pyelonephritis
